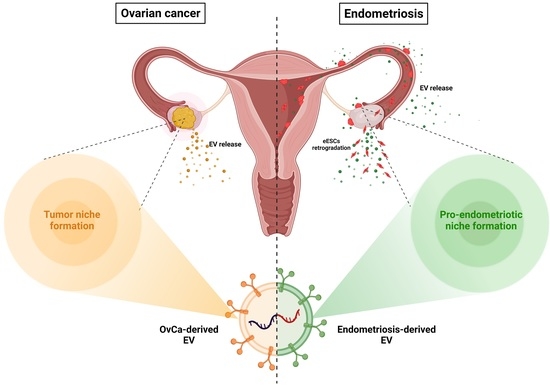
Immunosuppressive Extracellular Vesicles as a Linking Factor in the Development of Tumor and Endometriotic Lesions in the Gynecologic Tract
Abstract
:1. Introduction
2. Biogenesis, Components and Characterization of Extracellular Vesicles
3. Extracellular Vesicles in Ovarian Cancer
3.1. EVs as Mediators of Immunosuppression in Ovarian Cancer
3.1.1. EV-Mediated Suppression of T-Cells
3.1.2. EV-Mediated Suppression of NK-Cells and DCs
3.1.3. EV-Mediated Suppression of Macrophages
3.2. Indirect EV-Mediated Immunosuppression
3.2.1. EVs Promote Differentiation, Expansion and Functions of Tregs
3.2.2. EV-Mediated Remodeling of Other Cells of the TME towards Immunosuppression
3.3. Therapeutic Applications of EVs in Ovarian Cancer
3.3.1. EVs as Drug Carriers
3.3.2. EVs as Cancer Vaccines
3.3.3. EVs as Therapeutic Targets
4. Extracellular Vesicles in Endometrial Cancer
4.1. EVs as Mediators of Immunosuppression in Endometrial Cancer
4.1.1. EV-Mediated Suppression of Macrophages
4.1.2. EV-Mediated Immunosuppression of Other Non-Immune Cells
4.2. Therapeutic Applications of EVs in Endometrial Cancer
5. Epidemiologic and Genetic Connections between Ovarian Cancer, Endometrial Cancer and Endometriosis
6. Extracellular Vesicles in Endometriosis
6.1. EVs as Mediators of Immunosuppression in Endometriosis
6.1.1. EV-Mediated Suppression of Macrophages
6.1.2. EV-Mediated Suppression of T-Cells
6.1.3. EV-Mediated Immunosuppression of Other Non-Immune Cells
6.2. Therapeutic Applications of EVs in Endometriosis
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paget, S. The distribution of secondary growths in cancer of the breast. Cancer Metastasis Rev. 1989, 8, 98–101. [Google Scholar] [CrossRef] [Green Version]
- Sampson, J.A. Metastatic or Embolic Endometriosis, due to the Menstrual Dissemination of Endometrial Tissue into the Venous Circulation. Am. J. Pathol. 1927, 3, 93–110.43. [Google Scholar] [PubMed]
- Akhtar, M.; Haider, A.; Rashid, S.; Al-Nabet, A. Paget’s “Seed and Soil” Theory of Cancer Metastasis: An Idea Whose Time has Come. Adv. Anat. Pathol. 2019, 26, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.J.; Yang, H.L.; Shao, J.; Mei, J.; Chang, K.K.; Zhu, R.; Li, M.Q. Anti-inflammatory cytokines in endometriosis. Cell Mol. Life Sci. 2019, 76, 2111–2132. [Google Scholar] [CrossRef]
- Alsina-Sanchis, E.; Figueras, A.; Lahiguera, A.; Gil-Martin, M.; Pardo, B.; Piulats, J.M.; Marti, L.; Ponce, J.; Matias-Guiu, X.; Vidal, A.; et al. TGFbeta Controls Ovarian Cancer Cell Proliferation. Int. J. Mol. Sci. 2017, 18, 1658. [Google Scholar] [CrossRef]
- Basu, M.; Bhattacharya, R.; Ray, U.; Mukhopadhyay, S.; Chatterjee, U.; Roy, S.S. Invasion of ovarian cancer cells is induced byPITX2-mediated activation of TGF-beta and Activin-A. Mol. Cancer 2015, 14, 162. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Liu, G. Endometriosis-associated Ovarian Clear Cell Carcinoma: A Special Entity? J. Cancer 2021, 12, 6773–6786. [Google Scholar] [CrossRef]
- Nakamura, K.; Sawada, K.; Kobayashi, M.; Miyamoto, M.; Shimizu, A.; Yamamoto, M.; Kinose, Y.; Kimura, T. Role of the Exosome in Ovarian Cancer Progression and Its Potential as a Therapeutic Target. Cancers 2019, 11, 1147. [Google Scholar] [CrossRef] [Green Version]
- Nogués, L.; Benito-Martin, A.; Hergueta-Redondo, M.; Peinado, H. The influence of tumour-derived extracellular vesicles on local and distal metastatic dissemination. Mol. Asp. Med. 2018, 60, 15–26. [Google Scholar] [CrossRef]
- Freger, S.; Leonardi, M.; Foster, W.G. Exosomes and their cargo are important regulators of cell function in endometriosis. Reprod. Biomed. Online 2021, 43, 370–378. [Google Scholar] [CrossRef]
- Urbanelli, L.; Magini, A.; Buratta, S.; Brozzi, A.; Sagini, K.; Polchi, A.; Tancini, B.; Emiliani, C. Signaling pathways in exosomes biogenesis, secretion and fate. Genes 2013, 4, 152–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ji, Q.; Yang, Y.; Li, Q.; Wang, Z. Exosome: Function and Role in Cancer Metastasis and Drug Resistance. Technol. Cancer Res. Treat. 2018, 17, 1533033818763450. [Google Scholar] [CrossRef] [Green Version]
- Lotvall, J.; Hill, A.F.; Hochberg, F.; Buzas, E.I.; Di Vizio, D.; Gardiner, C.; Gho, Y.S.; Kurochkin, I.V.; Mathivanan, S.; Quesenberry, P.; et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles 2014, 3, 26913. [Google Scholar] [CrossRef]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- Mathivanan, S.; Simpson, R.J. ExoCarta: A compendium of exosomal proteins and RNA. Proteomics 2009, 9, 4997–5000. [Google Scholar] [CrossRef]
- Yanez-Mo, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borras, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [Green Version]
- Esfandyari, S.; Elkafas, H.; Chugh, R.M.; Park, H.S.; Navarro, A.; Al-Hendy, A. Exosomes as Biomarkers for Female Reproductive Diseases Diagnosis and Therapy. Int. J. Mol. Sci. 2021, 22, 2165. [Google Scholar] [CrossRef]
- Dorayappan, K.D.P.; Wanner, R.; Wallbillich, J.J.; Saini, U.; Zingarelli, R.; Suarez, A.A.; Cohn, D.E.; Selvendiran, K. Hypoxia-induced exosomes contribute to a more aggressive and chemoresistant ovarian cancer phenotype: A novel mechanism linking STAT3/Rab proteins. Oncogene 2018, 37, 3806–3821. [Google Scholar] [CrossRef]
- Czystowska-Kuzmicz, M.; Whiteside, T.L. The potential role of tumor-derived exosomes in diagnosis, prognosis, and response to therapy in cancer. Expert. Opin. Biol. 2021, 21, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Kalra, H.; Simpson, R.J.; Ji, H.; Aikawa, E.; Altevogt, P.; Askenase, P.; Bond, V.C.; Borras, F.E.; Breakefield, X.; Budnik, V.; et al. Vesiclepedia: A compendium for extracellular vesicles with continuous community annotation. PLoS Biol. 2012, 10, e1001450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keerthikumar, S.; Chisanga, D.; Ariyaratne, D.; Al Saffar, H.; Anand, S.; Zhao, K.; Samuel, M.; Pathan, M.; Jois, M.; Chilamkurti, N.; et al. ExoCarta: A Web-Based Compendium of Exosomal Cargo. J. Mol. Biol. 2016, 428, 688–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, D.D.; Gercel-Taylor, C.; Lyons, K.S.; Stanson, J.; Whiteside, T.L. T-cell apoptosis and suppression of T-cell receptor/CD3-zeta by Fas ligand-containing membrane vesicles shed from ovarian tumors. Clin. Cancer Res. 2003, 9, 5113–5119. [Google Scholar]
- Taylor, D.D.; Gercel-Taylor, C. Tumour-derived exosomes and their role in cancer-associated T-cell signalling defects. Br. J. Cancer 2005, 92, 305–311. [Google Scholar] [CrossRef]
- Meng, Y.; Kang, S.; Fishman, D.A. Lysophosphatidic acid stimulates fas ligand microvesicle release from ovarian cancer cells. Cancer Immunol. Immunother. 2005, 54, 807–814. [Google Scholar] [CrossRef]
- Webb, T.J.; Li, X.; Giuntoli, R.L., 2nd; Lopez, P.H.; Heuser, C.; Schnaar, R.L.; Tsuji, M.; Kurts, C.; Oelke, M.; Schneck, J.P. Molecular identification of GD3 as a suppressor of the innate immune response in ovarian cancer. Cancer Res. 2012, 72, 3744–3752. [Google Scholar] [CrossRef] [Green Version]
- Shenoy, G.N.; Loyall, J.; Berenson, C.S.; Kelleher, R.J., Jr.; Iyer, V.; Balu-Iyer, S.V.; Odunsi, K.; Bankert, R.B. Sialic Acid-Dependent Inhibition of T Cells by Exosomal Ganglioside GD3 in Ovarian Tumor Microenvironments. J. Immunol. 2018, 201, 3750–3758. [Google Scholar] [CrossRef] [Green Version]
- Asare-Werehene, M.; Communal, L.; Carmona, E.; Han, Y.; Song, Y.S.; Burger, D.; Mes-Masson, A.M.; Tsang, B.K. Plasma Gelsolin Inhibits CD8(+) T-cell Function and Regulates Glutathione Production to Confer Chemoresistance in Ovarian Cancer. Cancer Res. 2020, 80, 3959–3971. [Google Scholar] [CrossRef]
- Kelleher, R.J., Jr.; Balu-Iyer, S.; Loyall, J.; Sacca, A.J.; Shenoy, G.N.; Peng, P.; Iyer, V.; Fathallah, A.M.; Berenson, C.S.; Wallace, P.K.; et al. Extracellular Vesicles Present in Human Ovarian Tumor Microenvironments Induce a Phosphatidylserine-Dependent Arrest in the T-cell Signaling Cascade. Cancer Immunol. Res. 2015, 3, 1269–1278. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Yao, Y.; Jin, M. Circ-0001068 is a novel biomarker for ovarian cancer and inducer of PD1 expression in T cells. Aging 2020, 12, 19095–19106. [Google Scholar] [CrossRef]
- Peng, P.; Yan, Y.; Keng, S. Exosomes in the ascites of ovarian cancer patients: Origin and effects on anti-tumor immunity. Oncol. Rep. 2011, 25, 749–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muller, L.; Mitsuhashi, M.; Simms, P.; Gooding, W.E.; Whiteside, T.L. Tumor-derived exosomes regulate expression of immune function-related genes in human T cell subsets. Sci. Rep. 2016, 6, 20254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Yang, Y.; Xiong, A.; Wu, X.; Xie, J.; Han, S.; Zhao, S. Comparative Gene Expression Analysis of Lymphocytes Treated with Exosomes Derived from Ovarian Cancer and Ovarian Cysts. Front. Immunol. 2017, 8, 607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Zhou, J.; Zhuo, Q.; Zhang, J.; Xie, J.; Han, S.; Zhao, S. Malignant ascite-derived extracellular vesicles inhibit T cell activity by upregulating Siglec-10 expression. Cancer Manag. Res. 2019, 11, 7123–7134. [Google Scholar] [CrossRef] [Green Version]
- Muller, L.; Simms, P.; Hong, C.S.; Nishimura, M.I.; Jackson, E.K.; Watkins, S.C.; Whiteside, T.L. Human tumor-derived exosomes (TEX) regulate Treg functions via cell surface signaling rather than uptake mechanisms. Oncoimmunology 2017, 6, e1261243. [Google Scholar] [CrossRef]
- Shenoy, G.N.; Loyall, J.; Maguire, O.; Iyer, V.; Kelleher, R.J., Jr.; Minderman, H.; Wallace, P.K.; Odunsi, K.; Balu-Iyer, S.V.; Bankert, R.B. Exosomes Associated with Human Ovarian Tumors Harbor a Reversible Checkpoint of T-cell Responses. Cancer Immunol. Res. 2018, 6, 236–247. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Conrad, D.M.; Butler, J.J.; Zhao, C.; Blay, J.; Hoskin, D.W. Adenosine acts through A2 receptors to inhibit IL-2-induced tyrosine phosphorylation of STAT5 in T lymphocytes: Role of cyclic adenosine 3′,5′-monophosphate and phosphatases. J. Immunol. 2004, 173, 932–944. [Google Scholar] [CrossRef] [Green Version]
- Mandapathil, M.; Szczepanski, M.J.; Szajnik, M.; Ren, J.; Jackson, E.K.; Johnson, J.T.; Gorelik, E.; Lang, S.; Whiteside, T.L. Adenosine and prostaglandin E2 cooperate in the suppression of immune responses mediated by adaptive regulatory T cells. J. Biol. Chem. 2010, 285, 27571–27580. [Google Scholar] [CrossRef] [Green Version]
- Clayton, A.; Al-Taei, S.; Webber, J.; Mason, M.D.; Tabi, Z. Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production. J. Immunol. 2011, 187, 676–683. [Google Scholar] [CrossRef]
- Schuler, P.J.; Saze, Z.; Hong, C.S.; Muller, L.; Gillespie, D.G.; Cheng, D.; Harasymczuk, M.; Mandapathil, M.; Lang, S.; Jackson, E.K.; et al. Human CD4+ CD39+ regulatory T cells produce adenosine upon co-expression of surface CD73 or contact with CD73+ exosomes or CD73+ cells. Clin. Exp. Immunol. 2014, 177, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Czystowska-Kuzmicz, M.; Sosnowska, A.; Nowis, D.; Ramji, K.; Szajnik, M.; Chlebowska-Tuz, J.; Wolinska, E.; Gaj, P.; Grazul, M.; Pilch, Z.; et al. Small extracellular vesicles containing arginase-1 suppress T-cell responses and promote tumor growth in ovarian carcinoma. Nat. Commun. 2019, 10, 3000. [Google Scholar] [CrossRef] [PubMed]
- Del Vecchio, F.; Martinez-Rodriguez, V.; Schukking, M.; Cocks, A.; Broseghini, E.; Fabbri, M. Professional killers: The role of extracellular vesicles in the reciprocal interactions between natural killer, CD8+ cytotoxic T-cells and tumour cells. J. Extracell. Vesicles 2021, 10, e12075. [Google Scholar] [CrossRef] [PubMed]
- Labani-Motlagh, A.; Israelsson, P.; Ottander, U.; Lundin, E.; Nagaev, I.; Nagaeva, O.; Dehlin, E.; Baranov, V.; Mincheva-Nilsson, L. Differential expression of ligands for NKG2D and DNAM-1 receptors by epithelial ovarian cancer-derived exosomes and its influence on NK cell cytotoxicity. Tumour. Biol. 2016, 37, 5455–5466. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.; Konig, A.K.; Marme, F.; Runz, S.; Wolterink, S.; Koensgen, D.; Mustea, A.; Sehouli, J.; Altevogt, P. Systemic presence and tumor-growth promoting effect of ovarian carcinoma released exosomes. Cancer Lett. 2009, 278, 73–81. [Google Scholar] [CrossRef]
- Hosseini, R.; Asef-Kabiri, L.; Yousefi, H.; Sarvnaz, H.; Salehi, M.; Akbari, M.E.; Eskandari, N. The roles of tumor-derived exosomes in altered differentiation, maturation and function of dendritic cells. Mol. Cancer 2021, 20, 83. [Google Scholar] [CrossRef]
- Kryczek, I.; Zou, L.; Rodriguez, P.; Zhu, G.; Wei, S.; Mottram, P.; Brumlik, M.; Cheng, P.; Curiel, T.; Myers, L.; et al. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J. Exp. Med. 2006, 203, 871–881. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, J.; Li, X.; Wang, X.; Lin, Y.; Wang, X. Exosomes derived from hypoxic epithelial ovarian cancer cells deliver microRNAs to macrophages and elicit a tumor-promoted phenotype. Cancer Lett. 2018, 435, 80–91. [Google Scholar] [CrossRef]
- Chen, X.; Ying, X.; Wang, X.; Wu, X.; Zhu, Q.; Wang, X. Exosomes derived from hypoxic epithelial ovarian cancer deliver microRNA-940 to induce macrophage M2 polarization. Oncol. Rep. 2017, 38, 522–528. [Google Scholar] [CrossRef] [Green Version]
- Ying, X.; Wu, Q.; Wu, X.; Zhu, Q.; Wang, X.; Jiang, L.; Chen, X.; Wang, X. Epithelial ovarian cancer-secreted exosomal miR-222-3p induces polarization of tumor-associated macrophages. Oncotarget 2016, 7, 43076–43087. [Google Scholar] [CrossRef] [Green Version]
- Kanlikilicer, P.; Bayraktar, R.; Denizli, M.; Rashed, M.H.; Ivan, C.; Aslan, B.; Mitra, R.; Karagoz, K.; Bayraktar, E.; Zhang, X.; et al. Exosomal miRNA confers chemo resistance via targeting Cav1/p-gp/M2-type macrophage axis in ovarian cancer. EBioMedicine 2018, 38, 100–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu, A.; Sawada, K.; Kobayashi, M.; Yamamoto, M.; Yagi, T.; Kinose, Y.; Kodama, M.; Hashimoto, K.; Kimura, T. Exosomal CD47 Plays an Essential Role in Immune Evasion in Ovarian Cancer. Mol. Cancer Res. 2021, 19, 1583–1595. [Google Scholar] [CrossRef] [PubMed]
- Dutsch-Wicherek, M.M.; Szubert, S.; Dziobek, K.; Wisniewski, M.; Lukaszewska, E.; Wicherek, L.; Jozwicki, W.; Rokita, W.; Koper, K. Analysis of the treg cell population in the peripheral blood of ovarian cancer patients in relation to the long-term outcomes. Ginekol. Polska 2019, 90, 179–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curiel, T.J.; Coukos, G.; Zou, L.; Alvarez, X.; Cheng, P.; Mottram, P.; Evdemon-Hogan, M.; Conejo-Garcia, J.R.; Zhang, L.; Burow, M.; et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 2004, 10, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Szajnik, M.; Czystowska, M.; Szczepanski, M.J.; Mandapathil, M.; Whiteside, T.L. Tumor-derived microvesicles induce, expand and up-regulate biological activities of human regulatory T cells (Treg). PLoS ONE 2010, 5, e11469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef]
- Mabuchi, S.; Sasano, T.; Komura, N. Targeting Myeloid-Derived Suppressor Cells in Ovarian Cancer. Cells 2021, 10, 329. [Google Scholar] [CrossRef]
- Chalmin, F.; Ladoire, S.; Mignot, G.; Vincent, J.; Bruchard, M.; Remy-Martin, J.P.; Boireau, W.; Rouleau, A.; Simon, B.; Lanneau, D.; et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J. Clin. Investig. 2010, 120, 457–471. [Google Scholar] [CrossRef]
- Gobbo, J.; Marcion, G.; Cordonnier, M.; Dias, A.M.M.; Pernet, N.; Hammann, A.; Richaud, S.; Mjahed, H.; Isambert, N.; Clausse, V.; et al. Restoring Anticancer Immune Response by Targeting Tumor-Derived Exosomes with a HSP70 Peptide Aptamer. J. Natl. Cancer Inst. 2016, 108, djy330. [Google Scholar] [CrossRef]
- Rashid, M.H.; Borin, T.F.; Ara, R.; Piranlioglu, R.; Achyut, B.R.; Korkaya, H.; Liu, Y.; Arbab, A.S. Critical immunosuppressive effect of MDSCderived exosomes in the tumor microenvironment. Oncol. Rep. 2021, 45, 1171–1181. [Google Scholar] [CrossRef]
- Yeung, T.L.; Leung, C.S.; Li, F.; Wong, S.S.; Mok, S.C. Targeting Stromal-Cancer Cell Crosstalk Networks in Ovarian Cancer Treatment. Biomolecules 2016, 6, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giusti, I.; Di Francesco, M.; D’Ascenzo, S.; Palmerini, M.G.; Macchiarelli, G.; Carta, G.; Dolo, V. Ovarian cancer-derived extracellular vesicles affect normal human fibroblast behavior. Cancer Biol. 2018, 19, 722–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, J.A.; Park, H.; Lim, E.H.; Kim, K.H.; Choi, J.S.; Lee, J.H.; Shin, J.W.; Lee, K.W. Exosomes from ovarian cancer cells induce adipose tissue-derived mesenchymal stem cells to acquire the physical and functional characteristics of tumor-supporting myofibroblasts. Gynecol. Oncol. 2011, 123, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, X.; Wang, J.; Li, M.; Cao, C.; Tan, J.; Ma, D.; Gao, Q. TGFbeta1 in fibroblasts-derived exosomes promotes epithelial-mesenchymal transition of ovarian cancer cells. Oncotarget 2017, 8, 96035–96047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tai, Y.L.; Chen, K.C.; Hsieh, J.T.; Shen, T.L. Exosomes in cancer development and clinical applications. Cancer Sci. 2018, 109, 2364–2374. [Google Scholar] [CrossRef] [Green Version]
- Geng, T.; Pan, P.; Leung, E.; Chen, Q.; Chamley, L.; Wu, Z. Recent Advancement and Technical Challenges in Developing Small Extracellular Vesicles for Cancer Drug Delivery. Pharm. Res. 2021, 38, 179–197. [Google Scholar] [CrossRef]
- He, D.; Xu, X.; Li, L.; Chen, C.; Gong, K.; Guo, Q.; Liu, F.; Wang, Y.; Duan, Y.; Li, H. Functional Exosome-Mediated Delivery of Triptolide Endowed with Targeting Properties as Chemotherapy Carriers for Ovarian Carcinoma. J. Biomed. Nanotechnol. 2021, 17, 426–438. [Google Scholar] [CrossRef]
- Pisano, S.; Pierini, I.; Gu, J.; Gazze, A.; Francis, L.W.; Gonzalez, D.; Conlan, R.S.; Corradetti, B. Immune (Cell) Derived Exosome Mimetics (IDEM) as a Treatment for Ovarian Cancer. Front. Cell Dev. Biol. 2020, 8, 553576. [Google Scholar] [CrossRef]
- Giannopoulou, L.; Zavridou, M.; Kasimir-Bauer, S.; Lianidou, E.S. Liquid biopsy in ovarian cancer: The potential of circulating miRNAs and exosomes. Transl. Res. 2019, 205, 77–91. [Google Scholar] [CrossRef]
- Yoshida, K.; Yokoi, A.; Kato, T.; Ochiya, T.; Yamamoto, Y. The clinical impact of intra- and extracellular miRNAs in ovarian cancer. Cancer Sci. 2020, 111, 3435–3444. [Google Scholar] [CrossRef]
- Morse, M.A.; Garst, J.; Osada, T.; Khan, S.; Hobeika, A.; Clay, T.M.; Valente, N.; Shreeniwas, R.; Sutton, M.A.; Delcayre, A.; et al. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J. Transl. Med. 2005, 3, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Besse, B.; Charrier, M.; Lapierre, V.; Dansin, E.; Lantz, O.; Planchard, D.; Le Chevalier, T.; Livartoski, A.; Barlesi, F.; Laplanche, A.; et al. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology 2016, 5, e1071008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escudier, B.; Dorval, T.; Chaput, N.; Andre, F.; Caby, M.P.; Novault, S.; Flament, C.; Leboulaire, C.; Borg, C.; Amigorena, S.; et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: Results of thefirst phase I clinical trial. J. Transl. Med. 2005, 3, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, S.; Wei, D.; Wu, Z.; Zhou, X.; Wei, X.; Huang, H.; Li, G. Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol. Ther. 2008, 16, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Navabi, H.; Croston, D.; Hobot, J.; Clayton, A.; Zitvogel, L.; Jasani, B.; Bailey-Wood, R.; Wilson, K.; Tabi, Z.; Mason, M.D.; et al. Preparation of human ovarian cancer ascites-derived exosomes for a clinical trial. Blood Cells Mol. Dis. 2005, 35, 149–152. [Google Scholar] [CrossRef]
- Adams, M.; Navabi, H.; Croston, D.; Coleman, S.; Tabi, Z.; Clayton, A.; Jasani, B.; Mason, M.D. The rationale for combined chemo/immunotherapy using a Toll-like receptor 3 (TLR3) agonist and tumour-derived exosomes in advanced ovarian cancer. Vaccine 2005, 23, 2374–2378. [Google Scholar] [CrossRef] [PubMed]
- Rughetti, A.; Rahimi, H.; Belleudi, F.; Napoletano, C.; Battisti, F.; Zizzari, I.G.; Antonilli, M.; Bellati, F.; Wandall, H.H.; Benedetti Panici, P.; et al. Microvesicle cargo of tumor-associated MUC1 to dendritic cells allows cross-presentation and specific carbohydrate processing. Cancer Immunol. Res. 2014, 2, 177–186. [Google Scholar] [CrossRef] [Green Version]
- Taylor, D.D.; Gercel-Taylor, C.; Parker, L.P. Patient-derived tumor-reactive antibodies as diagnostic markers for ovarian cancer. Gynecol. Oncol. 2009, 115, 112–120. [Google Scholar] [CrossRef] [Green Version]
- Heubner, M.; Errico, D.; Kasimir-Bauer, S.; Herlyn, D.; Kimmig, R.; Wimberger, P. EpCAM-autoantibody levels in the course of disease of ovarian cancer patients. Med. Oncol. 2011, 28, 626–630. [Google Scholar] [CrossRef]
- Kim, J.H.; Herlyn, D.; Wong, K.K.; Park, D.C.; Schorge, J.O.; Lu, K.H.; Skates, S.J.; Cramer, D.W.; Berkowitz, R.S.; Mok, S.C. Identification of epithelial cell adhesion molecule autoantibody in patients with ovarian cancer. Clin. Cancer Res. 2003, 9, 4782–4791. [Google Scholar]
- Disis, M.L.; Pupa, S.M.; Gralow, J.R.; Dittadi, R.; Menard, S.; Cheever, M.A. High-titer HER-2/neu protein-specific antibody can be detected in patients with early-stage breast cancer. J. Clin. Oncol. 1997, 15, 3363–3367. [Google Scholar] [CrossRef] [PubMed]
- Battke, C.; Ruiss, R.; Welsch, U.; Wimberger, P.; Lang, S.; Jochum, S.; Zeidler, R. Tumour exosomes inhibit binding of tumour-reactive antibodies to tumour cells and reduce ADCC. Cancer Immunol. Immunother. 2011, 60, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Aung, T.; Chapuy, B.; Vogel, D.; Wenzel, D.; Oppermann, M.; Lahmann, M.; Weinhage, T.; Menck, K.; Hupfeld, T.; Koch, R.; et al. Exosomal evasion of humoral immunotherapy in aggressive B-cell lymphoma modulated by ATP-binding cassette transporter A3. Proc. Natl. Acad. Sci. USA 2011, 108, 15336–15341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De la Fuente, A.; Alonso-Alconada, L.; Costa, C.; Cueva, J.; Garcia-Caballero, T.; Lopez-Lopez, R.; Abal, M. M-Trap: Exosome-Based Capture of Tumor Cells as a New Technology in Peritoneal Metastasis. J. Natl. Cancer Inst. 2015, 107, djy184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catalano, M.; O’Driscoll, L. Inhibiting extracellular vesicles formation and release: A review of EV inhibitors. J. Extracell. Vesicles 2020, 9, 1703244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayatudin, R.; Fong, Z.; Ming, L.C.; Goh, B.H.; Lee, W.L.; Kifli, N. Overcoming Chemoresistance via Extracellular Vesicle Inhibition. Front. Mol. Biosci. 2021, 8, 629874. [Google Scholar] [CrossRef]
- Hoshino, A.; Costa-Silva, B.; Shen, T.L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef] [Green Version]
- Robert, C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat. Commun. 2020, 11, 3801. [Google Scholar] [CrossRef]
- Massaro, C.; Min, W.; Pegtel, D.M.; Baglio, S.R. Harnessing EV communication to restore antitumor immunity. Adv. Drug Deliv. Rev. 2021, 176, 113838. [Google Scholar] [CrossRef]
- Yin, Z.; Yu, M.; Ma, T.; Zhang, C.; Huang, S.; Karimzadeh, M.R.; Momtazi-Borojeni, A.A.; Chen, S. Mechanisms underlying low-clinical responses to PD-1/PD-L1 blocking antibodies in immunotherapy of cancer: A key role of exosomal PD-L1. J. Immunother. Cancer 2021, 9, e001698. [Google Scholar] [CrossRef]
- Poggio, M.; Hu, T.; Pai, C.C.; Chu, B.; Belair, C.D.; Chang, A.; Montabana, E.; Lang, U.E.; Fu, Q.; Fong, L.; et al. Suppression of Exosomal PD-L1 Induces Systemic Anti-tumor Immunity and Memory. Cell 2019, 177, 414–427.e13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.; Huang, A.C.; Zhang, W.; Zhang, G.; Wu, M.; Xu, W.; Yu, Z.; Yang, J.; Wang, B.; Sun, H.; et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 2018, 560, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Maiorano, B.A.; Maiorano, M.F.P.; Lorusso, D.; Maiello, E. Ovarian Cancer in the Era of Immune Checkpoint Inhibitors: State of the Art and Future Perspectives. Cancers 2021, 13, 4438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, C.; Wang, J.; Hu, Q.; Langworthy, B.; Ye, Y.; Sun, W.; Lin, J.; Wang, T.; Fine, J.; et al. PD-1 Blockade Cellular Vesicles for Cancer Immunotherapy. Adv. Mater. 2018, 30, e1707112. [Google Scholar] [CrossRef]
- Trojano, G.; Olivieri, C.; Tinelli, R.; Damiani, G.R.; Pellegrino, A.; Cicinelli, E. Conservative treatment in early stage endometrial cancer: A review. Acta Biomed. 2019, 90, 405–410. [Google Scholar] [CrossRef]
- Bruno, V.; Corrado, G.; Baci, D.; Chiofalo, B.; Carosi, M.A.; Ronchetti, L.; Piccione, E.; Albini, A.; Noonan, D.M.; Piaggio, G.; et al. Endometrial Cancer Immune Escape Mechanisms: Let Us Learn from the Fetal-Maternal Interface. Front. Oncol. 2020, 10, 156. [Google Scholar] [CrossRef]
- McDonald, M.E.; Bender, D.P. Endometrial Cancer: Obesity, Genetics, and Targeted Agents. Obstet. Gynecol. Clin. N. Am. 2019, 46, 89–105. [Google Scholar] [CrossRef]
- Brooks, N.; Pouniotis, D.S. Immunomodulation in endometrial cancer. Int. J. Gynecol. Cancer 2009, 19, 734–740. [Google Scholar] [CrossRef]
- Xiao, L.; He, Y.; Peng, F.; Yang, J.; Yuan, C. Endometrial Cancer Cells Promote M2-Like Macrophage Polarization by Delivering Exosomal miRNA-21 under Hypoxia Condition. J. Immunol. Res. 2020, 2020, 9731049. [Google Scholar] [CrossRef]
- Gu, X.; Shi, Y.; Dong, M.; Jiang, L.; Yang, J.; Liu, Z. Exosomal transfer of tumor-associated macrophage-derived hsa_circ_0001610 reduces radiosensitivity in endometrial cancer. Cell Death Dis. 2021, 12, 818. [Google Scholar] [CrossRef]
- Caloric Restriction before Surgery in Treating Patients with Endometrial, Prostate, or Breast Cancer. Available online: https://clinicaltrials.gov/show/NCT02983279 (accessed on 5 April 2022).
- Fayez, R.; Gupta, V. Imipramine. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557656/ (accessed on 5 April 2022).
- Pacher, P.; Kecskemeti, V. Cardiovascular side effects of new antidepressants and antipsychotics: New drugs, old concerns? Curr. Pharm. Des. 2004, 10, 2463–2475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.Z.; Ruan, J.S.; Jiang, Z.S.; Wang, L.; Wang, S.M. Extracellular Vesicles: A New Perspective in Tumor Therapy. Biomed. Res. Int. 2018, 2018, 2687954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zondervan, K.T.; Becker, C.M.; Koga, K.; Missmer, S.A.; Taylor, R.N.; Vigano, P. Endometriosis. Nat. Rev. Dis. Primers 2018, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Kajiyama, H.; Suzuki, S.; Yoshihara, M.; Tamauchi, S.; Yoshikawa, N.; Niimi, K.; Shibata, K.; Kikkawa, F. Endometriosis and cancer. Free Radic. Biol. Med. 2019, 133, 186–192. [Google Scholar] [CrossRef]
- Pearce, C.L.; Templeman, C.; Rossing, M.A.; Lee, A.; Near, A.M.; Webb, P.M.; Nagle, C.M.; Doherty, J.A.; Cushing-Haugen, K.L.; Wicklund, K.G.; et al. Association between endometriosis and risk of histological subtypes of ovarian cancer: A pooled analysis of case-control studies. Lancet. Oncol. 2012, 13, 385–394. [Google Scholar] [CrossRef] [Green Version]
- Murakami, K.; Kotani, Y.; Nakai, H.; Matsumura, N. Endometriosis-Associated Ovarian Cancer: The Origin and Targeted Therapy. Cancers 2020, 12, 1676. [Google Scholar] [CrossRef] [PubMed]
- Kvaskoff, M.; Mahamat-Saleh, Y.; Farland, L.V.; Shigesi, N.; Terry, K.L.; Harris, H.R.; Roman, H.; Becker, C.M.; As-Sanie, S.; Zondervan, K.T.; et al. Endometriosis and cancer: A systematic review and meta-analysis. Hum. Reprod. Update 2021, 27, 393–420. [Google Scholar] [CrossRef]
- Rowlands, I.J.; Nagle, C.M.; Spurdle, A.B.; Webb, P.M.; Australian National Endometrial Cancer Study, G.; Australian Ovarian Cancer Study, G. Gynecological conditions and the risk of endometrial cancer. Gynecol. Oncol. 2011, 123, 537–541. [Google Scholar] [CrossRef]
- Brinton, L.A.; Gridley, G.; Persson, I.; Baron, J.; Bergqvist, A. Cancer risk after a hospital discharge diagnosis of endometriosis. Am. J. Obstet. Gynecol. 1997, 176, 572–579. [Google Scholar] [CrossRef]
- Poole, E.M.; Lin, W.T.; Kvaskoff, M.; De Vivo, I.; Terry, K.L.; Missmer, S.A. Endometriosis and risk of ovarian and endometrial cancers in a large prospective cohort of U.S. nurses. Cancer Causes Control 2017, 28, 437–445. [Google Scholar] [CrossRef] [Green Version]
- Mogensen, J.B.; Kjaer, S.K.; Mellemkjaer, L.; Jensen, A. Endometriosis and risks for ovarian, endometrial and breast cancers: A nationwide cohort study. Gynecol. Oncol. 2016, 143, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Painter, J.N.; O’Mara, T.A.; Morris, A.P.; Cheng, T.H.T.; Gorman, M.; Martin, L.; Hodson, S.; Jones, A.; Martin, N.G.; Gordon, S.; et al. Genetic overlap between endometriosis and endometrial cancer: Evidence from cross-disease genetic correlation and GWAS meta-analyses. Cancer Med. 2018, 7, 1978–1987. [Google Scholar] [CrossRef] [PubMed]
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet 2004, 364, 1789–1799. [Google Scholar] [CrossRef]
- Panir, K.; Schjenken, J.E.; Robertson, S.A.; Hull, M.L. Non-coding RNAs in endometriosis: A narrative review. Hum. Reprod. Update 2018, 24, 497–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schjenken, J.E.; Panir, K.; Robertson, S.A.; Hull, M.L. Exosome-mediated intracellular signalling impacts the development of endometriosis-new avenues for endometriosis research. Mol. Hum. Reprod. 2019, 25, 2–4. [Google Scholar] [CrossRef] [PubMed]
- Burney, R.O. Biomarker development in endometriosis. Scand. J. Clin. Lab. Investig. Suppl. 2014, 244, 75–81, discussion 80. [Google Scholar] [CrossRef] [Green Version]
- Ahn, S.H.; Monsanto, S.P.; Miller, C.; Singh, S.S.; Thomas, R.; Tayade, C. Pathophysiology and Immune Dysfunction in Endometriosis. Biomed. Res. Int. 2015, 2015, 795976. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Wu, J.; Wang, W.; Xie, H.; Yao, S. Pro-endometriotic niche in endometriosis. Reprod. Biomed. Online 2019, 38, 549–559. [Google Scholar] [CrossRef] [Green Version]
- Ulukus, M.; Arici, A. Immunology of endometriosis. Minerva Ginecol. 2005, 57, 237–248. [Google Scholar]
- Munksgaard, P.S.; Blaakaer, J. The association between endometriosis and ovarian cancer: A review of histological, genetic and molecular alterations. Gynecol. Oncol. 2012, 124, 164–169. [Google Scholar] [CrossRef]
- Yang, H.L.; Zhou, W.J.; Chang, K.K.; Mei, J.; Huang, L.Q.; Wang, M.Y.; Meng, Y.; Ha, S.Y.; Li, D.J.; Li, M.Q. The crosstalk between endometrial stromal cells and macrophages impairs cytotoxicity of NK cells in endometriosis by secreting IL-10 and TGF-beta. Reproduction 2017, 154, 815–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lobb, R.J.; Lima, L.G.; Moller, A. Exosomes: Key mediators of metastasis and pre-metastatic niche formation. Semin. Cell Dev. Biol. 2017, 67, 3–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Li, H.; Yuan, M.; Li, D.; Sun, C.; Wang, G. Serum Exosomal MicroRNAs as Potential Circulating Biomarkers for Endometriosis. Dis. Markers 2020, 2020, 2456340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Huang, H.; Huang, W.; Wang, L.; Xia, X.; Fang, X. Analysis of exosomal lncRNA, miRNA and mRNA expression profiles and ceRNA network construction in endometriosis. Epigenomics 2020, 12, 1193–1213. [Google Scholar] [CrossRef]
- Nazri, H.M.; Imran, M.; Fischer, R.; Heilig, R.; Manek, S.; Dragovic, R.A.; Kessler, B.M.; Zondervan, K.T.; Tapmeier, T.T.; Becker, C.M. Characterization of exosomes in peritoneal fluid of endometriosis patients. Fertil. Steril. 2020, 113, 364–373.e2. [Google Scholar] [CrossRef] [Green Version]
- Khalaj, K.; Miller, J.E.; Lingegowda, H.; Fazleabas, A.T.; Young, S.L.; Lessey, B.A.; Koti, M.; Tayade, C. Extracellular vesicles from endometriosis patients are characterized by a unique miRNA-lncRNA signature. JCI Insight 2019, 4, e128846. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, K.; Xu, Y.; Guo, P.; Hong, B.; Cao, Y.; Wei, Z.; Xue, R.; Wang, C.; Jiang, H. Alteration of Myeloid-Derived Suppressor Cells, Chronic Inflammatory Cytokines, and Exosomal miRNA Contribute to the Peritoneal Immune Disorder of Patients with Endometriosis. Reprod. Sci. 2019, 26, 1130–1138. [Google Scholar] [CrossRef]
- Harp, D.; Driss, A.; Mehrabi, S.; Chowdhury, I.; Xu, W.; Liu, D.; Garcia-Barrio, M.; Taylor, R.N.; Gold, B.; Jefferson, S.; et al. Exosomes derived from endometriotic stromal cells have enhanced angiogenic effects in vitro. Cell Tissue Res. 2016, 365, 187–196. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Liu, M.; Zheng, C.; Zhang, D.; Li, M.; Zhang, L. Exosomal lncRNA CHL1-AS1 Derived from Peritoneal Macrophages Promotes the Progression of Endometriosis via the miR-610/MDM2 Axis. Int. J. Nanomed. 2021, 16, 5451–5464. [Google Scholar] [CrossRef]
- Zhang, L.; Li, H.H.; Yuan, M.; Li, D.; Wang, G.Y. Exosomal miR-22-3p derived from peritoneal macrophages enhances proliferation, migration, and invasion of ectopic endometrial stromal cells through regulation of the SIRT1/NF-kappaB signaling pathway. Eur. Rev. Med. Pharm. Sci. 2020, 24, 571–580. [Google Scholar] [CrossRef]
- Hutter, S.; Heublein, S.; Knabl, J.; Andergassen, U.; Vrekoussis, T.; Makrigiannakis, A.; Friese, K.; Mayr, D.; Jeschke, U. Macrophages: Are they involved in endometriosis, abortion and preeclampsia and how? J. Nippon Med. Sch. 2013, 80, 97–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bacci, M.; Capobianco, A.; Monno, A.; Cottone, L.; Di Puppo, F.; Camisa, B.; Mariani, M.; Brignole, C.; Ponzoni, M.; Ferrari, S.; et al. Macrophages are alternatively activated in patients with endometriosis and required for growth and vascularization of lesions in a mouse model of disease. Am. J. Pathol. 2009, 175, 547–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hull, M.L.; Johan, M.Z.; Hodge, W.L.; Robertson, S.A.; Ingman, W.V. Host-derived TGFB1 deficiency suppresses lesion development in a mouse model of endometriosis. Am. J. Pathol. 2012, 180, 880–887. [Google Scholar] [CrossRef]
- Sun, H.; Li, D.; Yuan, M.; Li, Q.; Zhen, Q.; Li, N.; Wang, G. Macrophages alternatively activated by endometriosis-exosomes contribute to the development of lesions in mice. Mol. Hum. Reprod. 2019, 25, 5–16. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, L.; Li, H.; Ye, J.; Lin, N.; Chen, M.; Pan, D.; Chen, Z. Endometriosis derived exosomal miR-301a-3p mediates macrophage polarization via regulating PTEN-PI3K axis. Biomed. Pharm. 2022, 147, 112680. [Google Scholar] [CrossRef] [PubMed]
- Camussi, G.; Deregibus, M.C.; Bruno, S.; Cantaluppi, V.; Biancone, L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010, 78, 838–848. [Google Scholar] [CrossRef] [Green Version]
- Ismail, N.; Wang, Y.; Dakhlallah, D.; Moldovan, L.; Agarwal, K.; Batte, K.; Shah, P.; Wisler, J.; Eubank, T.D.; Tridandapani, S.; et al. Macrophage microvesicles induce macrophage differentiation and miR-223 transfer. Blood 2013, 121, 984–995. [Google Scholar] [CrossRef] [Green Version]
- Ohlsson Teague, E.M.; Van der Hoek, K.H.; Van der Hoek, M.B.; Perry, N.; Wagaarachchi, P.; Robertson, S.A.; Print, C.G.; Hull, L.M. MicroRNA-regulated pathways associated with endometriosis. Mol. Endocrinol. 2009, 23, 265–275. [Google Scholar] [CrossRef]
- Shao, J.; Zhang, B.; Yu, J.J.; Wei, C.Y.; Zhou, W.J.; Chang, K.K.; Yang, H.L.; Jin, L.P.; Zhu, X.Y.; Li, M.Q. Macrophages promote the growth and invasion of endometrial stromal cells by downregulating IL-24 in endometriosis. Reproduction 2016, 152, 673–682. [Google Scholar] [CrossRef] [Green Version]
- Bronte, V.; Murray, P.J. Understanding local macrophage phenotypes in disease: Modulating macrophage function to treat cancer. Nat. Med. 2015, 21, 117–119. [Google Scholar] [CrossRef]
- Texido, L.; Romero, C.; Vidal, A.; Garcia-Valero, J.; Fernandez Montoli, M.E.; Baixeras, N.; Condom, E.; Ponce, J.; Garcia-Tejedor, A.; Martin-Satue, M. Ecto-nucleotidases activities in the contents of ovarian endometriomas: Potential biomarkers of endometriosis. Mediat. Inflamm. 2014, 2014, 120673. [Google Scholar] [CrossRef] [PubMed]
- Allard, B.; Longhi, M.S.; Robson, S.C.; Stagg, J. The ectonucleotidases CD39 and CD73: Novel checkpoint inhibitor targets. Immunol. Rev. 2017, 276, 121–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kodam, S.P.; Ullah, M. Diagnostic and Therapeutic Potential of Extracellular Vesicles. Technol. Cancer Res. Treat. 2021, 20, 15330338211041203. [Google Scholar] [CrossRef] [PubMed]
- Aalberts, M.; van Dissel-Emiliani, F.M.; van Adrichem, N.P.; van Wijnen, M.; Wauben, M.H.; Stout, T.A.; Stoorvogel, W. Identification of distinct populations of prostasomes that differentially express prostate stem cell antigen, annexin A1, and GLIPR2 in humans. Biol. Reprod. 2012, 86, 82. [Google Scholar] [CrossRef]
- Vanhie, A.; Tomassetti, C.; D’Hooghe, T.M. Peritoneal fluid exosomes as potential biomarkers for endometriosis: Mind and bridge the gap between innovation and validation/development into benefit for patients. Fertil. Steril. 2020, 113, 326–327. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wu, P.; Li, X.; Zeng, C.; Zhu, J.; Zhou, Y.; Lu, Y.; Xue, Q. Extracellular Vesicles Inhibit Proliferation and Invasion of Ovarian Endometrial Stromal Cells and Their Expression of SF-1, ERbeta, and Aromatase. Front. Endocrinol. 2021, 12, 666195. [Google Scholar] [CrossRef]
- Wu, D.; Lu, P.; Mi, X.; Miao, J. Exosomal miR-214 from endometrial stromal cells inhibits endometriosis fibrosis. Mol. Hum. Reprod. 2018, 24, 357–365. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Darcha, C. Antifibrotic properties of epigallocatechin-3-gallate in endometriosis. Hum. Reprod. 2014, 29, 1677–1687. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Darcha, C.; Maleysson, E.; Canis, M.; Mage, G. Impaired down-regulation of E-cadherin and beta-catenin protein expression in endometrial epithelial cells in the mid-secretory endometrium of infertile patients with endometriosis. J. Clin. Endocrinol. Metab. 2010, 95, 3437–3445. [Google Scholar] [CrossRef] [Green Version]
- Lv, J.W.; Wen, W.; Jiang, C.; Fu, Q.B.; Gu, Y.J.; Lv, T.T.; Li, Z.D.; Xue, W. Inhibition of microRNA-214 promotes epithelial-mesenchymal transition process and induces interstitial cystitis in postmenopausal women by upregulating Mfn2. Exp. Mol. Med. 2017, 49, e357. [Google Scholar] [CrossRef] [Green Version]
- Koippallil Gopalakrishnan, A.R.; Kishore, U.; Madan, T. Mesenchymal stem cells: A promising tool for targeted gene therapy of endometriosis. Regen. Med. 2017, 12, 69–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.Y.; Khanal, D.; Kalionis, B.; Chrzanowski, W. High-fidelity probing of the structure and heterogeneity of extracellular vesicles by resonance-enhanced atomic force microscopy infrared spectroscopy. Nat. Protoc. 2019, 14, 576–593. [Google Scholar] [CrossRef]
- Zhao, Y.; Tao, M.; Wei, M.; Du, S.; Wang, H.; Wang, X. UMesenchymal stem cells derived exosomal miR-323-3p promotes proliferation and inhibits apoptosis of cumulus cells in polycystic ovary syndrome (PCOS). Artif. Cells Nanomed Biotechnol. 2019, 47, 3804–3813. [Google Scholar] [CrossRef]
- Phinney, D.G.; Pittenger, M.F. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef] [Green Version]
- Baranov, V.; Malysheva, O.; Yarmolinskaya, M. Pathogenomics of Endometriosis Development. Int. J. Mol. Sci. 2018, 19, 1852. [Google Scholar] [CrossRef] [Green Version]
- Shacter, E.; Weitzman, S.A. Chronic inflammation and cancer. Oncology 2002, 16, 217–226, 229; discussion 230–212. [Google Scholar]
- Comen, E.A.; Bowman, R.L.; Kleppe, M. Underlying Causes and Therapeutic Targeting of the Inflammatory Tumor Microenvironment. Front. Cell Dev. Biol. 2018, 6, 56. [Google Scholar] [CrossRef]
- Ludwig, N.; Whiteside, T.L.; Reichert, T.E. Challenges in Exosome Isolation and Analysis in Health and Disease. Int. J. Mol. Sci. 2019, 20, 4684. [Google Scholar] [CrossRef] [Green Version]
- Tominaga, N.; Yoshioka, Y.; Ochiya, T. A novel platform for cancer therapy using extracellular vesicles. Adv. Drug Deliv. Rev. 2015, 95, 50–55. [Google Scholar] [CrossRef] [Green Version]


| Disease | Source of EVs | Studied Cargo | Cargo Type | Mechanism | Target Cells | References |
|---|---|---|---|---|---|---|
| Ovarian Cancer | Serum | FasL | protein | Apoptosis, down-regulation of CD3zeta | Jurkat T-cells | [24] |
| Ascites | FasL | protein | Apoptosis, down-regulation of CD3zeta and JAK-3 | Jurkat T-cells | [25] | |
| Ascites | GD3 | glycosphingolipid | NKTs arrest by competing with natural ligands for CD1b binding | NKTs | [27] | |
| OvCa cells (high grade serous and endometroid carcinoma) | plasma gelsolin | protein | Apoptosis of CD8+ T-cells via FLIP downregulation and CASP-3 activation, polarization of CD4+ T-cells towards Th2 | T-cells | [29] | |
| Ascites | phosphatidylserine | phospholipid | Blocking of T-cell activation by inhibiting NF-kb/NFAT signaling | CD8+ T-cells | [30] | |
| OvCa cells (ovarian endometroid adenocarcinoma), serum | circ-0001068 | circRNA | T-cell exhaustion and PD-1 induction by competing with mir-28-5p | CD8+ T-cells | [31] | |
| Ascites | Singlec-10 | protein | Blocking T-cell activation | CD8+ T-cells | [35] | |
| Ascites | not specified | not specified | Proliferation arrest, down-regulation of CD69, CD107a, cytokine production | CD8+ T-cells | [37] | |
| OvCa cells (adenocarcinoma), serum, ascites | ARG1 | protein | Proliferation arrest, down-regulation of CD3zeta | CD8+ and CD4+T-cells | [42] | |
| OvCa cells (epithelial ovarian carcinoma) | NKG2D ligands | protein | Down-regulation of NKG2D receptors | NK-cells | [44] | |
| OvCa cells (epithelial ovarian carcinoma) | not specified | M2 polarization through the (SOCS)4/5/STAT3 pathway | Macrophages | [48] | ||
| OvCa cells (epithelial ovarian carcinoma) | miR-940 | miRNA | M2 polarization | Macrophages | [49] | |
| OvCa cells (epithelial ovarian carcinoma) | miR-222-3p | miRNA | M2 polarization by inducing STAT3 expression | Macrophages | [50] | |
| OvCa cells (serous adenocarcinoma, serous cystadenocarcinoma, endometrioid adenocarcinoma) | miR-1246 | miRNA | M2 polarization by down-regulation of Cav1, chemoresistance | Macrophages, OvCa cells | [51] | |
| OvCa cells (epithelial ovarian carcinoma) | CD47 | protein | Decreased tumor-infiltration phagocytosis by M1 macrophages | Macrophages | [52] | |
| Ascites | TGFβ, IL-10 | protein | Induction of Tregs, enhancement of Treg suppressive function | Tregs | [55] | |
| Urine | HSP70 | protein | Activation of mdscs through TLR2-binding | MDSCs | [59] | |
| OvCa cells (serous surface papillary adenocarcinoma, serous cystadenocarcinoma) | TGFβ | protein | Transition of normal fibroblasts into cafs | CAFs | [62] | |
| OvCa cells (serous cystadenocaricnoma, high grade serous adenocarcinoma) | TGFβ | protein | Generation of tumor-associated myofibroblastic cells from AT-mscs through induction PI3K/AKT signaling pathways | AT-MSCs | [63] | |
| Endometrial cancer | EC cells (poorly differentiated grade 3 (G3) endometrial carcinoma) | miR-21 | miRNA | Monocytes polarization into M2 phenotype | Monocytes | [99] |
| M2 macrophages | hsa_circ_0001610 | lncRNA | Enhancement of EC radioresistance | EC cells | [100] | |
| Endometriosis | Immortalized endometriotic, ectopic epithelial cells | not specified | lncRNA | Upregulation of proinflammatory cytokine production-tnfα, upregulation of G-CSF, downregulation of MDC | Endothelial cells | [128] |
| eESCs | not specified | M2 polarization, suppression of phagocytic ability, increased M2 macrophage recruitment into the ectopic lesions | Peritoneal macrophages | [136] | ||
| eESCs | miR-301a-3p | miRNA | M2 polarization through the PTEN/PI3Kγ signaling pathway, upregulation of Arg-1 expression on macrophages | Macrophages | [137] | |
| M2 macrophages | miR-223 | miRNA | Naive monocyte differentiation into M2 phenotype, terminal maturation of other progenitor lineages into granulocytes or megakaryocytes, uptake by endothelial, epithelial, and fibroblast cells | Naive monocytes, progenitor cells, endothelial, epithelial, and fibroblast cells | [139] | |
| Peritoneal macrophages | miR-22-3p | miRNA | Increase of proliferation and migration in eesc via SIRT1/NF-kβ pathway | eESCs | [132] | |
| Peritoneal macrophages | CHL1-A | lncRNA | Promotion of proliferation, migration, and invasion of eescs, and inhibition of their apoptosis via downregulating mir-610 and upregulating MDM2 | eESC | [131] | |
| Endometrioma aspirates | CD39 and CD73 | protein | Inhibition T-cell and NK-cell response, promotion of Treg proliferation and upregulation of CTLA-4 and PD-1 expression | T-cells, NK-cells, Treg cells | [143] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soroczynska, K.; Zareba, L.; Dlugolecka, M.; Czystowska-Kuzmicz, M. Immunosuppressive Extracellular Vesicles as a Linking Factor in the Development of Tumor and Endometriotic Lesions in the Gynecologic Tract. Cells 2022, 11, 1483. https://doi.org/10.3390/cells11091483
Soroczynska K, Zareba L, Dlugolecka M, Czystowska-Kuzmicz M. Immunosuppressive Extracellular Vesicles as a Linking Factor in the Development of Tumor and Endometriotic Lesions in the Gynecologic Tract. Cells. 2022; 11(9):1483. https://doi.org/10.3390/cells11091483
Chicago/Turabian StyleSoroczynska, Karolina, Lukasz Zareba, Magdalena Dlugolecka, and Malgorzata Czystowska-Kuzmicz. 2022. "Immunosuppressive Extracellular Vesicles as a Linking Factor in the Development of Tumor and Endometriotic Lesions in the Gynecologic Tract" Cells 11, no. 9: 1483. https://doi.org/10.3390/cells11091483
APA StyleSoroczynska, K., Zareba, L., Dlugolecka, M., & Czystowska-Kuzmicz, M. (2022). Immunosuppressive Extracellular Vesicles as a Linking Factor in the Development of Tumor and Endometriotic Lesions in the Gynecologic Tract. Cells, 11(9), 1483. https://doi.org/10.3390/cells11091483