Decreased Plasma Levels of Kynurenine and Kynurenic Acid in Previously Treated and First-Episode Antipsychotic-Naive Schizophrenia Patients
Abstract
:1. Introduction
2. Materials and Methods
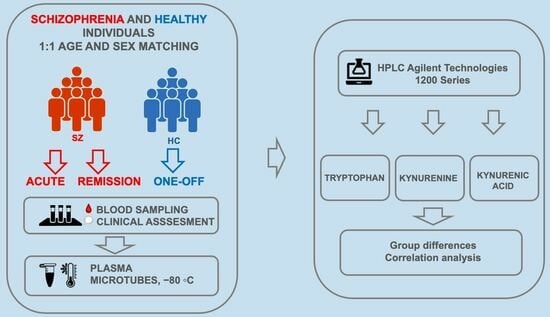
2.1. Participants
2.2. Study Design
2.3. Blood Sampling
2.4. Tryptophan, Kynurenine and Kynurenic Acid Measurement
2.4.1. Sample Preparation
2.4.2. Chromatographic Conditions
2.5. Statistical Analysis
3. Results
3.1. Sociodemographic and Baseline Characteristics
3.2. Clinical Characterization of Schizophrenia Patients
3.3. Levels of Tryptophan, Kynurenine and Kynurenic Acid
3.4. Plasma Levels of Kynurenine, Kynurenic Acid and Tryptophan in Subgroups of Schizophrenia Patients Treated and Non-Treated with Clozapine
3.5. Correlations among Plasma Concentrations of Kynurenine, Kynurenic Acid and Tryptophan in Sschizophrenia Patients and Clinical Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Foster, A.C.; Vezzani, A.; French, E.D.; Schwarcz, R. Kynurenic acid blocks neurotoxicity and seizures induced in rats by the related brain metabolite quinolinic acid. Neurosci. Lett. 1984, 48, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Perkins, M.N.; Stone, T.W. An iontophoretic investigation of the actions of convulsant kynurenines and their interaction with the endogenous excitant quinolinic acid. Brain Res. 1982, 247, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, E.X.; Schwarcz, R. Kynurenic acid as an antagonist of alpha7 nicotinic acetylcholine receptors in the brain: Facts and challenges. Biochem. Pharmacol. 2013, 85, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Moroni, F.; Cozzi, A.; Sili, M.; Mannaioni, G. Kynurenic acid: A metabolite with multiple actions and multiple targets in brain and periphery. J. Neural. Transm. 2012, 119, 133–139. [Google Scholar] [CrossRef]
- Parsons, C.G.; Danysz, W.; Quack, G.; Hartmann, S.; Lorenz, B.; Wollenburg, C.; Baran, L.; Przegalinski, E.; Kostowski, W.; Krzascik, P.; et al. Novel systemically active antagonists of the glycine site of the N-methyl-D-aspartate receptor: Electrophysiological, biochemical and behavioral characterization. J. Pharmacol. Exp. Ther. 1997, 283, 1264–1275. [Google Scholar]
- Schwarcz, R.; Du, F.; Schmidt, W.; Turski, W.A.; Gramsbergen, J.B.; Okuno, E.; Roberts, R.C. Kynurenic acid: A potential pathogen in brain disorders. Ann. N. Y. Acad. Sci. 1992, 648, 140–153. [Google Scholar] [CrossRef]
- Schwarcz, R. Kynurenines and Glutamate: Multiple Links and Therapeutic Implications. Adv. Pharmacol. 2016, 76, 13–37. [Google Scholar]
- Schwarcz, R.; Stone, T.W. The kynurenine pathway and the brain: Challenges, controversies and promises. Neuropharmacology 2017, 112, 237–247. [Google Scholar] [CrossRef]
- Bender, D.A. Biochemistry of tryptophan in health and disease. Mol. Asp. Med. 1983, 6, 101–197. [Google Scholar] [CrossRef]
- Schwarcz, R.; Bruno, J.P.; Muchowski, P.J.; Wu, H.Q. Kynurenines in the mammalian brain: When physiology meets pathology. Nat. Rev. Neurosci. 2012, 13, 465–477. [Google Scholar] [CrossRef]
- Dang, Y.; Dale, W.E.; Brown, O.R. Comparative effects of oxygen on indoleamine 2,3-dioxygenase and tryptophan 2,3-dioxygenase of the kynurenine pathway. Free Radic. Biol. Med. 2000, 28, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Fukui, S.; Schwarcz, R.; Rapoport, S.I.; Takada, Y.; Smith, Q.R. Blood-brain barrier transport of kynurenines: Implications for brain synthesis and metabolism. J. Neurochem. 1991, 56, 2007–2017. [Google Scholar] [CrossRef] [PubMed]
- Lexchin, J.L.; Cude-Simpson, K.D.; Stancer, H.C. Brain and blood indole metabolites after peripheral administration of(14)C-5-HT in rat. Neurochem. Res. 1977, 2, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Gál, E.M.; Sherman, A.D. L-kynurenine: Its synthesis and possible regulatory function in brain. Neurochem. Res. 1980, 5, 223–239. [Google Scholar] [CrossRef]
- Savitz, J. The kynurenine pathway: A finger in every pie. Mol. Psychiatry 2020, 25, 131–147. [Google Scholar] [CrossRef]
- Campbell, B.M.; Charych, E.; Lee, A.W.; Möller, T. Kynurenines in CNS disease: Regulation by inflammatory cytokines. Front. Neurosci. 2014, 8, 12. [Google Scholar] [CrossRef]
- Pedraz-Petrozzi, B.; Elyamany, O.; Rummel, C.; Mulert, C. Effects of inflammation on the kynurenine pathway in schizophrenia—A systematic review. J. Neuroinflamm. 2020, 17, 56. [Google Scholar] [CrossRef]
- Salter, M.; Pogson, C.I. The role of tryptophan 2,3-dioxygenase in the hormonal control of tryptophan metabolism in isolated rat liver cells. Effects ofglucocorticoids and experimental diabetes. Biochem. J. 1985, 229, 499–504. [Google Scholar] [CrossRef]
- Chiappelli, J.; Notarangelo, F.M.; Pocivavsek, A.; Thomas, M.A.R.; Rowland, L.M.; Schwarcz, R.; Hong, L.E. Influence of plasma cytokines on kynurenine and kynurenic acid in schizophrenia. Neuropsychopharmacology 2018, 43, 1675–1680. [Google Scholar] [CrossRef]
- Upthegrove, R.; Manzanares-Teson, N.; Barnes, N.M. Cytokine function in medication-naive first episode psychosis: A systematic review and meta-analysis. Schizophr. Res. 2014, 155, 101–108. [Google Scholar] [CrossRef]
- Frydecka, D.; Krzystek-Korpacka, M.; Lubeiro, A.; Stramecki, F.; Stańczykiewicz, B.; Beszłej, J.A.; Piotrowski, P.; Kotowicz, K.; Szewczuk-Bogusławska, M.; Pawlak-Adamska, E.; et al. Profiling inflammatory signatures of schizophrenia: A cross-sectional and meta-analysis study. Brain Behav. Immun. 2018, 71, 28–36. [Google Scholar] [CrossRef]
- Mithaiwala, M.N.; Santana-Coelho, D.; Porter, G.A.; O’Connor, J.C. Neuroinflammation and the Kynurenine Pathway in CNS Disease: Molecular Mechanisms and Therapeutic Implications. Cells 2021, 10, 1548. [Google Scholar] [CrossRef]
- Erhardt, S.; Blennow, K.; Nordin, C.; Skogh, E.; Lindström, L.H.; Engberg, G. Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci. Lett. 2001, 313, 96–98. [Google Scholar] [CrossRef]
- Schwarcz, R.; Rassoulpour, A.; Wu, H.Q.; Medoff, D.; Tamminga, C.A.; Roberts, R.C. Increased cortical kynurenate content in schizophrenia. Biol. Psychiatry 2001, 50, 521–530. [Google Scholar] [CrossRef]
- Miller, C.L.; Llenos, I.C.; Dulay, J.R.; Weis, S. Upregulation of the initiating step of the kynurenine pathway in postmortem anterior cingulate cortex from individuals with schizophrenia and bipolar disorder. Brain Res. 2006, 1073, 25–37. [Google Scholar] [CrossRef]
- Sathyasaikumar, K.V.; Stachowski, E.K.; Wonodi, I.; Roberts, R.C.; Rassoulpour, A.; McMahon, R.P.; Schwarcz, R. Impaired kynurenine pathway metabolism in the prefrontal cortex of individuals with schizophrenia. Schizophr. Bull. 2011, 37, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Linderholm, K.R.; Skogh, E.; Olsson, S.K.; Dahl, M.L.; Holtze, M.; Engberg, G.; Samuelsson, M.; Erhardt, S. Increased levels of kynurenine and kynurenic acid in the CSF of patients with schizophrenia. Schizophr. Bull. 2012, 38, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Marx, W.; McGuinness, A.J.; Rocks, T.; Ruusunen, A.; Cleminson, J.; Walker, A.J.; Gomes-da-Costa, S.; Lane, M.; Sanches, M.; Diaz, A.P.; et al. The kynurenine pathway in major depressive disorder, bipolar disorder, and schizophrenia: A meta-analysis of 101 studies. Mol. Psychiatry 2021, 26, 4158–4178. [Google Scholar] [CrossRef] [PubMed]
- Plitman, E.; Iwata, Y.; Caravaggio, F.; Nakajima, S.; Chung, J.K.; Gerretsen, P.; Kim, J.; Takeuchi, H.; Chakravarty, M.M.; Remington, G.; et al. Kynurenic Acid in Schizophrenia: A Systematic Review and Meta-analysis. Schizophr. Bull. 2017, 43, 764–777. [Google Scholar] [CrossRef]
- Morrens, M.; De Picker, L.; Kampen, J.K.; Coppens, V. Blood-based kynurenine pathway alterations in schizophrenia spectrum disorders: A meta-analysis. Schizophr. Res. 2020, 223, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Sathyasaikumar, K.V.; Notarangelo, F.M.; Kelly, D.L.; Rowland, L.M.; Hare, S.M.; Chen, S.; Mo, C.; Buchanan, R.W.; Schwarcz, R. Tryptophan Challenge in Healthy Controls and People with Schizophrenia: Acute Effects on Plasma Levels of Kynurenine, Kynurenic Acid and 5-Hydroxyindoleacetic Acid. Pharmaceuticals 2022, 15, 1003. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). The ICD-10 Classification of Mental and Behavioral Disorders; WHO: Geneva, Switzerland, 1993. [Google Scholar]
- Leucht, S.; Davis, J.M.; Engel, R.R.; Kissling, W.; Kane, J.M. Definitions of response and remission in schizophrenia: Recommendations fore their use and their presentation. Acta Psychiatr. Scand. 2009, 119, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Gao, P.; Zhu, D. Optimization of Zn2+-containing mobile phase for simultaneous determination of kynurenine, kynurenic acid and tryptophan in human plasma by high performance liquid chromatography. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010, 878, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Mandi, Y.; Vecsei, L. The kynurenine system and immunoregulation. J. Neural Transm. 2012, 119, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Fujigaki, H.; Saito, K.; Fujigaki, S.; Takemura, M.; Sudo, K.; Ishiguro, H.; Seishima, M. The signal transducer and activator of transcription 1alpha and interferon regulatory factor 1 are not essential for the induction of indoleamine 2,3-dioxygenase by lipo- polysaccharide: Involvement of p38 mitogen-activated protein kinase and nuclear factor-kappaB pathways, and synergistic effect of several proinflammatory cytokines. J. Biochem. 2006, 139, 655–662. [Google Scholar] [PubMed]
- Guillemin, G.J.; Smythe, G.; Takikawa, O.; Brew, B.J. Expression of indoleamine 2,3-dioxygenase and production of quinolinic acid by human microglia, astrocytes, and neurons. Glia 2005, 49, 15–23. [Google Scholar] [CrossRef] [PubMed]
- de Bie, J.; Lim, C.K.; Guillemin, G.J. Kynurenines, gender and neuroinflammation; showcase schizophrenia. Neurotox. Res. 2016, 30, 285–294. [Google Scholar] [CrossRef]
- Halstead, S.; Siskind, D.; Amft, M.; Wagner, E.; Yakimov, V.; Shih-Jung Liu, Z.; Walder, K.; Warren, N. Alteration patterns of peripheral concentrations of cytokines and associated inflammatory proteins in acute and chronic stages of schizophrenia: A systematic review and network meta-analysis. Lancet Psychiatry 2023, 10, 260–271. [Google Scholar] [CrossRef]
- Wang, A.K.; Miller, B.J. Meta-analysis of Cerebrospinal Fluid Cytokine and Tryptophan Catabolite Alterations in Psychiatric Patients: Comparisons Between Schizophrenia, Bipolar Disorder, and Depression. Schizophr. Bull. 2018, 13, 75–83. [Google Scholar] [CrossRef]
- Cao, B.; Chen, Y.; Ren, Z.; Pan, Z.; McIntyre, R.S.; Wang, D. Dysregulation of kynurenine pathway and potential dynamic changes of kynurenine in schizophrenia: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2021, 123, 203–214. [Google Scholar] [CrossRef]
- Szymona, K.; Zdzisińska, B.; Karakuła-Juchnowicz, H.; Kocki, T.; Kandefer-Szerszeń, M.; Flis, M.; Rosa, W.; Urbańska, E.M. Correlations of Kynurenic Acid, 3-Hydroxykynurenine, sIL-2R, IFN-α, and IL-4 with Clinical Symptoms During Acute Relapse of Schizophrenia. Neurotox. Res. 2017, 32, 17–26. [Google Scholar] [CrossRef]
- Kuuskmäe, C.; Philips, M.A.; Kilk, K.; Haring, L.; Kangro, R.; Seppo, I.; Zilmer, M.; Vasar, E. Kynurenine pathway dynamics in patients with schizophrenia spectrum disorders across the disease trajectory. Psychiatry Res. 2023, 328, 115423. [Google Scholar] [CrossRef] [PubMed]
- Peluso, M.J.; Lu, S.; Tang, A.F.; Durstenfeld, M.S.; Ho, H.E.; Goldberg, S.A.; Forman, C.A.; Munter, S.E.; Hoh, R.; Tai, V.; et al. Markers of Immune Activation and Inflammation in Individuals With Postacute Sequelae of Severe Acute Respiratory Syndrome Coronavirus 2 Infection. J. Infect. Dis. 2021, 224, 1839–1848. [Google Scholar] [CrossRef] [PubMed]
- Wellen, K.E.; Hotamisligil, G.S. Inflammation, stress, and diabetes. J. Clin. Invest. 2005, 115, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A. Blood-brain barrier transport of cytokines: A mechanism for neuropathology. Curr. Pharm. Des. 2005, 11, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Malkiewicz, M.A.; Szarmach, A.; Sabisz, A.; Cubala, W.J.; Szurowska, E.; Winklewski, P.J. Blood-brain barrier permeability and physical exercise. J. Neuroinflamm. 2019, 16, 15. [Google Scholar] [CrossRef] [PubMed]
- Myint, A.M.; Schwarz, M.J.; Verkerk, R.; Mueller, H.H.; Zach, J.; Scharpé, S.; Steinbusch, H.W.M.; Leonard, B.E.; Kim, Y.K. Reversal of imbalance between kynurenic acid and 3-hydroxykynurenine by antipsychotics in medication-naïve and medication-free schizophrenic patients. Brain Behav. Immun. 2011, 25, 1576–1581. [Google Scholar] [CrossRef]
- Kindler, J.; Lim, C.K.; Weickert, C.S.; Boerrigter, D.; Galletly, C.; Liu, D.; Jacobs, K.R.; Balzan, R.; Bruggemann, J.; O’Donnell, M.; et al. Dysregulation of kynurenine metabolism is related to proinflammatory cytokines, attention, and prefrontal cortex volume in schizophrenia. Mol. Psychiatry 2020, 25, 2860–2872. [Google Scholar] [CrossRef]
- Skorobogatov, K.; Autier, V.; Foiselle, M.; Richard, J.R.; Boukouaci, W.; Wu, C.L.; Raynal, S.; Carbonne, C.; Laukens, K.; Meysman, P.; et al. Kynurenine pathway abnormalities are state-specific but not diagnosis-specific in schizophrenia and bipolar disorder. Brain Behav. Immun. Health 2023, 2, 100584. [Google Scholar] [CrossRef]
- Strasser, B.; Becker, K.; Fuchs, D.; Gostner, J.M. Kynurenine pathway metabolism and immune activation: Peripheral measure- ments in psychiatric and co-morbid conditions. Neuropharmacology 2017, 112, 286–296. [Google Scholar] [CrossRef]
- Chiappelli, J.; Postolache, T.T.; Kochunov, P.; Rowland, L.M.; Wijtenburg, S.A.; Shukla, D.K.; Tagamets, M.; Du, X.; Savransky, A.; Lowry, C.A.; et al. Tryptophan metabolism and white matter integrity in schizophrenia. Neuropsychopharmacology 2016, 41, 2587–2595. [Google Scholar] [CrossRef] [PubMed]
- Barry, S.; Clarke, G.; Scully, P.; Dinan, T.G. Kynurenine pathway in psychosis: Evidence of increased tryptophan degradation. J. Psychopharmacol. 2009, 23, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Okusaga, O.; Fuchs, D.; Reeves, G.; Giegling, I.; Hartmann, A.M.; Konte, B.; Friedl, M.; Groer, M.; Cook, T.B.; Stearns-Yoder, K.A.; et al. Kynurenine and tryptophan levels in patients with schi- zophrenia and elevated antigliadin immunoglobulin G antibodies. Psychosom. Med. 2016, 78, 931–939. [Google Scholar] [CrossRef]
- Almulla, A.F.; Vasupanrajit, A.; Tunvirachaisakul, C.; Al-Hakeim, H.K.; Solmi, M.; Verkerk, R.; Maes, M. The tryptophan catabolite or kynurenine pathway in schizophrenia: Meta-analysis reveals dissociations between central, serum, and plasma compartments. Mol. Psychiatry 2022, 27, 3679–3691. [Google Scholar] [CrossRef]
- Eggertsen, P.P.; Hansen, J.; Andersen, M.L.; Nielsen, J.F.; Olsen, R.K.J.; Palmfeldt, J. Simultaneous measurement of kynurenine metabolites and explorative metabolomics using liquid chromatography-mass spectrometry: A novel accurate method applied to serum and plasma samples from a large healthy cohort. J. Pharm. Biomed. Anal. 2023, 1, 115304. [Google Scholar] [CrossRef]
- Pollak, T.A.; Drndarski, S.; Stone, J.M.; David, A.S.; McGuire, P.; Abbott, N.J. The blood-brain barrier in psychosis. Lancet Psychiatry 2018, 5, 79–92. [Google Scholar] [CrossRef]
- Chang, C.Y.; Luo, D.Z.; Pei, J.C.; Kuo, M.C.; Hsieh, Y.C.; Lai, W.S. Not just a bystander: The rmerging role of astrocytes and research tools in studying cognitive dysfunctions in schizophrenia. Int. J. Mol. Sci. 2021, 22, 5343. [Google Scholar] [CrossRef]
- Fujigaki, H.; Mouri, A.; Yamamoto, Y.; Nabeshima, T.; Saito, K. Linking phencyclidine intoxication to the tryptophan-kynurenine pathway: Therapeutic implications for schizophrenia. Neurochem. Int. 2019, 125, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Erhardt, S.; Schwieler, L.; Imbeault, S.; Engberg, G. The kynurenine pathway in schizophrenia and bipolar disorder. Neuropharmacology 2017, 112, 297–306. [Google Scholar] [CrossRef]
- Schubert, K.O.; Föcking, M.; Cotter, D.R. Proteomic pathway analysis of the hippocampus in schizophrenia and bipolar affective disorder implicates 14-3-3 signaling, aryl hydrocarbon receptor signaling, and glucose metabolism: Potential roles in GABAergic interneuron pathology. Schizophr. Res. 2015, 167, 64–72. [Google Scholar] [CrossRef]
- Berlinguer-Palmini, R.; Masi, A.; Narducci, R.; Cavone, L.; Maratea, D.; Cozzi, A.; Sili, M.; Moroni, F.; Mannaioni, G. GPR35 activation reduces Ca2+ transients and contributes to the kynurenic acid-dependent reduction of synaptic activity at CA3-CA1 synapses. PLoS ONE 2013, 8, e82180. [Google Scholar] [CrossRef] [PubMed]
- DiNatale, B.C.; Murray, I.A.; Schroeder, J.C.; Flaveny, C.A.; Lahoti, T.S.; Laurenzana, E.M.; Omiecinski, C.J.; Perdew, G.H. Kynurenic Acid Is a Potent Endogenous Aryl Hydrocarbon Receptor Ligand that Synergistically Induces Interleukin-6 in the Presence of Inflammatory Signaling. Toxicol. Sci. 2010, 115, 89–97. [Google Scholar] [CrossRef]
- Adesso, S.; Magnus, T.; Cuzzocrea, S.; Campolo, M.; Rissiek, B.; Paciello, O.; Autore, G.; Pinto, A.; Marzocco, S. Indoxyl Sulfate Affects Glial Function Increasing Oxidative Stress and Neuroinflammation in Chronic Kidney Disease: Interaction between Astrocytes and Microglia. Front. Pharmacol. 2017, 12, 370. [Google Scholar] [CrossRef] [PubMed]
- Fazio, F.; Lionetto, L.; Curto, M.; Iacovelli, L.; Cavallari, M.; Zappulla, C.; Ulivieri, M.; Napoletano, F.; Capi, M.; Corigliano, V.; et al. Xanthurenic acid activates mGlu2/3 metabotropic glutamate receptors and is a potential trait marker for schizophrenia. Sci. Rep. 2015, 5, 17799. [Google Scholar] [CrossRef]
- De Picker, L.; Fransen, E.; Coppens, V.; Timmers, M.; de Boer, P.; Oberacher, H.; Fuchs, D.; Verkerk, R.; Sabbe, B.; Morrens, M. Immune and Neuroendocrine Trait and State Markers in Psychotic Illness: Decreased Kynurenines Marking Psychotic Exacerbations. Front. Immunol. 2020, 10, 2971. [Google Scholar] [CrossRef] [PubMed]
- Schlittler, M.; Goiny, M.; Agudelo, L.Z.; Venckunas, T.; Brazaitis, M.; Skurvydas, A.; Kamandulis, S.; Ruas, J.L.; Erhardt, S.; Westerblad, H.; et al. Endurance exercise increases skeletal muscle kynurenine aminotransferases and plasma kynurenic acid in humans. Am. J. Physiol. Cell Physiol. 2016, 310, C836–C840. [Google Scholar] [CrossRef]
- Theofylaktopoulou, D.; Midttun, Ø.; Ulvik, A.; Ueland, P.M.; Tell, G.S.; Vollset, S.E.; Nygård, O.; Eussen, S.J.P.M. A community-based study on determinants of circulating markers of cellular immune activation and kynurenines: The Hordaland Health Study. Clin. Exp. Immunol. 2013, 173, 121–130. [Google Scholar] [CrossRef]
- Marszalek-Grabska, M.; Walczak, K.; Gawel, K.; Wicha-Komsta, K.; Wnorowska, S.; Wnorowski, A.; Turski, W.A. Kynurenine emerges from the shadows—Current knowledge on its fate and function. Pharmacol. Ther. 2021, 225, 107845. [Google Scholar] [CrossRef]
| Variable | Group | |
|---|---|---|
| Control (n = 64) | Schizophrenia (n = 64) | |
| Gender, n (%) | ||
| Male | 37 (57.8) | 37 (57.8) |
| Female | 27 (42.2) | 27 (42.2) |
| Age, mean ± SD | 36.61 ± 10.51 | 36.72 ± 10.46 |
| Smoking status, n (%) | ||
| Non-smoker/Ex-smoker | 43 (67.2) | 25 (39.1) |
| Smoker | 21 (32.8) | 39 (60,9) |
| Comorbidities, n (%) | 16 (25.0) | 10 (16.1) |
| Family history, n (%) | ||
| Psychosis | / | 36 (56.3) |
| Suicide | / | 11 (17.2) |
| Illness duration (months), median (25th–75th percentile) | / | 96 (37–204) |
| Drug naive, n (%) | / | 16 (25.0) |
| Clozapine, n (%) | / | 20 (31.3) |
| Number of hospitalizations, median (25th–75th percentile) | / | 3 (1–5) |
| BMI, mean ± SD | 25.45 ± 4.28 | 24.58 ± 4.40 |
| Leukocytes (109/L), mean ± SD | 5.58 ± 1.52 | 6.93 ± 2.32 |
| Variable | Score (Mean ± SD) | |
|---|---|---|
| Acute | Remission | |
| PANSS positive scale | 32.17 ± 5.54 | 14.56 ± 4.01 * |
| PANSS negative scale | 26.09 ± 6.75 | 16.66 ± 5.49 * |
| PANSS general psychopathology | 60.34 ± 8.22 | 32.61 ± 7.03 * |
| Total PANSS score | 118.45 ± 17.86 | 64.09 ± 13.80 * |
| CGI | 5.73 ± 0.76 | 1.70 ± 0.46 * |
| GAF | 24.95 ± 8.32 | 58.81 ± 8.91 * |
| CDSS | 5.06 ± 4.46 | 2.47 ± 2.48 * |
| PSP | 26.78 ± 9.04 | 62.64 ± 10.80 * |
| Metabolite | Acute Phase | Remission | Healthy Controls |
|---|---|---|---|
| Kynurenine (μmol/L) | |||
| All SZ patients | 1.52 (1.27–1.70) a | 1.40 (1.16–1.80) a | 1.90 (1.52–2.29) |
| Drug naive | 1.45 (1.07–1.66) a | 1.42 (1.08–1.80) a | |
| Kynurenic acid (nmol/L) | |||
| All SZ patients | 21.30 (16.22–28.83) a | 19.88 (14.60–26.58) a | 31.81 (27.12–41.32) |
| Drug naive | 22.36 (19.89–31.11) a | 20.87 (15.45–29.31) a | |
| Tryptophan (μmol/L) | |||
| All SZ patients | 40.77 (33.81–46.13) a | 33.32 (26.02–40.58) a,b | 46.88 (41.97–53.62) |
| Drug naive | 43.21 (38.98–48.56) | 33.81 (26.92–42.46) a | |
| KYN/TRP ratio | |||
| All SZ patients | 0.04 (0.03–0.05) | 0.04 (0.03–0.06) | 0.04 (0.03–0.05) |
| Drug naive | 0.03 (0.03–0.04) | 0.04 (0.04–0.05) b |
| Substance | Acute | Remission | Control | ||
|---|---|---|---|---|---|
| W/o CLO | CLO | W/o CLO | CLO | ||
| KYN (μmol/L) | 1.54 a (1.31–1.75) | 1.43 a (1.24–1.62) | 1.44 (1.20–1.84) | 1.18 * (1.10–1.42) | 1.90 (1.52–2.29) |
| KYNA (nmol/L) | 21.75 a (17.32–28.83) | 19.15 a (15.31–29.24) | 20.76 (15.22–26.63) | 16.86 (12.04–26.08) | 31.81 (27.12–41.32) |
| TRP (μmol/L) | 41.71 a (34.38–46.13) | 38.45 a (33.81–47.08) | 35.86 (26.92–41.02) | 29.54 (23.60–36.90) | 46.88 (41.97–53.62) |
| KYN/TRP ratio | 0.04 (0.04–0.06) | 0.04 (0.03–0.06) | 0.04 (0.04–0.06) | 0.04 (0.03–0.06) | 0.04 (0.03–0.05) |
| PANSS | ΔPANSS | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Acute | Remission | ||||||||
| Positive Scale | Negative Scale | General Psychopathology | Total Score | Positive Scale | Negative Scale | General Psychopathology | Total Score | ||
| Acute | |||||||||
| KYN | −0.113 | 0.147 | −0.022 | 0.005 | −0.102 | 0.009 | −0.011 | −0.025 | 0.066 |
| KYNA | −0.364 * | −0.189 | −0.310 * | −0.323 * | −0.315 * | −0.222 | −0.268 * | −0.299 * | −0.103 |
| TRP | −0.162 | −0.034 | −0.048 | −0.069 | −0.216 | −0.123 | −0.221 | −0.219 | 0.113 |
| KYN/TRP ratio | 0.132 | 0.162 | 0.092 | 0.129 | 0.177 | 0.095 | 0.182 | 0.189 | 0.001 |
| Remission | |||||||||
| KYN | −0.806 | −0.133 | −0.180 | −0.162 | 0.207 | ||||
| KYNA | −0.257 * | −0.076 | −0.250 * | −0.204 | 0.029 | ||||
| TRP | −0.119 | −0.067 | −0.127 | −0.118 | 0.119 | ||||
| KYN/TRP ratio | −0.007 | −0.144 | −0.091 | −0.093 | 0.029 | ||||
| Illness Duration (Months) | Number of Hospitalizations | |
|---|---|---|
| Acute | ||
| Kynurenine | 0.051 | −0.125 |
| KYNA | −0.095 | −0.194 |
| Tryptophan | −0.329 * | −0.287 * |
| KYN/TRP ratio | 0.262 | 0.136 |
| Remission | ||
| Kynurenine | 0.149 | −0.021 |
| KYNA levels | −0.038 | −0.095 |
| Tryptophan | 0.003 | −0.054 |
| KYN/TRP ratio | 0.145 | 0.048 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marković, M.; Petronijević, N.; Stašević, M.; Stašević Karličić, I.; Velimirović, M.; Stojković, T.; Ristić, S.; Stojković, M.; Milić, N.; Nikolić, T. Decreased Plasma Levels of Kynurenine and Kynurenic Acid in Previously Treated and First-Episode Antipsychotic-Naive Schizophrenia Patients. Cells 2023, 12, 2814. https://doi.org/10.3390/cells12242814
Marković M, Petronijević N, Stašević M, Stašević Karličić I, Velimirović M, Stojković T, Ristić S, Stojković M, Milić N, Nikolić T. Decreased Plasma Levels of Kynurenine and Kynurenic Acid in Previously Treated and First-Episode Antipsychotic-Naive Schizophrenia Patients. Cells. 2023; 12(24):2814. https://doi.org/10.3390/cells12242814
Chicago/Turabian StyleMarković, Miloš, Nataša Petronijević, Milena Stašević, Ivana Stašević Karličić, Milica Velimirović, Tihomir Stojković, Slavica Ristić, Mina Stojković, Nataša Milić, and Tatjana Nikolić. 2023. "Decreased Plasma Levels of Kynurenine and Kynurenic Acid in Previously Treated and First-Episode Antipsychotic-Naive Schizophrenia Patients" Cells 12, no. 24: 2814. https://doi.org/10.3390/cells12242814
APA StyleMarković, M., Petronijević, N., Stašević, M., Stašević Karličić, I., Velimirović, M., Stojković, T., Ristić, S., Stojković, M., Milić, N., & Nikolić, T. (2023). Decreased Plasma Levels of Kynurenine and Kynurenic Acid in Previously Treated and First-Episode Antipsychotic-Naive Schizophrenia Patients. Cells, 12(24), 2814. https://doi.org/10.3390/cells12242814