Opposing Effects of Adenosine and Inosine in Human Subcutaneous Fibroblasts May Be Regulated by Third Party ADA Cell Providers
Abstract
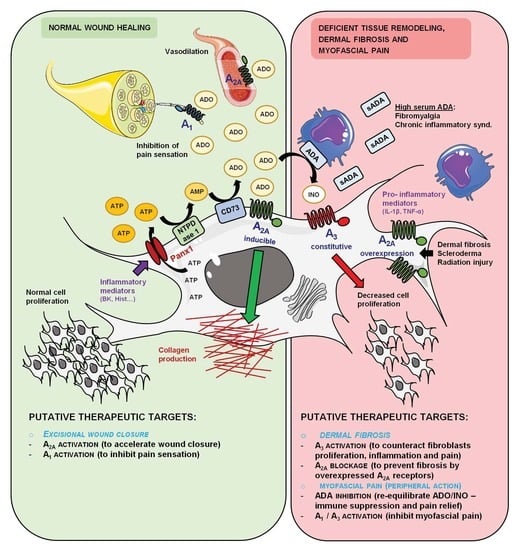
:1. Introduction
2. Experimental Procedures
2.1. Cell Cultures
2.2. Human CD73 Antibody Production
2.3. Immunocytochemistry
2.4. SDS-PAGE and Western Blotting
2.5. Enzymatic Kinetic Experiments and HPLC Analysis
2.6. Cell Viability/Proliferation
2.7. Total Collagen Determination
2.8. Materials and Reagents
2.9. Presentation of Data and Statistical Analysis
3. Results
3.1. Human Subcutaneous Fibroblasts Express Ecto-5′-Nucleotidase/CD73 and Adenosine A2A and A3 Receptor Subtypes
3.2. Adenosine Formation from AMP Overcomes its Deamination into Inosine Favoring Accumulation of the Nucleoside in HSCF Cultures
3.3. AMP Favors Collagen Production via Adenosine A2A Receptors Activation Coupled to the Adenylyl Cyclase (AC)/Exchange Protein Activated by Cyclic AMP (EPAC) Pathway in HSCF
3.4. Effect of Adenosine A2A and A3 Receptor Agonists on Proliferation/Viability and Collagen Production by HSCF
3.5. Inosine Exerts an Anti-Proliferative Role on HSCF through Activation of A3 Receptors, but its Formation Requires Exogenous ADA Application
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Langevin, H.M.; Sherman, K.J. Pathophysiological model for chronic low back pain integrating connective tissue and nervous system mechanisms. Med. Hypotheses 2007, 68, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Langevin, H.M.; Stevens-Tuttle, D.; Fox, J.R.; Badger, G.J.; Bouffard, N.A.; Krag, M.H.; Wu, J.; Henry, S.M. Ultrasound evidence of altered lumbar connective tissue structure in human subjects with chronic low back pain. BMC Musculoskelet Disord. 2009, 10, 151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langevin, H.M.; Fox, J.R.; Koptiuch, C.; Badger, G.J.; Greenan-Naumann, A.C.; Bouffard, N.A.; Konofagou, E.E.; Lee, W.N.; Triano, J.J.; Henry, S.M. Reduced thoracolumbar fascia shear strain in human chronic low back pain. BMC Musculoskelet Disord. 2011, 12, 203. [Google Scholar] [CrossRef] [PubMed]
- Bordoni, B.; Bordoni, G. Reflections on osteopathic fascia treatment in the peripheral nervous system. J. Pain Res. 2015, 8, 735–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldman, N.; Chandler-Militello, D.; Langevin, H.M.; Nedergaard, M.; Takano, T. Purine receptor mediated actin cytoskeleton remodeling of human fibroblasts. Cell. Calcium 2013, 53, 297–301. [Google Scholar] [CrossRef] [Green Version]
- Langevin, H.M.; Fujita, T.; Bouffard, N.A.; Takano, T.; Koptiuch, C.; Badger, G.J.; Nedergaard, M. Fibroblast cytoskeletal remodeling induced by tissue stretch involves ATP signaling. J. Cell Physiol. 2013, 228, 1922–1926. [Google Scholar] [CrossRef] [Green Version]
- Pinheiro, A.R.; Paramos-de-Carvalho, D.; Certal, M.; Costa, C.; Magalhães-Cardoso, M.T.; Ferreirinha, F.; Costa, M.A.; Correia-de-Sá, P. Bradykinin-induced Ca2+ signaling in human subcutaneous fibroblasts involves ATP release via hemichannels leading to P2Y12 receptors activation. Cell Commun. Signal. 2013, 11, 70. [Google Scholar] [CrossRef] [Green Version]
- Pinheiro, A.R.; Paramos-de-Carvalho, D.; Certal, M.; Costa, M.A.; Costa, C.; Magalhães-Cardoso, M.T.; Ferreirinha, F.; Sévigny, J.; Correia-de-Sá, P. Histamine induces ATP release from human subcutaneous fibroblasts via pannexin-1 hemichannels leading to Ca2+ mobilization and cell proliferation. J. Biol. Chem. 2013, 288, 27571–27583. [Google Scholar] [CrossRef] [Green Version]
- Perez-Aso, M.; Fernandez, P.; Mediero, A.; Chan, E.S.; Cronstein, B.N. Adenosine 2A receptor promotes collagen production by human fibroblasts via pathways involving cyclic AMP and AKT but independent of Smad2/3. FASEB J. 2014, 28, 802–812. [Google Scholar] [CrossRef] [Green Version]
- Burnstock, G. Purinergic Signalling: Therapeutic Developments. Front. Pharm. 2017, 8, 661. [Google Scholar] [CrossRef] [Green Version]
- Pastor-Anglada, M.; Pérez-Torras, S. Who Is Who in Adenosine Transport. Front. Pharm. 2018, 9, 627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cronstein, B.N. Adenosine receptors and fibrosis: A translational review. F1000 Biol. Rep. 2011, 3, 21. [Google Scholar] [CrossRef] [PubMed]
- Hasko, G.; Linden, J.; Cronstein, B.; Pacher, P. Adenosine receptors: Therapeutic aspects for inflammatory and immune diseases. Nat. Rev. Drug Discov. 2008, 7, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Borea, P.A.; Varani, K.; Vincenzi, F.; Baraldi, P.G.; Tabrizi, M.A.; Merighi, S.; Gessi, S. The A3 adenosine receptor: History and perspectives. Pharm. Rev. 2015, 67, 74–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burnstock, G. Purinergic signalling: From discovery to current developments. Exp. Physiol. 2014, 99, 16–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bessa-Gonçalves, M.; Braganca, B.; Martins-Dias, E.; Correia-de-Sá, P.; Fontes-Sousa, A.P. Is the adenosine A2B ‘biased’ receptor a valuable target for the treatment of pulmonary arterial hypertension? Drug Discov. Today 2018, 23, 1285–1292. [Google Scholar] [CrossRef]
- Chan, E.S.; Liu, H.; Fernandez, P.; Luna, A.; Perez-Aso, M.; Bujor, A.M.; Trojanowska, M.; Cronstein, B.N. Adenosine A(2A) receptors promote collagen production by a Fli1- and CTGF-mediated mechanism. Arthritis Res. 2013, 15, R58. [Google Scholar] [CrossRef] [Green Version]
- Borea, P.A.; Gessi, S.; Merighi, S.; Varani, K. Adenosine as a Multi-Signalling Guardian Angel in Human Diseases: When, Where and How Does it Exert its Protective Effects? Trends Pharm. Sci. 2016, 37, 419–434. [Google Scholar] [CrossRef]
- Chen, Z.; Janes, K.; Chen, C.; Doyle, T.; Bryant, L.; Tosh, D.K.; Jacobson, K.A.; Salvemini, D. Controlling murine and rat chronic pain through A3 adenosine receptor activation. FASEB J. 2012, 26, 1855–1865. [Google Scholar] [CrossRef] [Green Version]
- Paoletta, S.; Tosh, D.K.; Finley, A.; Gizewski, E.T.; Moss, S.M.; Gao, Z.G.; Auchampach, J.A.; Salvemini, D.; Jacobson, K.A. Rational design of sulfonated A3 adenosine receptor-selective nucleosides as pharmacological tools to study chronic neuropathic pain. J. Med. Chem. 2013, 56, 5949–5963. [Google Scholar] [CrossRef] [Green Version]
- Faria, M.; Magalhães-Cardoso, T.; Lafuente-de-Carvalho, J.M.; Correia-de-Sá, P. Corpus cavernosum from men with vasculogenic impotence is partially resistant to adenosine relaxation due to endothelial A(2B) receptor dysfunction. J. Pharm. Exp. 2006, 319, 405–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, M.A.; Barbosa, A.; Neto, E.; Sá-e-Sousa, A.; Freitas, R.; Neves, J.M.; Magalhães-Cardoso, T.; Ferreirinha, F.; Correia-de-Sá, P. On the role of subtype selective adenosine receptor agonists during proliferation and osteogenic differentiation of human primary bone marrow stromal cells. J. Cell Physiol. 2011, 226, 1353–1366. [Google Scholar] [CrossRef] [PubMed]
- Tullberg-Reinert, H.; Jundt, G. In situ measurement of collagen synthesis by human bone cells with a sirius red-based colorimetric microassay: Effects of transforming growth factor beta2 and ascorbic acid 2-phosphate. Histochem. Cell Biol. 1999, 112, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Perez-Aso, M.; Mediero, A.; Cronstein, B.N. Adenosine A2A receptor (A(2A)R) is a fine-tune regulator of the collagen1:collagen3 balance. Purinergic Signal. 2013, 9, 573–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cinalli, A.R.; Guarracino, J.F.; Fernandez, V.; Roquel, L.I.; Losavio, A.S. Inosine induces presynaptic inhibition of acetylcholine release by activation of A3 adenosine receptors at the mouse neuromuscular junction. Br. J. Pharm. 2013, 169, 1810–1823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez, G.; Sitkovsky, M.V. Differential requirement for A2a and A3 adenosine receptors for the protective effect of inosine in vivo. Blood 2003, 102, 4472–4478. [Google Scholar] [CrossRef]
- Jin, X.; Shepherd, R.K.; Duling, B.R.; Linden, J. Inosine binds to A3 adenosine receptors and stimulates mast cell degranulation. J. Clin Investig. 1997, 100, 2849–2857. [Google Scholar] [CrossRef] [Green Version]
- Tilley, S.L.; Wagoner, V.A.; Salvatore, C.A.; Jacobson, M.A.; Koller, B.H. Adenosine and inosine increase cutaneous vasopermeability by activating A(3) receptors on mast cells. J. Clin. Investig. 2000, 105, 361–367. [Google Scholar] [CrossRef] [Green Version]
- Sheth, S.; Brito, R.; Mukherjea, D.; Rybak, L.P.; Ramkumar, V. Adenosine receptors: Expression, function and regulation. Int. J. Mol. Sci. 2014, 15, 2024–2052. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, R.J.; Tomé, A.R.; Cunha, R.A. ATP as a multi-target danger signal in the brain. Front. Neurosci. 2015, 9, 148. [Google Scholar] [CrossRef] [Green Version]
- Kukulski, F.; Levesque, S.A.; Lavoie, E.G.; Lecka, J.; Bigonnesse, F.; Knowles, A.F.; Robson, S.C.; Kirley, T.L.; Sevigny, J. Comparative hydrolysis of P2 receptor agonists by NTPDases 1, 2, 3 and 8. Purinergic Signal. 2005, 1, 193–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colgan, S.P.; Eltzschig, H.K.; Eckle, T.; Thompson, L.F. Physiological roles for ecto-5′-nucleotidase (CD73). Purinergic Signal. 2006, 2, 351–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noronha-Matos, J.B.; Correia-de-Sá, P. Mesenchymal Stem Cells Ageing: Targeting the “Purinome” to Promote Osteogenic Differentiation and Bone Repair. J. Cell Physiol. 2016, 231, 1852–1861. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, A.M.; Schetinger, M.R.; Correia-de-Sá, P.; Sevigny, J. Impact of ectonucleotidases in autonomic nervous functions. Auton Neurosci. 2015, 191, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Araújo, M.; Nascimento, C.; Timóteo, M.A.; Magalhães-Cardoso, M.T.; Correia-de-Sá, P. Relative contribution of ecto-ATPase and ecto-ATPDase pathways to the biphasic effect of ATP on acetylcholine release from myenteric motoneurons. Br. J. Pharm. 2009, 156, 519–533. [Google Scholar] [CrossRef] [Green Version]
- Magalhães-Cardoso, M.T.; Pereira, M.F.; Oliveira, L.; Ribeiro, J.A.; Cunha, R.A.; Correia-de-Sá, P. Ecto-AMP deaminase blunts the ATP-derived adenosine A2A receptor facilitation of acetylcholine release at rat motor nerve endings. J. Physiol. 2003, 549, 399–408. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, L.; Correia, A.; Cristina Costa, A.; Guerra-Gomes, S.; Ferreirinha, F.; Magalhães-Cardoso, M.T.; Vilanova, M.; Correia-de-Sá, P. Deficits in endogenous adenosine formation by ecto-5′-nucleotidase/CD73 impair neuromuscular transmission and immune competence in experimental autoimmune myasthenia gravis. Mediat. Inflamm. 2015, 2015, 460610. [Google Scholar] [CrossRef]
- Silva-Ramos, M.; Silva, I.; Faria, M.; Magalhães-Cardoso, M.T.; Correia, J.; Ferreirinha, F.; Correia-de-Sá, P. Impairment of ATP hydrolysis decreases adenosine A1 receptor tonus favoring cholinergic nerve hyperactivity in the obstructed human urinary bladder. Purinergic Signal. 2015, 11, 595–606. [Google Scholar] [CrossRef] [Green Version]
- Vieira, C.; Ferreirinha, F.; Magalhães-Cardoso, M.T.; Silva, I.; Marques, P.; Correia-de-Sá, P. Post-inflammatory Ileitis Induces Non-neuronal Purinergic Signaling Adjustments of Cholinergic Neurotransmission in the Myenteric Plexus. Front. Pharm. 2017, 8, 811. [Google Scholar] [CrossRef] [Green Version]
- Luttikhuizen, D.T.; Harmsen, M.C.; de Leij, L.F.; van Luyn, M.J. Expression of P2 receptors at sites of chronic inflammation. Cell Tissue Res. 2004, 317, 289–298. [Google Scholar] [CrossRef]
- Chunn, J.L.; Mohsenin, A.; Young, H.W.; Lee, C.G.; Elias, J.A.; Kellems, R.E.; Blackburn, M.R. Partially adenosine deaminase-deficient mice develop pulmonary fibrosis in association with adenosine elevations. Am. J. Physiol. Lung Cell Mol. Physiol. 2006, 290, L579–L587. [Google Scholar] [CrossRef] [PubMed]
- Fernández, P.; Trzaska, S.; Wilder, T.; Chiriboga, L.; Blackburn, M.R.; Cronstein, B.N.; Chan, E.S.L. Pharmacological blockade of A2A receptors prevents dermal fibrosis in a model of elevated tissue adenosine. Am. J. Pathol. 2008, 172, 1675–1682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klotz, K.N.; Hessling, J.; Hegler, J.; Owman, C.; Kull, B.; Fredholm, B.B.; Lohse, M.J. Comparative pharmacology of human adenosine receptor subtypes-characterization of stably transfected receptors in CHO cells. Naunyn. Schmiedeberg’s Arch. Pharm. 1998, 357, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Augusto, E.; Matos, M.; Sevigny, J.; El-Tayeb, A.; Bynoe, M.S.; Muller, C.E.; Cunha, R.A.; Chen, J.F. Ecto-5′-nucleotidase (CD73)-mediated formation of adenosine is critical for the striatal adenosine A2A receptor functions. J. Neurosci. 2013, 33, 11390–11399. [Google Scholar] [CrossRef] [Green Version]
- Barros-Barbosa, A.R.; Ferreirinha, F.; Oliveira, A.; Mendes, M.; Lobo, M.G.; Santos, A.; Rangel, R.; Pelletier, J.; Sévigny, J.; Cordeiro, J.M.; et al. Adenosine A2A receptor and ecto-5′-nucleotidase/CD73 are upregulated in hippocampal astrocytes of human patients with mesial temporal lobe epilepsy (MTLE). Purinergic Signal. 2016, 12, 719–734. [Google Scholar] [CrossRef] [Green Version]
- Cunha, R.A.; Correia-de-Sá, P.; Sebastiao, A.M.; Ribeiro, J.A. Preferential activation of excitatory adenosine receptors at rat hippocampal and neuromuscular synapses by adenosine formed from released adenine nucleotides. Br. J. Pharm. 1996, 119, 253–260. [Google Scholar] [CrossRef] [Green Version]
- Lazzerini, P.E.; Natale, M.; Gianchecchi, E.; Capecchi, P.L.; Montilli, C.; Zimbone, S.; Castrichini, M.; Balistreri, E.; Ricci, G.; Selvi, E.; et al. Adenosine A2A receptor activation stimulates collagen production in sclerodermic dermal fibroblasts either directly and through a cross-talk with the cannabinoid system. J. Mol. Med. 2012, 90, 331–342. [Google Scholar] [CrossRef]
- Fede, C.; Pirri, C.; Fan, C.; Albertin, G.; Porzionato, A.; Macchi, V.; De Caro, R.; Stecco, C. Sensitivity of the fasciae to sex hormone levels: Modulation of collagen-I, collagen-III and fibrillin production. PLoS ONE 2019, 14, e0223195. [Google Scholar] [CrossRef]
- Qu, F.; Cui, Y.; Zeng, J.; Zhang, M.; Qiu, S.; Huang, X.; Chen, A. Acupuncture induces adenosine in fibroblasts through energy metabolism and promotes proliferation by activating MAPK signaling pathway via adenosine3 receptor. J. Cell Physiol. 2020, 235, 2441–2451. [Google Scholar] [CrossRef]
- Perez-Aso, M.; Mediero, A.; Low, Y.C.; Levine, J.; Cronstein, B.N. Adenosine A2A receptor plays an important role in radiation-induced dermal injury. FASEB J. 2016, 30, 457–465. [Google Scholar] [CrossRef] [Green Version]
- Fais, A.; Cacace, E.; Corda, M.; Era, B.; Peri, M.; Utzeri, S.; Ruggiero, V. Purine metabolites in fibromyalgia syndrome. Clin. Biochem. 2013, 46, 37–39. [Google Scholar] [CrossRef] [PubMed]
- Hedley, G.D. Demonstration of the integrity of human superficial fascia as an autonomous organ. J. Bodyw. Mov. Ther. 2008, 12, 258. [Google Scholar] [CrossRef]
- Langevin, H.M.; Cornbrooks, C.J.; Taatjes, D.J. Fibroblasts form a body-wide cellular network. Histochem. Cell Biol. 2004, 122, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Janes, K.; Symons-Liguori, A.M.; Jacobson, K.A.; Salvemini, D. Identification of A3 adenosine receptor agonists as novel non-narcotic analgesics. Br. J. Pharmacol. 2016, 173, 1253–1267. [Google Scholar] [CrossRef] [Green Version]
- Coppi, E.; Cherchi, F.; Fusco, I.; Failli, P.; Vona, A.; Dettori, I.; Gaviano, L.; Lucarini, E.; Jacobson, K.A.; Tosh, D.K.; et al. Adenosine A3 receptor activation inhibits pronociceptive N-type Ca2+ currents and cell excitability in dorsal root ganglion neurons. Pain 2019, 160, 1103–1118. [Google Scholar] [CrossRef]
- Takano, T.; Chen, X.; Luo, F.; Fujita, T.; Ren, Z.; Goldman, N.; Zhao, Y.; Markman, J.D.; Nedergaard, M. Traditional acupuncture triggers a local increase in adenosine in human subjects. J. Pain 2012, 13, 1215–1223. [Google Scholar] [CrossRef] [Green Version]
- Langevin, H.M.; Bouffard, N.A.; Badger, G.J.; Churchill, D.L.; Howe, A.K. Subcutaneous tissue fibroblast cytoskeletal remodeling induced by acupuncture: Evidence for a mechanotransduction-based mechanism. J. Cell Physiol. 2006, 207, 767–774. [Google Scholar] [CrossRef]
- Nedeljkovic, N. Complex regulation of ecto-5′-nucleotidase/CD73 and A2AR-mediated adenosine signaling at neurovascular unit: A link between acute and chronic neuroinflammation. Pharmacol. Res. 2019, 144, 99–115. [Google Scholar] [CrossRef]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herman-de-Sousa, C.; Pinheiro, A.R.; Paramos-de-Carvalho, D.; Costa, M.A.; Ferreirinha, F.; Magalhães-Cardoso, T.; Ribeiro, S.; Pelletier, J.; Sévigny, J.; Correia-de-Sá, P. Opposing Effects of Adenosine and Inosine in Human Subcutaneous Fibroblasts May Be Regulated by Third Party ADA Cell Providers. Cells 2020, 9, 651. https://doi.org/10.3390/cells9030651
Herman-de-Sousa C, Pinheiro AR, Paramos-de-Carvalho D, Costa MA, Ferreirinha F, Magalhães-Cardoso T, Ribeiro S, Pelletier J, Sévigny J, Correia-de-Sá P. Opposing Effects of Adenosine and Inosine in Human Subcutaneous Fibroblasts May Be Regulated by Third Party ADA Cell Providers. Cells. 2020; 9(3):651. https://doi.org/10.3390/cells9030651
Chicago/Turabian StyleHerman-de-Sousa, Carina, Ana Rita Pinheiro, Diogo Paramos-de-Carvalho, Maria Adelina Costa, Fátima Ferreirinha, Teresa Magalhães-Cardoso, Severino Ribeiro, Julie Pelletier, Jean Sévigny, and Paulo Correia-de-Sá. 2020. "Opposing Effects of Adenosine and Inosine in Human Subcutaneous Fibroblasts May Be Regulated by Third Party ADA Cell Providers" Cells 9, no. 3: 651. https://doi.org/10.3390/cells9030651
APA StyleHerman-de-Sousa, C., Pinheiro, A. R., Paramos-de-Carvalho, D., Costa, M. A., Ferreirinha, F., Magalhães-Cardoso, T., Ribeiro, S., Pelletier, J., Sévigny, J., & Correia-de-Sá, P. (2020). Opposing Effects of Adenosine and Inosine in Human Subcutaneous Fibroblasts May Be Regulated by Third Party ADA Cell Providers. Cells, 9(3), 651. https://doi.org/10.3390/cells9030651