1. Introduction
The genus
Echinochloa includes about approximately 50 annual summer species, widespread in both tropical and temperate regions and in dry (e.g., maize and soybean) or water flooded soils (e.g., rice) [
1]. Several classification keys have been proposed in the last century, but none of them have been able to adequately fulfill the task;
Echinochloa species are often very difficult to distinguish due to wide intraspecific morphological and phenological variability [
2]. A rough first classification can be done on the basis of macro-phenological differences, dividing
Echinochloa species in two groups: the “red” (i.e.,
E. crus-galli (L.) P. Beav. and
E. hispidula (Retz.) Nees) and “white” species (e.g.,
E. oryzicola (Vasinger) Vasing. and
E. oryzoides (Ard.) Fritsch).
E. oryzicola (late watergrass) and
E. crus-galli (barnyard grass) are the most common members of the two categories in Italian paddy fields.
E. oryzicola is the dominant and most persistent due to its complex survival strategy in flooded rice [
3]. The species of both groups are annual with a C
4 photosynthetic pathway [
3,
4] and show a great competitive advantage when they grow together with C
3 crops.
Echinochloa spp. are weedy plants that have evolved resistance to several herbicide classes, among which acetolactate synthase (ALS) inhibitors are the most used for their control. The ALS-inhibiting herbicides are commonly used because of their high activity at low doses against a broad spectrum of weeds, the possibility of post-emergence grass weed control, the low mammalian toxicity and low costs [
5]. Furthermore, the introduction of the Clearfield
® technology, i.e., imidazolinone-tolerant rice varieties, has further increased the use of these herbicides, reducing the diversity of sites of action used in rice crops [
6]. Their highly specific target, along with their repetitive use on the same cropping system, have favored the selection of resistant populations. In most cases, the resistance to ALS inhibitors is due to point mutations in the
ALS gene (i.e., target-site resistance, TSR), that change specific amino acids of the target protein (ALS), thus reducing its affinity for this class of herbicides [
7]. So far, TSR to ALS inhibitors in
Echinochloa involve three amino acid positions: Ala-122, with three allelic variants (Val [
8], Thr [
8] and Asn [
9], in
E. crus-galli), Pro-197, with two allelic variants (Ser and Leu, in
E. cru-galli [
10]), and Trp-574, with one allelic variant (Leu, in
E. crus-galli [
10,
11] and
E. oryzicola [
12]).
On the basis of cytological evidence,
E. crus-galli is an allohexaploid assumed to have derived from a hybridization between the allotetraploid
E. oryzicola and an unknown diploid species [
13]. In polyploid species a variable number of each gene can occur. Therefore, the genomic organization is more complex than that of diploid species [
14,
15].
E. crus-galli and
E. oryzicola are allopolyploid species, which contain three and two homologous
ALS genes, respectively [
16], and each gene copy encoding a protein could evolve resistance-endowing mutations [
7]. It is still unclear how many gene copies, and which specific copy/ies, are responsible for the evolution of herbicide resistance in
Echinochloa and other polyploid species.
In this study, molecular analyses were performed on several Echinochloa spp. field populations to achieve a better taxonomic classification of “red” E. crus-galli and “white” E. oryzicola species using a CAPS Cleaved Amplified Polymorphic Sequence (CAPS) molecular marker based on the DNA barcoding method. The mutations endowing resistance to ALS inhibitors in the different species were detected and associated with the different ALS gene copies. Lastly, the relative expression of the ALS gene copies was evaluated to explain the high level of ALS inhibitor resistance in these polyploid species.
2. Materials and Methods
2.1. Plant Material
Seven
Echinochloa spp. populations were analyzed: 16S, 44R, 45R, 46R, 95R, 100R and 161S (note: S = susceptible and R = resistant). The different populations were preliminarily classified using the Pignatti (1982) [
17], Costea and Tardif (2002) [
18] and Viggiani and Tabacchi (2017) [
19] classification keys. All populations were collected in Italian rice fields (
Table S1), and tested for herbicide resistance through greenhouse assays following the protocol described in Panozzo et al. (2015) [
20]. Leaf tissue from the survivors of the recommended field dose of penoxsulam (40.8 g a.i. ha
−1) were sampled, frozen in liquid nitrogen and conserved at −80 °C until nucleic acids extraction.
2.2. DNA Barcoding and CAPS-rbcL
A DNA barcoding marker was established to assign the tested plants into the two classes of Echinochloa species (“red” or “white”). This was important to avoid misidentifications due to the high heterogeneity of field-collected populations, which can include plants from different species, and because correct identification of the plants was required for the molecular analyses.
A preliminary analysis using MEGA X
® software [
21] was performed including several nucleotide sequences of
Echinochloa spp. cpDNA (chloroplast DNA) gene
rbcL [
22,
23] reported in GenBank database (
www.ncbi.nlm.nih.gov/nucleotide accessed on 10 December 2021) and in the Barcode of Life Data Systems (BOLD,
http://www.barcodinglife.org/ accessed on 1 September 2021, vouchered specimens only), as well as some
rbcL sequences obtained from
Echinochloa purified and classified accessions [
24].
Genomic DNA (gDNA) was extracted from leaf tissue of five plants for each population using the CTAB method described in Doyle and Doyle (1987) [
25]. Concentration and quality of gDNA was determined using a NanoDrop™ 2000 (ThermoFisher Scientific, Waltham, MA, USA). Each sample was diluted with ddH
2O to reach a final concentration of 100 ng µL
−1 and then stored at −20 °C until use.
PCR amplification was conducted using the GoTaq
® G2 Hot Start DNA Polymerase (Promega, Madison, WI, USA) in a 25 µL final volume mixture including 5 µL 5× Go Colorless GoTaq
® Reaction Buffer, 2.5 µL MgCl
2 25 mM, 0.5 µL dNTPs mix 10 mM, 1 µL of primer rbcL_F1 (5′-GCA GCA TTC CGA GTA ACT CCT CA-3′) [
24] 10 µM and 1 µL of primer rbcL_R2 (5′-TTG GTG GAG GAA CTT TAG GAC ATC-3′) 10 µM, 0.2 µL GoTaq
® G2 Hot Start DNA Polymerase and 1 µL of gDNA. PCR reaction was conducted in a T1 Thermocycler (Biometra, Göttingen, Germany) using the following program: 2 min at 95 °C, 35 cycles with 30 s at 95 °C, 30 s at 60 °C, and 80 s at 72 °C, plus a final extension step at 72 °C for 5 min. Amplicons were purified using the NucleoSpin
® Gel and PCR Clean-Up Kit (Macherey-Nagel, Allentown, PA, USA) and sequenced by BMR genomics (Padova, Italy). Sequences were analyzed with Finch TV 1.4.0 software; consensus sequences were built using the SeqMan software included in the package DNASTAR
®. MEGA X
® software was used to align the sequences and to build UPGMA dendrograms.
To quickly distinguish the “red” from “white” plants in a heterogeneous population avoiding longer and more expensive sequencing experiments, a chloroplast cpDNA-based Cleaved Amplified Polymorphic Sequence (CAPS) system was designed based on the results of the DNA barcoding marker [
26]. Two non-species-specific primers were designed on the multi-species alignment of the
rbcL gene sequences, namely CAPS-rbcl-F (5′-CAACTGTTTGGACTGATGGAC-3′) and CAPS-rbcl-R (5′-CGTAGATCCTCCAAACGTAGAGC-3′); only the
rbcL sequence of “white” plants had a restriction site for the endonuclease TasI (AATT) (
Figure S1).
The PCR of the CAPS assay was performed using the Go Taq® G2 DNA Polymerase (Promega) in a 25 µL final volume mixture adding the required reagents. Amplification was conducted using the following program: DNA denaturation for 2 min at 95 °C, 25 cycles of 30 s at 95 °C, 30 s at 55 °C, and 20 s at 72 °C, and a final extension step of 5 min at 72 °C. PCR products were purified as described above and digestion with the endonuclease TasI (ThermoFisher Scientific, Waltham, MA, USA) was set as follows: purified PCR reaction mixture (5 µL), 10× Buffer B (1 µL) and TasI (1 µL) in a total volume of 16 µL. The reaction mix was incubated at 65 °C for 1 h and the inactivation of TasI was done by incubation at 80 °C for 20 min. EDTA 0.5M pH 8.0 (0.64 µL) was added to prepare the digested DNA for electrophoresis and the run was performed in a 2% agarose gel at 75 V for 90 min. Six to ten individual plants of each population were tested with the CAPS-rbcL to validate the method and then all plants used in the following experiments were preliminarily checked with the molecular marker.
2.3. Southern Blotting with ALS Gene
The plant material consisted of one E. crus-galli population (16S) susceptible to ALS inhibitors and three E. oryzicola populations, one susceptible (161S) and two resistant to ALS inhibitors (45R and 46R) collected in two sites far away from each other. This choice was made to determine whether the different number of copies of the gene could be related to the species and/or to the resistance status and/or to the site of collection.
Seeds of each population were chemically scarified [
11] and placed in soil. When seedlings were at three-leaf stage, one gram of the youngest leaf tissue (pooled from 10 plants) per population was ground in liquid nitrogen using a mortar and pestle and gDNA was extracted as described in
Section 2.2.
Digoxigenin (DIG)-labeled probe was generated by amplification of a conserved region (around B domain) of the ALS gene from gDNA of susceptible plants (population 16S) using PCR DIG Probe Synthesis Mix (Roche, Basel, Switzerland) and SB-F2 (5′-TGAGTTGGATCAGCAGAAGAG-3′) and SB-R2 (5′-AAGACCTTCACTGGGAGGTTC-3′) primers. GoTaq® Flexi DNA Polymerase (Promega) was used for the amplification. To a final volume of 50 µL, the following reagents were added: 10 µL 5× Colorless GoTaq® Flexi buffer, 2 µL MgCl2 solution 25 mM, 5 µL PCR DIG Probe Synthesis Mix (Roche), 2 µL of each primer 10 µM, 2.5 U of GoTaq® Hot Start DNA Polymerase and 200 ng of gDNA. Amplification was conducted using the following program: 2 min at 95 °C, 30 cycles of 30 s at 95 °C, 30 s at 54 °C, and 40 s at 72 °C, and 5 min of final extension time at 72 °C. The probe was purified using the MinElute PCR purification kit (Qiagen, Hilden, Germany) and stored at −20 °C until the digestion with endonucleases.
Five, 6-nucleotide-recognizing endonucleases that did not cut the probe sequence were selected to perform Southern blotting analysis on the four Echinochloa populations. Three experiments were conducted: (1) gDNA of populations 16S and 45R was digested with two restriction endonucleases, HindIII (Invitrogen, Waltham, MA, USA) and EcoRV (Invitrogen); (2) gDNA of populations 16S and 45R was digested with three restriction endonucleases, XbaI (Invitrogen), KpnI (Invitrogen) and BamHI (NEB); (3) populations 16S, 161S, 45R and 46R gDNA digestion was repeated with HindIII, to confirm the results obtained in the first two experiments.
The genomic DNA (30 μg per sample) was digested overnight at 37 °C in a total volume of 400 μL, following manufacturer’s instructions for each restriction enzyme. Aliquots of the digestion were checked in 1% agarose gel and the digestion stopped when the gDNA was completely digested. DNA was precipitated and re-suspended in 30 μL of ddH2O. Digested DNA and an undigested control were run on a 0.8% agarose gel in TBE buffer overnight using the DNA molecular weight market III DIG-labeled (Roche) for reference.
The gel was immersed in HCl 0.25 M stirring for 15 min at room temperature, followed by 20 min in denaturation buffer (0.5 M NaOH, 1.5 M NaCl). It was then washed in neutralization buffer (0.5 M -Tris-HCl pH 7.5, 1.5 M NaCl) for 30 min. DNA on gel was transferred to positively charged nylon membrane (Roche) by capillary in a 20× SSC buffer (3 M NaCl, 0.3 M sodium citrate, pH 7 and autoclaved) overnight. On the following day, DNA was fixed on the membrane using the Stratalinker® UV Crosslinker with the appropriate program and the hybridization was carried out following the manufacturer’s instructions. DIG-labeled probe was hybridized at 49 °C overnight in DIG Easy Hyb solution (Roche) to detect the homologous DNA fragments on the DNA blot.
2.4. ALS Gene Sequencing and Cloning
Two cloning experiments, with different aims, were conducted. In the first one, the full-length sequences of the ALS gene of two plants of each population were cloned, sequenced and aligned to detect the SNPs and mutations endowing resistance. In the second cloning experiment, the aim was to identify the diverse ALS gene copies of each species and their phylogenetic relationships. As it is technically difficult to clone a 2 kbp amplicon, in this experiment the ALS gene was amplified and cloned in two parts: 620 bp starting from the 5′ of the gene and including the aa position 122, and 1000 bp ending at the 3′ of the gene and including the aa position 574.
Total RNA was extracted from 100 mg of one fresh young leaf using the commercial InviTrap Spin Plant RNA Mini Kit (Invitek, Berlin, Germany). cDNA was synthesized using the ImProm-II
TM Reverse Transcriptase System (Promega) following the manufacturer’s instructions and the different parts of the
ALS gene were amplified using the primer pairs reported in
Table 1. PCR amplifications were conducted using the Advantage 2 PCR Kit (Clontech, Takara) in a 50 µL mixture of 1× Advantage 2 SA PCR Buffer, 1× dNTP mix (10 mM each), 0.2 µM of each primer, 1× Advantage 2 Polymerase Mix and 100 ng cDNA. Amplifications were conducted using the following program: 1 min at 95 °C; 35 cycles of 30 s at 95 °C, 30 s at 60 °C, and 40–120 s (depending on the length of the amplicon) at 68 °C; 3 min at 68 °C. PCR products were purified through columns using MinElute PCR purification kit (Qiagen) and cloned using the TOPO TA Cloning
® kit (Invitrogen) following the manufacturer’s instructions. In the first experiment, six colonies in total were selected from two plants per population. Plasmids were extracted from
E. coli bacterial cells using the PureYieldTM Plasmid Miniprep System (Promega), quantified in 1% agarose gel and sequenced with universal M13 primers and a forward primer positioned in the middle of the gene (ECH_5F). In the second experiment, 10–20 colonies were selected for each of two plants per population and only the universal M13 primers were used for the sequencing. The nucleotide sequences were edited using DNASTAR
® software and phylogenetic analysis was conducted using MEGA X
® software to determine specific motifs in the sequences able to distinguish the homologous gene copies. The analysis was hierarchically performed: in the first step cloned sequences of a single plant were analyzed together: then, those of different plants belonging to the same population: then, those of populations belonging to the same species and then all together.
2.5. Relative Expression of ALS Gene Copies
This study was used to analyze the relative expression of the ALS gene copy carrying the mutations responsible for target-site resistance to the ALS inhibitor penoxsulam (ALS1) with respect to the expression of the other ALS gene copies (ALS2-3). Comparisons were also made among populations belonging to different Echinochloa species, having different resistance pattern and collected in sites far away from each other.
Two S populations (16S and 161S) and three R populations (44R, 45R and 46R) were included in the analyses. Twenty seedlings for each population were transplanted into pots (one plant per pot) containing a soil substrate and maintained in a greenhouse until they reached the three-leaf stage. Ten plants for each population were sprayed with penoxsulam 240 g L
−1 at the dose of 250 mL ha
−1. After 24 h, 50–100 mg of leaf tissue was sampled from three treated (T) and three non-treated (NT) plants of each population. Total RNA was extracted using the TRIzol method [
27], purified using the DNase I (Roche) and cDNA was synthesized using the SuperScript
® III Reverse Transcriptase (Invitrogen) following the manufacturer’s instructions.
For qPCR analyses, specific primers for the different
ALS gene copies were designed based on an
ALS gene part including two allele-specific SNPs (
Table 2). The resulting amplicon was 140 bp long and included a cutting site for the restriction endonuclease FokI only in
ALS1. Therefore, a preliminary check was performed on cDNA samples of different
Echinochloa populations to check the specificity of each primer pair.
Eight reference genes (
Rubisco,
Actin,
18S,
CAP,
Tubuline,
GAPDH,
EF1 and
Ubiquitine) chosen among those previously tested on
Echinochloa spp. [
28] were tested on the cDNA of two populations (161S and 45R). Serial dilutions of cDNA template (1:100, 1:50, 1:25 and 1:6.25) were tested to set up the appropriate experimental conditions. Finally, cDNA of T and NT plants of all populations included in the experiment was tested in the qPCR analyses to determine the relative expression of the different
ALS gene copies using two reference genes (
Rubisco and
18S). qPCR was carried out in three technical replicates using the SYBR Green
® kit in a 7300 Real-Time PCR System (Applied Biosystems, Corning, NY, USA) on 96-well plates PCR-96M2-HS-C
® (Axygen, Corning, NY, USA) with a sealer MicroAmp
® Optical Adhesive Film (Applied Biosystems, Corning, NY, USA). The reactions were performed in a final volume of 20 μL consisting of 10 μL of cDNA (diluted 1:25), 2 μL of 10× buffer, 1.2 μL of MgCl
2 solution (50 mM), 0.5 μL of dNTPs (10 μM of each nucleotide), 2 μL of SYBR Green
® (Invitrogen), 0.2 μL of ROX Reference Dye (Invitrogen), 0.1 μL of Taq Platinum
® (Invitrogen) and 0.4 μL of each of the forward and reverse primers (10 μM). The amplification steps included an initial cycle of 95 °C for 5 min, followed by 40 cycles including a three-step amplification sequence (94 °C for 15 s, 60 °C for 10 s, 72 °C for 15 s), a fourth step where the instrument detects the fluorescence emitted at each cycle (60 °C for 35 s), and a final elongation step of 95 °C for 15 s and 60 °C for 60 s.
Data were analyzed using the Sequence Detection Software (SDS) 1.4 and the Ct values means, the standard deviation and confidence interval per treatment were calculated. The relative expression was calculated on the mean Ct values using the ∆Ct method [
29] by the following equation:
where ∆∆
Ct is the relative expression of the different
ALS gene copies in our case, and the application of the result in 2
−(∆∆Ct) gives the variation dimension (Relative Quantity, RQ). The Ct reference values were determined from the average of the two reference genes considered.
Table 2.
List of primers used for the study of relative expression of the different ALS gene copies.
Table 2.
List of primers used for the study of relative expression of the different ALS gene copies.
| Primer Name | Primer Sequence (5′–3′) | Target |
|---|
| F1_590Tm | GAG CAC ACA CAT ACT TGG GGC AT 1 | Forward primer, pos. 590
Specific for ALS1 |
| F2_590Cm | AGC ACA CAC ATA CTT GGG GCA C 1 | Forward primer, pos. 590
Specific for ALS2-3 |
| R1_621 | GAG CAT CTT CTT AAT TGC TGC ACG G | Reverse primer, pos. 621
Specific for ALS1 |
| R2_621 | GAG CAT CTT CTT GAT TGC TGC ACG T | Reverse primer, pos. 621
Specific for ALS2-3 |
4. Discussion
In this study we considered a series of
Echinochloa spp. populations collected in rice fields from different areas of Italy with a broad high resistance level to ALS inhibitors. In fact, both phenotypes associated with Ala122Asn and Trp574Leu mutations showed, on average, high resistance levels to all ALS inhibitors tested: penoxsulam, azimsulfuron and imazamox (note that population 100R was tested only for resistance to penoxsulam, therefore no speculation can be made regarding the other ALS inhibitors). An exception was population 44R which showed a slightly different pattern, it was cross-resistant to imazamox and penoxsulam but controlled by azimsulfuron. Furthermore, population 44R was one of the first cases of
Echionochloa resistant to the ACCase inhibitor profoxydim detected in Italy [
11]. The broad cross-resistance pattern was previously found in other species for mutations in position 122 [
33] and also in
Echinochloa spp. carrying a mutation in position 574 [
11,
12], whereas the resistance pattern of population 44R was firstly associated with the mutation in position 574 [
31].
The aim of this study was to clearly identify the main
Echinochloa species involved in ALS inhibitors resistance in Italian rice fields and to characterize the associated target-site resistance mechanism. Previous studies identified mutations on the
ALS gene associated with herbicide resistance, but information about the effect of mutations on the different genomes of this polyploid genus is not available. The morphological observations made according to different classification keys and the molecular data obtained analyzing the
rbcL gene of several
Echinochloa populations indicate that the two prevalent
Echinochloa species infesting Italian rice fields are
E. crus-galli and
E. oryzicola. This conclusion is supported also by the studies on ITS and cpDNA conducted by Aoki and Hirofumi on several
Echinochloa spp. accessions collected in Japan [
34]. They demonstrated that
E. phyllopogon and
E. oryzicola belong to the same species (i.e., similar morphological, cytogenetic, and isozymic features, as well as cross-compatibility) and, therefore,
E. phyllopogon is a synonym of
E. oryzicola.
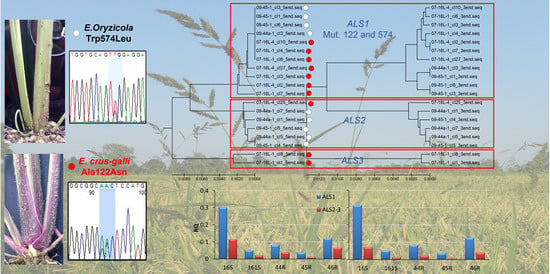
DNA-blotting analysis revealed differences in the
ALS gene locus number in the different populations investigated. The results of the hybridization of DNA fragments cut with HindIII showed that two copies of the
ALS gene are present in the genome of the
E. oryzicola susceptible population 161S and resistant populations 45R and 46R, whereas one more copy was detected in the genome of the
E. crus-galli susceptible population 16S. These results were confirmed by the
ALS gene cloning analyses, which revealed the presence of two shared homologs in all species (
ALS1 and
ALS2) to which a third copy (
ALS3) was added when
E. crus-galli populations were included as described also by Runge et al. [
35]. The differences in the gene copy numbers are in accordance with the genetic background of
E. crus-galli (6n), which is assumed to be evolutionarily derived from interspecific crossing between tetraploid
E. oryzicola (4n) and an unidentified diploid
Echinochloa spp. (2n) [
16]. The additional
ALS gene whose orthologue is not in
E. oryzicola is likely from the genome of the unidentified diploid
Echinochloa spp.
ALS1 was demonstrated to be the gene copy that carries the mutations responsible for target-site resistance. In a study on
E. crus-galli [
36] it was asserted that all three
ALS gene copies identified are involved in the evolution of target-site resistance, even if the results showed that mainly one
ALS gene copy (named
ALS3) was involved in conferring resistance. This second finding is in line with our qPCR results which identified
ALS1 as the gene copy, on average, that was more expressed in both non-treated and herbicide treated samples. Another study on a different weed,
Monochoria vaginalis, identified five copies of
ALS genes, two of which contributed to the evolution of target-site resistance and were largely more abundant than the other
ALS gene copies [
37].
It seems that in Echinochloa spp. the higher expression of ALS1 was not linked neither to the species, nor to the resistance status or the origin of the populations. ALS1 was expressed more highly in both population 16S, which belongs to E. crus-galli and in all other populations that belong E. oryzicola. At the same time, ALS1 was expressed more highly in both susceptible, 16S and 161S, and resistant populations 44R, 45R and 46R, which are originated from different parts of Italy. The relatively higher expression of ALS1 was expected considering the outcome of the cloning of the ALS gene. It was observed that, at least for E. oryzicola, ALS1 clones were preferentially selected in the random sampling over ALS2 clones.
The identification of resistance-endowing mutations only in
ALS1 is not entirely unexpected [
38], since this copy was also found to be the most highly expressed. In a polyploid species with multiple
ALS copies, a dilution effect will occur if only one of the copies carries resistance [
15,
39,
40]. The less the resistance-conferring gene is expressed, relative to the other copies, the greater the dilution will be, resulting in a lower level of resistance. Instead, in
Avena fatua [
39] single
ACCase resistance mutations confer relatively low resistance levels, likely due to a dilution effect by susceptible
ACCase expressed by homoeologs which led to a slow resistance evolution. The lack of dilution effect observed in
Echinochloa spp. [
36] may be associated with the rapid evolution of resistance seen in recent years in these species.
Although NTSR mechanisms cannot be ruled out [
10,
41], TS
ALS1 mutations seem to be the most evolutionarily favored mechanism endowing ALS inhibitor resistance in
Echinochloa spp. It would be interesting to conduct a broader survey of
Echinochloa spp. to determine whether resistance-conferring mutations in the other
ALS copies are occasionally present and, if so, to quantify the level of whole-plant resistance that they confer. Other questions that would be interesting to answer are: which ancestral genome does
ALS1 belong to? In the case of multiple-resistance, do different resistance-endowing variations occur on the same ancestral genome?