Framing Effects on Decision-Making for Diagnostic Genetic Testing: Results from a Randomized Trial
Abstract
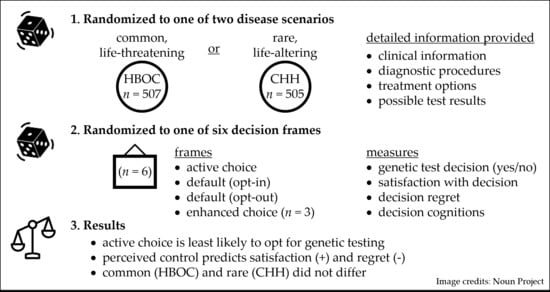
:1. Introduction
2. Materials and Methods
2.1. Trial Design
2.2. Participants
2.3. Interventions
2.4. Outcomes
2.5. Sample Size
2.6. Randomization/Sequence Generation
2.7. Statistical Methods
3. Results
3.1. Participant Characteristics
3.2. Effect of Choice Architecture (Framing) on Genetic Testing Decisions
3.3. Common, Life-Altering Scenario vs. Rare, Life-Altering Scenario
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adams, D.R.; Eng, C.M. Next-Generation Sequencing to Diagnose Suspected Genetic Disorders. N. Engl. J. Med. 2018, 379, 1353–1362. [Google Scholar] [CrossRef] [Green Version]
- Legare, F.; Robitaille, H.; Gane, C.; Hebert, J.; Labrecque, M.; Rousseau, F. Improving Decision Making about Genetic Testing in the Clinic: An Overview of Effective Knowledge Translation Interventions. PLoS ONE 2016, 11, e0150123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Academies of Sciences Engineering and Medicine. Understanding Disparities in Access to Genomic Medicine: Proceedings of a Workshop; National Academies Press: Washington, DC, USA, 2018. [Google Scholar] [CrossRef]
- Dwyer, A.A.; Hesse-Biber, S.; Flynn, B.; Remick, S. Parent of Origin Effects on Family Communication of Risk in BRCA+ Women: A Qualitative Investigation of Human Factors in Cascade Screening. Cancers 2020, 12, 2316. [Google Scholar] [CrossRef] [PubMed]
- Pacyna, J.E.; Radecki Breitkopf, C.; Jenkins, S.M.; Sutton, E.J.; Horrow, C.; Kullo, I.J.; Sharp, R.R. Should pretest genetic counselling be required for patients pursuing genomic sequencing? Results from a survey of participants in a large genomic implementation study. J. Med. Genet. 2019, 56, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Jamal, L.; Schupmann, W.; Berkman, B.E. An ethical framework for genetic counseling in the genomic era. J. Genet. Couns. 2020, 29, 718–727. [Google Scholar] [CrossRef]
- Ramos, E.; Weissman, S.M. The dawn of consumer-directed testing. Am. J. Med. Genet. C Semin. Med. Genet. 2018, 178, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Middleton, A.; Mendes, A.; Benjamin, C.M.; Howard, H.C. Direct-to-consumer genetic testing: Where and how does genetic counseling fit? Per. Med. 2017, 14, 249–257. [Google Scholar] [CrossRef]
- Thaler, R.H.; Sunstein, C.R. Nudge: Improving Decisions about Health, Wealth, and Happiness; Penguin Group: New York, NY, USA, 2009. [Google Scholar]
- Akl, E.A.; Oxman, A.D.; Herrin, J.; Vist, G.E.; Terrenato, I.; Sperati, F.; Costiniuk, C.; Blank, D.; Schunemann, H. Framing of health information messages. Cochrane Database Syst. Rev. 2011, CD006777. [Google Scholar] [CrossRef]
- Abhyankar, P.; Summers, B.A.; Velikova, G.; Bekker, H.L. Framing Options as Choice or Opportunity: Does the Frame Influence Decisions? Med. Decis. Mak. 2014, 34, 567–582. [Google Scholar] [CrossRef]
- Voorwinden, J.S.; Buitenhuis, A.H.; Birnie, E.; Lucassen, A.M.; Verkerk, M.A.; van Langen, I.M.; Plantinga, M.; Ranchor, A.V. Expanded carrier screening: What determines intended participation and can this be influenced by message framing and narrative information? Eur. J. Hum. Genet. 2017, 25, 793–800. [Google Scholar] [CrossRef] [Green Version]
- Lillie, S.E.; Tarini, B.A.; Janz, N.K.; Zikmund-Fisher, B.J. Framing optional genetic testing in the context of mandatory newborn screening tests. BMC Med. Inform. Decis. Mak. 2015, 15, 50. [Google Scholar] [CrossRef] [Green Version]
- Ajzen, I. The theory of planned behavior. Org. Behav. Hum. Dec. Process. 1991, 50, 179–211. [Google Scholar] [CrossRef]
- Gooding, H.C.; Organista, K.; Burack, J.; Biesecker, B.B. Genetic susceptibility testing from a stress and coping perspective. Soc. Sci. Med. 2006, 62, 1880–1890. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Tenhunen, H.; Torkki, P.; Heinonen, S.; Lillrank, P.; Stefanovic, V. Considering medical risk information and communicating values: A mixed-method study of women’s choice in prenatal testing. PLoS ONE 2017, 12, e0173669. [Google Scholar] [CrossRef] [PubMed]
- Atkins, R.; Kelly, T.A.; Johnson, S.; Williams, W.; Nelson, Y.; Joseph, P.V.; Jackson, D.; King, D.; Stellmacher, T.; Halty, N.D.; et al. Eliciting Willingness and Beliefs towards Participation in Genetic Psychiatric Testing in Black/African American Mothers at Risk for Depression. Behav. Sci. 2020, 10, 181. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, P.; Grant, S.; Mayo-Wilson, E.; Macdonald, G.; Michie, S.; Hopewell, S.; Moher, D.; Group, C.-S. Reporting randomised trials of social and psychological interventions: The CONSORT-SPI 2018 Extension. Trials 2018, 19, 407. [Google Scholar] [CrossRef]
- Mason, W.; Suri, S. Conducting behavioral research on Amazon’s Mechanical Turk. Behav. Res. Methods 2012, 44, 1–23. [Google Scholar] [CrossRef]
- Bohannon, J. PSYCHOLOGY. Mechanical Turk upends social sciences. Science 2016, 352, 1263–1264. [Google Scholar] [CrossRef]
- Crump, M.J.; McDonnell, J.V.; Gureckis, T.M. Evaluating Amazon’s Mechanical Turk as a tool for experimental behavioral research. PLoS ONE 2013, 8, e57410. [Google Scholar] [CrossRef] [Green Version]
- Bartneck, C.; Duenser, A.; Moltchanova, E.; Zawieska, K. Comparing the similarity of responses received from studies in Amazon’s Mechanical Turk to studies conducted online and with direct recruitment. PLoS ONE 2015, 10, e0121595. [Google Scholar] [CrossRef]
- Mortensen, K.; Hughes, T.L. Comparing Amazon’s Mechanical Turk Platform to Conventional Data Collection Methods in the Health and Medical Research Literature. J. Gen. Intern. Med. 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tversky, A.; Kahneman, D. The framing of decisions and the psychology of choice. Science 1981, 211, 453–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armitage, C.J.; Conner, M. Efficacy of the Theory of Planned Behaviour: A meta-analytic review. Br. J. Soc. Psychol. 2001, 40, 471–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fonteyn, M.E.; Kuipers, B.; Grobe, S.J. A description of think aloud method and protocol analysis. Qual. Health Res. 1993, 3, 430–441. [Google Scholar] [CrossRef]
- Holmes-Rovner, M.; Kroll, J.; Schmitt, N.; Rovner, D.R.; Breer, M.L.; Rothert, M.L.; Padonu, G.; Talarczyk, G. Patient satisfaction with health care decisions: The satisfaction with decision scale. Med. Decis. Mak. 1996, 16, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Wills, C.E.; Holmes-Rovner, M. Preliminary validation of the Satisfaction with Decision scale with depressed primary care patients. Health Expect 2003, 6, 149–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Connor, A.M. User Manual—Decision Regret Scale. Available online: http://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_Refret_Scale.pdf (accessed on 8 November 2020).
- Chew, L.D.; Griffin, J.M.; Partin, M.R.; Noorbaloochi, S.; Grill, J.P.; Snyder, A.; Bradley, K.A.; Nugent, S.M.; Baines, A.D.; Vanryn, M. Validation of screening questions for limited health literacy in a large VA outpatient population. J. Gen. Intern. Med. 2008, 23, 561–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallace, L.S.; Rogers, E.S.; Roskos, S.E.; Holiday, D.B.; Weiss, B.D. Brief report: Screening items to identify patients with limited health literacy skills. J. Gen. Intern. Med. 2006, 21, 874–877. [Google Scholar] [CrossRef] [Green Version]
- Weiss, B.D.; Mays, M.Z.; Martz, W.; Castro, K.M.; DeWalt, D.A.; Pignone, M.P.; Mockbee, J.; Hale, F.A. Quick assessment of literacy in primary care: The newest vital sign. Ann. Fam. Med. 2005, 3, 514–522. [Google Scholar] [CrossRef] [Green Version]
- Shendure, J.; Findlay, G.M.; Snyder, M.W. Genomic Medicine-Progress, Pitfalls, and Promise. Cell 2019, 177, 45–57. [Google Scholar] [CrossRef] [Green Version]
- Horton, R.; Lucassen, A.; Fenwick, A. Unpacking the Concept of a Genomic Result. Am. J. Bioeth. 2019, 19, 70–71. [Google Scholar] [CrossRef]
- Niemiec, E.; Kalokairinou, L.; Howard, H.C. Current ethical and legal issues in health-related direct-to-consumer genetic testing. Per. Med. 2017, 14, 433–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcon, A.R.; Bieber, M.; Caulfield, T. Representing a “revolution”: How the popular press has portrayed personalized medicine. Genet. Med. 2018, 20, 950–956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, K.M. Genetic Pointillism versus Physiological Form. Perspect. Biol. Med. 2018, 61, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.T.; Lee, K.; Chung, W.K.; Gordon, A.S.; Herman, G.E.; Klein, T.E.; Stewart, D.R.; Amendola, L.M.; Adelman, K.; Bale, S.J.; et al. ACMG SF v3.0 list for reporting of secondary findings in clinical exome and genome sequencing: A policy statement of the American College of Medical Genetics and Genomics (ACMG). Genet. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.C.; Dotson, W.D.; DeVore, C.S.; Bednar, E.M.; Bowen, D.J.; Ganiats, T.G.; Green, R.F.; Hurst, G.M.; Philp, A.R.; Ricker, C.N.; et al. Delivery Of Cascade Screening For Hereditary Conditions: A Scoping Review Of The Literature. Health Aff. 2018, 37, 801–808. [Google Scholar] [CrossRef]
- Fattahi Ardakani, M.; Salehi-Abargouei, A.; Sotoudeh, A.; Esmaeildokht, S.; Bahrevar, V. Do Subjective Norms Predict the Screening of Cancer Patients’ First-Degree Relatives? A Systematic Review and Meta-Analysis. Asian Pac. J. Cancer Prev. 2020, 21, 1521–1530. [Google Scholar] [CrossRef]
- United States Preventive Services Taskforce; Owens, D.K.; Davidson, K.W.; Krist, A.H.; Barry, M.J.; Cabana, M.; Caughey, A.B.; Doubeni, C.A.; Epling, J.W., Jr.; Kubik, M.; et al. Risk Assessment, Genetic Counseling, and Genetic Testing for BRCA-Related Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2019, 322, 652–665. [Google Scholar] [CrossRef] [Green Version]
- Boehm, U.; Bouloux, P.M.; Dattani, M.T.; de Roux, N.; Dode, C.; Dunkel, L.; Dwyer, A.A.; Giacobini, P.; Hardelin, J.P.; Juul, A.; et al. Expert consensus document: European Consensus Statement on congenital hypogonadotropic hypogonadism—Pathogenesis, diagnosis and treatment. Nat. Rev. Endocrinol. 2015, 11, 547–564. [Google Scholar] [CrossRef] [Green Version]
- Abhyankar, P.; Volk, R.J.; Blumenthal-Barby, J.; Bravo, P.; Buchholz, A.; Ozanne, E.; Vidal, D.C.; Col, N.; Stalmeier, P. Balancing the presentation of information and options in patient decision aids: An updated review. BMC Med. Inform. Decis. Mak. 2013, 13 (Suppl. 2), S6. [Google Scholar] [CrossRef] [Green Version]



| HBOC (n = 507) | CHH (n = 505) | Total (n = 1012) | |
|---|---|---|---|
| Age (years) | |||
| Mean ± SD | 36.1 ± 10.7 | 36.3 ± 10.8 | 36.2 ± 10.7 |
| (95% CI) | (36.4–37.3) | (32.4–47.8) | (34.4–42.1) |
| Sex | |||
| Male | 304 (60%) | 300 (59%) | 604 (60%) |
| Female | 203 (40%) | 205 (41%) | 408 (40%) |
| Race | |||
| White | 207 (68%) | 217 (69%) | 424 (68%) |
| Asian | 73 (24%) | 69 (22%) | 142 (23%) |
| Black/African-American | 19 (6%) | 22 (7%) | 41 (7%) |
| Other * | 7 (2%) | 8 (2%) | 15 (2%) |
| Marital Status | |||
| Single | 242 (48%) | 234 (46%) | 476 (47%) |
| Married | 265 (52%) | 271 (54%) | 536 (53%) |
| Children | 246 (80%) | 256 (81%) | 502 (81%) |
| Education | |||
| Less than college | 107 (21%) | 120 (24%) | 227 (22%) |
| College graduate | 306 (60%) | 288 (57%) | 594 (59%) |
| Post-graduate | 94 (19%) | 97 (19%) | 191 (19%) |
| Subjective health literacy † | |||
| Adequate (n, %) | 413 (81%) | 409 (80%) | 822 (81%) |
| Inadequate (n, %) | 94 (19%) | 96 (20%) | 1990 (19%) |
| Objective health literacy (NVS) | |||
| Mean ± SD | 3.06 ± 0.80 | 2.87 ± 0.80 | 2.97 ± 0.06 |
| (95% CI) | (2.90–3.22) | (2.71–3.03) | (2.85–3.08) |
| Past experience | |||
| Breast cancer (n, %) | 91 (18%) | n/a | 91 (18%) |
| Rare disease (n, %) | n/a | 86 (17%) | 86 (17%) |
| Theory of Planned Behavior Item | Satisfaction † Bi (SE) | Regret ‡ Bi (SE) |
|---|---|---|
| Perceived risk | ||
| This health scenario would effect me personally | B = 0.071 (0.065) p = 0.27 | B = 0.100 (0.049) p = 0.042 |
| Context/Consequences | ||
| GT would have physical consequences for me | B = 0.018 (0.055) p = 0.74 | B = 0.124 (0.042) p = 0.003 |
| GT would have psychological consequences for me | B = 0.017 (0.053) p = 0.75 | B = 0.049 (0.040) p = 0.22 |
| GT would have social consequences for me (discrimination) | B = 0.018 (0.059) p = 0.76 | B = 0.291 (0.045) p < 0.001 |
| Attitudes | ||
| Having GT would be an easy decision | B = 0.508 (0.069) p < 0.001 | B = 0.258 (0.053) p < 0.001 |
| Having GT would be good/bad | B = 0.195 (0.075) p = 0.010 | B = 0.285 (0.057) p < 0.001 |
| For me, having GT would be pleasant/unpleasant | B = 0.156 (0.090) p = 0.08 | B = 0.050 (0.068) p = 0.46 |
| Norms | ||
| Having GT would be important for people I care about | B = 0.53 (0.088) p < 0.001 | B = 0.091 (0.067) p = 0.17 |
| Having GT would be important for my healthcare provider | B = 0.105 (0.059) p = 0.08 | B = 0.083 (0.045) p = 0.06 |
| For me, having GT would be valuable | B = -0.143 (0.091) p = 0.12 | B = 0.089 (0.069) p = 0.20 |
| Behavioral control | ||
| Having GT is entirely up to me | B = 0.811 (0.085) p < 0.001 | B = 0.126 (0.064) p = 0.05 |
| If my doctor offers GT, it would be difficult for me to say no | B = -0.2 (0.045) p = 0.66 | B = 0.031 (0.34) p = 0.37 |
| I feel I have no control over my decision to have GT | B = -0.254 (0.049) p < 0.001 | B = 0.346 (0.037) p < 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dwyer, A.A.; Shen, H.; Zeng, Z.; Gregas, M.; Zhao, M. Framing Effects on Decision-Making for Diagnostic Genetic Testing: Results from a Randomized Trial. Genes 2021, 12, 941. https://doi.org/10.3390/genes12060941
Dwyer AA, Shen H, Zeng Z, Gregas M, Zhao M. Framing Effects on Decision-Making for Diagnostic Genetic Testing: Results from a Randomized Trial. Genes. 2021; 12(6):941. https://doi.org/10.3390/genes12060941
Chicago/Turabian StyleDwyer, Andrew A., Hongjie Shen, Ziwei Zeng, Matt Gregas, and Min Zhao. 2021. "Framing Effects on Decision-Making for Diagnostic Genetic Testing: Results from a Randomized Trial" Genes 12, no. 6: 941. https://doi.org/10.3390/genes12060941
APA StyleDwyer, A. A., Shen, H., Zeng, Z., Gregas, M., & Zhao, M. (2021). Framing Effects on Decision-Making for Diagnostic Genetic Testing: Results from a Randomized Trial. Genes, 12(6), 941. https://doi.org/10.3390/genes12060941