Fe0/H2O Systems for Environmental Remediation: The Scientific History and Future Research Directions
Abstract
:1. Introduction
2. The Interactions within Fe0/H2O Systems
Mass Transfer within Fe0/H2O Systems
3. The Importance of Indirect Reduction
3.1. Aniline and the Fe0/H2O System
3.2. MnO2 and the Fe0/H2O System
4. Long-Term Permeability of Fe0 Walls
5. Ion-Selective Nature of Fe0/H2O Systems (Coulomb’s Law)
6. Discussion
7. Fe0 for Environmental Remediation
8. Lessons from the History and Future Directions
- (1)
- Understanding the role and mechanisms of interfering inorganic and organic species typically occurring in natural multi-component aqueous systems under relevant environmental conditions,
- (2)
- Elucidating the processes occurring on the various material phases (solid, liquid, and solid–liquid interface), and their effects on the formation and persistence of the iron oxide film. Recent advances in surface analytical techniques for solid-state characterization enable such detailed studies,
- (3)
- Long-term studies using typical multi-component contaminated aqueous media conducted in a quiescent mode are required to overcome some of the limitations associated with short-term studies based on artificial solutions and ideal experimental conditions (e.g., agitation, constant temperature),
- (4)
- Development and evaluation of tailor-made Fe0/H2O systems that accounts for the expansive nature of iron corrosion, ion-selectivity, and the role of co-solutes/agents. This will overcome the limitations associated with the use of materials not purposively developed for Fe0 remediation.
9. Conclusions
Supplementary Materials
Supplementary File 1Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gillham, R.W.; O’Hannesin, S.F. Enhanced degradation of halogenated aliphatics by zero-valent iron. Groundwater 1994, 32, 958–967. [Google Scholar] [CrossRef]
- Burris, D.R.; Campbell, T.J.; Manoranjan, V.S. Sorption of trichloroethylene and tetrachloroethylene in a batch reactive metallic iron-water system. Environ. Sci. Technol. 1995, 29, 2850–2855. [Google Scholar] [CrossRef] [PubMed]
- Cantrell, K.J.; Kaplan, D.I.; Wietsma, T.W. Zero-valent iron for the in situ remediation of selected metals in groundwater. J. Hazard. Mater. 1995, 42, 201–212. [Google Scholar] [CrossRef]
- Muftikian, R.; Fernando, Q.; Korte, N. A method for the rapid dechlorination of low molecular weight chlorinated hydrocarbons in water. Water Res. 1995, 29, 2434–2439. [Google Scholar] [CrossRef]
- Wilson, E.K. Zero-valent metals provide possible solution to groundwater problems. Chem. Eng. News 1995, 73, 19–22. [Google Scholar] [CrossRef]
- Dwyer, B.P.; Marozas, D.C.; Cantrell, K.; Stewart, W. Laboratory and Field Scale Demonstration of Reactive Barrier Systems; SAND–96-2500; Sandia National Laboratories: Albuquerque, NM, USA, 1996; p. 13. [Google Scholar]
- Fairweather, V. When toxics meet metal. Civ. Eng. 1996, 66, 44–48. [Google Scholar]
- Tratnyek, P.G. Putting corrosion to use: Remediating contaminated groundwater with zero-valent metals. Chem. Ind. 1996, 13, 499–503. [Google Scholar]
- Morrison, S.J.; Mushovic, P.S.; Niesen, P.L. Early breakthrough of molybdenum and uranium in a permeable reactive barrier. Environ. Sci. Technol. 2006, 40, 2018–2024. [Google Scholar] [CrossRef] [PubMed]
- Gillham, R.W. Development of the granular iron permeable reactive barrier technology (good science or good fortune). In Advances in Environmental Geotechnics: Proceedings of the International Symposium on Geoenvironmental Engineering, Hangzhou, China, 8–10 September 2007; Chen, Y., Tang, X., Zhan, L., Eds.; Springer: Berlin, Germany; London, UK, 2008; pp. 5–15. [Google Scholar]
- Reynolds, G.W.; Hoff, J.T.; Gillham, R.W. Sampling bias caused by materials used to monitor halocarbons in groundwater. Environ. Sci. Technol. 1990, 24, 135–142. [Google Scholar] [CrossRef]
- Richardson, J.P.; Nicklow, J.W. In situ permeable reactive barriers for groundwater contamination. Soil Sediment Contam. 2002, 11, 241–268. [Google Scholar] [CrossRef]
- Obiri-Nyarko, F.; Grajales-Mesa, S.J.; Malina, G. An overview of permeable reactive barriers for in situ sustainable groundwater remediation. Chemosphere 2014, 111, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sinha, A. Biphasic reduction model for predicting the impacts of dye-bath constituents on the reduction of tris-azo dye Direct Green-1 by zero valent iron (Fe0). J. Environ. Sci. 2017, 52, 160–1699. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, J.; Huang, T.; Guan, X. The influences of iron characteristics, operating conditions and solution chemistry on contaminants removal by zero-valent iron: A review. Water Res. 2016, 100, 277–295. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Sun, Y.; Qin, H.; Li, J.; Lo, I.M.C.; He, D.; Dong, H. The limitations of applying zero-valent iron technology in contaminants sequestration and the corresponding countermeasures: The development in zero-valent iron technology in the last two decades (1994–2014). Water Res. 2015, 75, 224–248. [Google Scholar] [CrossRef] [PubMed]
- James, B.R.; Rabenhorst, M.C.; Frigon, G.A. Phosphorus sorption by peat and sand amended with iron oxides or steel wool. Water Environ. Res. 1992, 64, 699–705. [Google Scholar] [CrossRef]
- Campos, V. The effect of carbon steel-wool in removal of arsenic from drinking water. Environ. Geol. 2002, 42, 81–82. [Google Scholar] [CrossRef]
- Henderson, A.D.; Demond, A.H. Long-term performance of zero-valent iron permeable reactive barriers: A critical review. Environ. Eng. Sci. 2007, 24, 401–423. [Google Scholar] [CrossRef]
- Antia, D.D.J. Sustainable zero-valent metal (ZVM) water treatment associated with diffusion, infiltration, abstraction and recirculation. Sustainability 2010, 2, 2988–3073. [Google Scholar] [CrossRef]
- Birke, V.; Schuett, C.; Burmeier, H.; Friedrich, H.-J. Impact of trace elements and impurities in technical zero-valent iron brands on reductive dechlorination of chlorinated ethenes in groundwater. In Permeable Reactive Barrier: Sustainable Groundwater Remediation; Naidu, R., Birke, V., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 87–98. ISBN 978-1-4822-2447-4. [Google Scholar]
- Wilkin, R.T.; Acree, S.D.; Ross, R.R.; Puls, R.W.; Lee, T.R.; Woods, L.L. Fifteen-year assessment of a permeable reactive barrier for treatment of chromate and trichloroethylene in groundwater. Sci. Total Environ. 2014, 468–469, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Alonso, F.; Beletskaya, I.P.; Yus, M. Metal-mediated reductive hydrodehalogenation of organic halides. Chem. Rev. 2002, 102, 4009–4092. [Google Scholar] [CrossRef] [PubMed]
- Matheson, L.J.; Tratnyek, P.G. Reductive dehalogenation of chlorinated methanes by iron metal. Environ. Sci. Technol. 1994, 28, 2045–2053. [Google Scholar] [CrossRef] [PubMed]
- Weber, E.J. Iron-mediated reductive transformations: Investigation of reaction mechanism. Environ. Sci. Technol. 1996, 30, 716–719. [Google Scholar] [CrossRef]
- Fu, F.; Dionysiou, D.D.; Liu, H. The use of zero-valent iron for groundwater remediation and wastewater treatment: A review. J. Hazard. Mater. 2014, 267, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Colombo, A.; Dragonetti, C.; Magni, M.; Roberto, D. Degradation of toxic halogenated organic compounds by iron-containing mono-, bi- and tri-metallic particles in water. Inorg. Chim. Acta 2015, 431, 48–60. [Google Scholar] [CrossRef]
- Jiao, Y.; Qiu, C.; Huang, L.; Wu, K.; Ma, H.; Chen, S.; Ma, L.; Wu, L. Reductive dechlorination of carbon tetrachloride by zero-valent iron and related iron corrosion. Appl. Catal. B 2009, 91, 434–440. [Google Scholar] [CrossRef]
- Gheju, M. Progress in understanding the mechanism of CrVI Removal in Fe0-based filtration systems. Water 2018, 10, 651. [Google Scholar] [CrossRef]
- Noubactep, C. Relevant reducing agents in remediation Fe0/H2O systems. Clean Soil Air Water 2013, 41, 493–502. [Google Scholar] [CrossRef]
- Gheju, M.; Balcu, I. Sustaining the efficiency of the Fe(0)/H2O system for Cr(VI) removal by MnO2 amendment. Chemosphere 2019, 214, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Sarr, D. Zero-valent-iron permeable reactive barriers—How long will they last? Remediation 2001, 11, 1–18. [Google Scholar]
- Noubactep, C. Metallic iron for environmental remediation: A review of reviews. Water Res. 2015, 85, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Sareyed-Dim, N.A. The Cementation of Nickel onto Iron at Elevated Temperatures. Ph.D. Thesis, Monash University, Clayton, Australia, 1974. [Google Scholar]
- Burris, D.R.; Allen-King, R.M.; Manoranjan, V.S.; Campbell, T.J.; Loraine, G.A.; Baolin, D. Chlorinated ethene reduction by cast iron: Sorption and mass transfer. J. Environ. Eng. 1998, 124, 1012–1019. [Google Scholar] [CrossRef]
- Cho, H.-H.; Lee, T.; Hwang, S.-J.; Park, J.-W. Iron and organo-bentonite for the reduction and sorption of trichloroethylene. Chemosphere 2005, 58, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Popat, V.; Padhiyar, N. Kinetic study of Bechamp Process for p-nitrotoluene reduction to p-toluidine. Int. J. Chem. Eng. Appl. 2013, 4, 401–405. [Google Scholar] [CrossRef]
- Firdous, R.; Devlin, J.F. BEARKIMPE-2: A VBA Excel program for characterizing granular iron in treatability studies. Comput. Geosci. 2014, 63, 54–61. [Google Scholar] [CrossRef]
- Noubactep, C. Processes of contaminant removal in “Fe0–H2O” systems revisited. The importance of co-precipitation. Open Environ. Sci. 2007, 1, 9–13. [Google Scholar] [CrossRef]
- Noubactep, C. A critical review on the mechanism of contaminant removal in Fe0–H2O systems. Environ. Technol. 2008, 29, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Noubactep, C. An analysis of the evolution of reactive species in Fe0/H2O systems. J. Hazard. Mater. 2009, 168, 1626–1631. [Google Scholar] [CrossRef] [PubMed]
- Noubactep, C. The fundamental mechanism of aqueous contaminant removal by metallic iron. Water SA 2010, 36, 663–670. [Google Scholar] [CrossRef]
- Noubactep, C. Aqueous contaminant removal by metallic iron: Is the paradigm shifting? Water SA 2011, 37, 419–426. [Google Scholar] [CrossRef]
- Noubactep, C. Investigating the processes of contaminant removal in Fe0/H2O systems. Korean J. Chem. Eng. 2012, 29, 1050–1056. [Google Scholar] [CrossRef]
- Noubactep, C. Metallic iron for water treatment: A critical review. Clean Soil Air Water 2013, 41, 702–710. [Google Scholar] [CrossRef]
- Ghauch, A. Iron-based metallic systems: An excellent choice for sustainable water treatment. Freib. Online Geosci. 2015, 38, 1–80. [Google Scholar]
- Noubactep, C. Research on metallic iron for environmental remediation: Stopping growing sloppy science. Chemosphere 2016, 153, 528–530. [Google Scholar] [CrossRef] [PubMed]
- Makota, S.; Nde-Tchoupe, A.I.; Mwakabona, H.T.; Tepong-Tsindé, R.; Noubactep, C.; Nassi, A.; Njau, K.N. Metallic iron for water treatment: Leaving the valley of confusion. Appl. Water Sci. 2017, 7, 4177–4196. [Google Scholar] [CrossRef]
- Noubactep, C.; Makota, S.; Bandyopadhyay, A. Rescuing Fe0 remediation research from its systemic flaws. Res. Rev. Insights 2017, 1, 1–8. [Google Scholar] [CrossRef]
- Ghauch, A.; Abou Assi, H.; Baydoun, H.; Tuqan, A.M.; Bejjani, A. Fe0-based trimetallic systems for the removal of aqueous diclofenac: Mechanism and kinetics. Chem. Eng. J. 2011, 172, 1033–1044. [Google Scholar] [CrossRef]
- Naseri, E.; Ndé-Tchoupé, A.I.; Mwakabona, H.T.; Nanseu-Njiki, C.P.; Noubactep, C.; Njau, K.N.; Wydra, K.D. Making Fe0-based filters a universal solution for safe drinking water provision. Sustainability 2017, 9, 1224. [Google Scholar] [CrossRef]
- Gheju, M. Hexavalent chromium reduction with zero-valent iron (ZVI) in aquatic systems. Water Air Soil Pollut. 2011, 222, 103–148. [Google Scholar] [CrossRef]
- Gheju, M.; Balcu, I. Removal of chromium from Cr(VI) polluted wastewaters by reduction with scrap iron and subsequent precipitation of resulted cations. J. Hazard. Mater. 2011, 196, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Gheju, M.; Balcu, I.; Vancea, C. An investigation of Cr(VI) removal with metallic iron in the co-presence of sand and/or MnO2. J. Environ. Manag. 2016, 170, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M. The formation and properties of passive films on iron. Can. J. Chem. 1959, 37, 286–291. [Google Scholar] [CrossRef]
- Cohen, M. Thin oxide films on iron. J. Electrochem. Soc. 1974, 121, 191C–197C. [Google Scholar] [CrossRef]
- Vetter, K.J. General kinetics of passive layers on metals. Electrochim. Acta 1971, 16, 1923–1937. [Google Scholar] [CrossRef]
- Sato, N. 1989 Whitney Award Lecture: Toward a more fundamental understanding of corrosion processes. Corrosion 1989, 45, 354–368. [Google Scholar] [CrossRef]
- Sato, N. An overview on the passivity of metals. Corros. Sci. 1990, 31, 1–19. [Google Scholar] [CrossRef]
- Knowlton, L.G. Some experiments on iron. J. Phys. Chem. 1928, 32, 1572–1595. [Google Scholar] [CrossRef]
- Wang, L.; Li, P.; Wu, Z.; Yan, J.; Wang, M.; Ding, Y. Reduction of nitroarenes to aromatic amines with nanosized activated metallic iron powder in water. Synthesis 2003, 13, 2001–2004. [Google Scholar] [CrossRef]
- Gould, J.P. The kinetics of hexavalent chromium reduction by metallic iron. Water Res. 1982, 16, 871–877. [Google Scholar] [CrossRef]
- Murphy, A.P. Chemical removal of nitrate from water. Nature 1991, 350, 223–225. [Google Scholar] [CrossRef]
- Brondel, D.; Edwards, R.; Hayman, A.; Hill, D.; Mehta, S.; Semerad, T. Corrosion in the oil industry. Oilfield Rev. 1994, 6, 4–18. [Google Scholar]
- Nesic, S. Key issues related to modelling of internal corrosion of oil and gas pipelines—A review. Corros. Sci. 2007, 49, 4308–4338. [Google Scholar] [CrossRef]
- Lipczynska-Kochany, E.; Harms, S.; Milburn, R.; Sprah, G.; Nadarajah, N. Degradation of carbon tetrachloride in the presence of iron and sulphur containing compounds. Chemosphere 1994, 29, 1477–1489. [Google Scholar] [CrossRef]
- Khudenko, B.M. Feasibility evaluation of a novel method for destruction of organics. Water Sci. Technol. 1991, 23, 1873–1881. [Google Scholar] [CrossRef]
- Warren, K.D.; Arnold, R.G.; Bishop, T.L.; Lindholm, L.C.; Betterton, E.A. Kinetics and mechanism of reductive dehalogenation of carbon tetrachloride using zero-valence metals. J. Hazard. Mater. 1995, 41, 217–227. [Google Scholar] [CrossRef]
- Qiu, S.R.; Lai, H.-F.; Roberson, M.J.; Hunt, M.L.; Amrhein, C.; Giancarlo, L.C.; Flynn, G.W.; Yarmoff, J.A. Removal of contaminants from aqueous solution by reaction with iron surfaces. Langmuir 2000, 16, 2230–2236. [Google Scholar] [CrossRef]
- Farrell, J.; Wang, J.; O’Day, P.; Conklin, M. Electrochemical and spectroscopic study of arsenate removal from water using zero-valent iron media. Environ. Sci. Technol. 2001, 35, 2026–2032. [Google Scholar] [CrossRef] [PubMed]
- Noubactep, C.; Licha, T.; Scott, T.B.; Fall, M.; Sauter, M. Exploring the influence of operational parameters on the reactivity of elemental iron materials. J. Hazard. Mater. 2009, 172, 943–951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavine, B.K.; Auslander, G.; Ritter, J. Polarographic studies of zero valent iron as a reductant for remediation of nitroaromatics in the environment. Microchem. J. 2001, 70, 69–83. [Google Scholar] [CrossRef]
- Odziemkowski, M. Spectroscopic studies and reactions of corrosion products at surfaces and electrodes. In Spectroscopic Properties of Inorganic and Organometallic Compounds; The Royal Society of Chemistry: Cambridge, UK, 2009; Volume 40, pp. 385–450. [Google Scholar]
- Chen, L.; Jin, S.; Fallgren, P.H.; Liu, F.; Colberg, P.J.S. Passivation of zero-valent iron by denitrifying bacteria and the impact on trichloroethene reduction in groundwater. Water Sci. Technol. 2013, 67, 1254–1259. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Fan, D.; Tratnyek, P.G. Novel contaminant transformation pathways by abiotic reductants. Environ. Sci. Technol. Lett. 2014, 1, 432–436. [Google Scholar] [CrossRef]
- McKaveney, J.P.; Fassinger, W.P.; Stivers, D.A. Removal of heavy metals from water and brine using silicon alloys. Environ. Sci. Technol. 1972, 6, 1109–1113. [Google Scholar] [CrossRef]
- Pilling, N.B.; Bedworth, R.E. The oxidation of metals at high temperatures. J. Inst. Met. 1923, 29, 529–591. [Google Scholar]
- Caré, S.; Crane, R.; Calabro, P.S.; Ghauch, A.; Temgoua, E.; Noubactep, C. Modelling the permeability loss of metallic iron water filtration systems. Clean Soil Air Water 2013, 41, 275–282. [Google Scholar] [CrossRef]
- Domga, R.; Togue-Kamga, F.; Noubactep, C.; Tchatchueng, J.-B. Discussing porosity loss of Fe0 packed water filters at ground level. Chem. Eng. J. 2015, 263, 127–134. [Google Scholar] [CrossRef]
- Phukan, M. Characterizing the Fe0/sand system by the extent of dye discoloration. Freib. Online Geosci 2015, 42, 1–80. [Google Scholar]
- Phukan, M.; Noubactep, C.; Licha, T. Characterizing the ion-selective nature of Fe0-based filters using azo dyes. Chem. Eng. J. 2015, 259, 481–491. [Google Scholar] [CrossRef]
- Phukan, M.; Noubactep, C.; Licha, T. Characterizing the ion-selective nature of Fe0/H2O systems in batch experiments. J. Environ. Chem. Eng. 2016, 4, 65–72. [Google Scholar] [CrossRef]
- Gatcha-Bandjun, N.; Noubactep, C.; Loura-Mbenguela, B. Mitigation of contamination in effluents by metallic iron: The role of iron corrosion products. Environ. Technol. Innov. 2017, 8, 71–83. [Google Scholar] [CrossRef]
- Snowdon, R.C. The electrolytic reduction of nitrobenzene. J. Phys. Chem. 1911, 15, 797–844. [Google Scholar] [CrossRef]
- Lyons, R.E.; Smith, L.T. Die Reduktion von Nitroverbindungen mit Eisen und löslichen Chloriden. Berichte der Deutschen Chemischen Gesellschaft 1927, 60, 173–182. [Google Scholar] [CrossRef]
- Linnenbom, V.J. The Reaction between iron and water in the absence of oxygen. J. Electrochem. Soc. 1958, 105, 322–324. [Google Scholar] [CrossRef]
- Stratmann, M.; Müller, J. The mechanism of the oxygen reduction on rust-covered metal substrates. Corros. Sci. 1994, 36, 327–359. [Google Scholar] [CrossRef]
- Cole, I.S.; Marney, D. The science of pipe corrosion: A review of the literature on the corrosion of ferrous metals in soils. Corros. Sci. 2012, 56, 5–16. [Google Scholar] [CrossRef]
- Vollprecht, D.; Krois, L.-M.; Sedlazeck, K.P.; Müller, P.; Mischitz, R.; Olbrich, T.; Pomberger, R. Removal of critical metals from waste water by zero-valent iron. J. Clean. Prod. 2019, 208, 1409–1420. [Google Scholar] [CrossRef]
- Goetz, R.; MacDougall, B.; Graham, M.J. An AES and SIMS study of the influence of chloride on the passive oxide film on iron. Electrochim. Acta 1986, 1, 1299–1303. [Google Scholar] [CrossRef]
- Taylor, D.C. Atomistic modeling of corrosion events at the interface between a metal and its environment. Int. J. Corros. 2012, 2012, 204640. [Google Scholar] [CrossRef]
- Tepong-Tsindé, R.; Phukan, M.; Nassi, A.; Noubactep, C.; Ruppert, H. Validating the efficiency of the MB discoloration method for the characterization of Fe0/H2O systems using accelerated corrosion by chloride ions. Chem. Eng. J. 2015, 279, 353–362. [Google Scholar] [CrossRef]
- Taxén, C.; Letelier, M.V.; Lagos, G. Model for estimation of copper release to drinking water from copper pipes. Corros. Sci. 2012, 58, 267–277. [Google Scholar] [CrossRef]
- Fisher, W.W. Fluidized cathode cementation of copper. Hydrometallurgy 1986, 16, 55–67. [Google Scholar] [CrossRef]
- Noubactep, C.; Schöner, A.; Sauter, M. Significance of oxide-film in discussing the mechanism of contaminant removal by elemental iron materials. In Photo-Electrochemistry & Photo-Biology for the Sustainability; Union Press: Osaka, Japan, 2012; pp. 97–122, ISBN-10 4946428615; ISBN-13 978-4946428616. [Google Scholar]
- White, A.F.; Paterson, M.L. Reduction of aqueous transition metal species on the surface of Fe(II)-containing oxides. Geochim. Cosmochim. Acta 1996, 60, 3799–3814. [Google Scholar] [CrossRef]
- Béchamp, A. Bechamp reduction. Ann. J. Am. Chem. Phys. 1854, 42, 186, cited in Popat and Padhiyar (2013). [Google Scholar]
- Muspratt, S.; Kerl, B.; Beckmann, E.O.; Bunte, H.; Neumann, B.; Binz, A.H.; Hayduch, F.; Stohmann, F.K.A. Handbuch der Technischen Chemie; F. Vieweg & Sohn: Braunschweig, Germany, 1888; p. 942, cited in Lyons and Smith (1927). [Google Scholar]
- Noubactep, C. Investigations for the Passive In-Situ Immobilization of Uranium (VI) from Water. Ph.D. Thesis, TU Bergakademie Freiberg, Freiberg, Germany, 2003; p. 140. (In German). [Google Scholar]
- Noubactep, C.; Meinrath, G.; Dietrich, P.; Merkel, B. Mitigating uranium in ground water: Prospects and limitations. Environ. Sci. Technol. 2003, 37, 4304–4308. [Google Scholar] [CrossRef] [PubMed]
- Noubactep, C.; Meinrath, G.; Merkel, J.B. Investigating the mechanism of uranium removal by zerovalent iron materials. Environ. Chem. 2005, 2, 235–242. [Google Scholar] [CrossRef]
- Noubactep, C.; Schöner, A.; Meinrath, G. Mechanism of uranium (VI) fixation by elemental iron. J. Hazard. Mater. 2006, 132, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Burghardt, D.; Kassahun, A. Development of a reactive zone technology for simultaneous in situ immobilisation of radium and uranium. Environ. Geol. 2005, 49, 314–320. [Google Scholar] [CrossRef]
- Noubactep, C. Characterizing the reactivity of metallic iron upon methylene blue discoloration in Fe0/MnO2/H2O systems. J. Hazard. Mater. 2009, 168, 1613–1616. [Google Scholar] [CrossRef] [PubMed]
- Ghauch, A.; Abou Assi, H.; Bdeir, S. Aqueous removal of diclofenac by plated elemental iron: Bimetallic systems. J. Hazard. Mater. 2010, 182, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Noubactep, C.; Caré, S.; Btatkeu-K, B.D.; Nanseu-Njiki, C.P. Enhancing the sustainability of household Fe0/sand filters by using bimetallics and MnO2. Clean Soil Air Water 2012, 40, 100–109. [Google Scholar] [CrossRef]
- Btatkeu-K, B.D.; Olvera-Vargas, H.; Tchatchueng, J.B.; Noubactep, C.; Caré, S. Characterizing the impact of MnO2 on the efficiency of Fe0-based filtration systems. Chem. Eng. J. 2014, 250, 416–422. [Google Scholar] [CrossRef]
- Bafghi, M.S.; Zakeri, A.; Ghasemi, Z.; Adeli, M. Reductive dissolution of manganese ore in sulfuric acid in the presence of iron metal. Hydrometallurgy 2008, 90, 207–212. [Google Scholar] [CrossRef]
- Westerhoff, P.; James, J. Nitrate removal in zero-valent iron packed columns. Water Res. 2003, 37, 1818–1830. [Google Scholar] [CrossRef]
- Bi, E.; Devlin, J.F.; Huang, B. Effects of mixing granular iron with sand on the kinetics of trichloroethylene reduction. Groundw. Monit. Remediat. 2009, 29, 56–62. [Google Scholar] [CrossRef]
- Ulsamer, S. A Model to Characterize the Kinetics of Dechlorination of Tetrachloroethylene and Trichloroethylene by a Zero Valent Iron Permeable Reactive Barrier. Master’s Thesis, Worcester Polytechnic Institute, Worcester, MA, USA, 2011; 73p. [Google Scholar]
- Tepong-Tsindé, R.; Crane, R.; Noubactep, C.; Nassi, A.; Ruppert, H. Testing metallic iron filtration systems for decentralized water treatment at pilot scale. Water 2015, 7, 868–897. [Google Scholar] [CrossRef]
- O´Hannesin, S.F.; Gillham, R.W. Long-term performance of an in situ “iron wall” for remediation of VOCs. Groundwater 1998, 36, 164–170. [Google Scholar] [CrossRef]
- Erickson, A.J. Enhanced Sand Filtration for Storm Water Phosphorus Removal. Master’s Thesis, University of Minnesota, Minneapolis, MN, USA, 2005. [Google Scholar]
- Erickson, A.J.; Gulliver, J.S.; Weiss, P.T. Enhanced sand filtration for storm water phosphorus removal. J. Environ. Eng. ASCE 2007, 133, 485–497. [Google Scholar] [CrossRef]
- Erickson, A.J.; Gulliver, J.S.; Weiss, P.T. Capturing phosphates with iron enhanced sand filtration. Water Res. 2012, 46, 3032–3042. [Google Scholar] [CrossRef] [PubMed]
- Bradley, I.; Straub, A.; Maraccini, P.; Markazi, S.; Nguyen, T.H. Iron oxide amended biosand filters for virus removal. Water Res. 2011, 45, 4501–4510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazzari, L. General aspects of corrosion. In Encyclopedia of Hydrocarbons; Istituto Enciclopedia Italiana: Rome, Italy, 2008; Volume V, Chapter 9.1. [Google Scholar]
- Kosmulski, M. Compilation of PZC and IEP of sparingly soluble metal oxides and hydroxides from literature. Adv. Colloid Interface Sci. 2009, 152, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Kosmulski, M. Isoelectric points and points of zero charge of metal (hydr)oxides: 50 years after Parks’ review. Adv. Colloid Interface Sci. 2016, 238, 1–61. [Google Scholar] [CrossRef] [PubMed]
- Frost, R.L.; Xi, Y.; He, H. Synthesis, characterization of palygorskite supported zero-valent iron and its application for methylene blue adsorption. J. Colloid Interf. Sci. 2010, 341, 153–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arabi, S.; Sohrabi, M.R. Removal of methylene blue, a basic dye, from aqueous solutions using nano-zerovalent iron. Water Sci. Technol. 2014, 70, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Scherer, M.M.; Richter, S.; Valentine, R.L.; Alvarez, P.J.J. Chemistry and microbiology of permeable reactive barriers for in situ groundwater clean up. Crit. Rev. Environ. Sci. Technol. 2000, 30, 363–411. [Google Scholar] [CrossRef]
- Comba, S.; Di Molfetta, A.; Sethi, R. A Comparison between field applications of nano-, micro-, and millimetric zero-valent iron for the remediation of contaminated aquifers. Water Air Soil Pollut. 2011, 215, 595–607. [Google Scholar] [CrossRef]
- Phillips, D.H. Permeable reactive barriers: A sustainable technology for cleaning contaminated groundwater in developing countries. Desalination 2009, 248, 352–359. [Google Scholar] [CrossRef]
- Phillips, D.H.; Van Nooten, T.; Bastiaens, L.; Russell, M.I.; Dickson, K.; Plant, S.; Ahad, J.M.E.; Newton, T.; Elliot, T.; Kalin, R.M. Ten year performance evaluation of a field-scale zero-valent iron permeable reactive barrier installed to remediate trichloroethene contaminated groundwater. Environ. Sci. Technol. 2010, 44, 3861–3869. [Google Scholar] [CrossRef] [PubMed]
- Naidu, R.; Birke, V. Permeable Reactive Barrier: Sustainable Groundwater Remediation; CRC Press: Boca Raton, FL, USA, 2015; 333p, ISBN 978-1-4822-2447-4. [Google Scholar]
- Mueller, N.C.; Braun, J.; Bruns, J.; Cerník, M.; Rissing, P.; Rickerby, D.; Nowack, B. Application of nanoscale zero valent iron (NZVI) for groundwater remediation in Europe. Environ. Sci. Pollut. Res. 2011, 19, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Ruhl, A.S.; Ünal, N.; Jekel, M. Evaluation of two-component Fe(0) fixed bed filters with porous materials for reductive dechlorination. Chem. Eng. J. 2012, 209, 401–406. [Google Scholar] [CrossRef]
- Bartzas, G.; Komnitsas, K. Solid phase studies and geochemical modelling of low-cost permeable reactive barriers. J. Hazard. Mater. 2010, 183, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Benson, C.H. Evaluation of five strategies to limit the impact of fouling in permeable reactive barriers. J. Hazard. Mater. 2010, 181, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Firdous, R.; Devlin, J.F. Visualizations and optimization of iron–sand mixtures for permeable reactive barriers. Groundw. Monit. Remediat. 2015, 35, 75–84. [Google Scholar] [CrossRef]
- Miyajima, K.; Noubactep, C. Impact of Fe0 amendment on methylene blue discoloration by sand columns. Chem. Eng. J. 2013, 217, 310–319. [Google Scholar] [CrossRef]
- Lee, G.; Rho, S.; Jahng, D. Design considerations for groundwater remediation using reduced metals. Korean J. Chem. Eng. 2004, 21, 621–628. [Google Scholar] [CrossRef]
- Guo, X.; Yang, Z.; Dong, H.; Guan, X.; Ren, Q.; Lv, X.; Jin, X. Simple combination of oxidants with zero-valent-iron (ZVI) achieved very rapid and highly efficient removal of heavy metals from water. Water Res. 2016, 88, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Karottu Ansaf, K.V.; Ambika, S.; Nambi, I.M. Performance enhancement of zero valent iron based systems using depassivators: Optimization and kinetic mechanisms. Water Res. 2016, 102, 436–444. [Google Scholar] [CrossRef] [PubMed]
- McGuire, M.M.; Carlson, D.L.; Vikesland, P.J.; Kohn, T.; Grenier, A.C.; Langley, L.A.; Roberts, A.L.; Fairbrother, D.H. Applications of surface analysis in the environmental sciences: Dehalogenation of chlorocarbons with zero-valent iron and iron-containing mineral surfaces. Anal. Chim. Acta 2003, 496, 301–313. [Google Scholar] [CrossRef]
- Kim, H.; Yang, H.; Kim, J. Standardization of the reducing power of zero-valent iron using iodine. J. Environ. Sci. Health A 2014, 49, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ding, Y.; Wang, W.; Lei, H. A facile method for determining the Fe(0) content and reactivity of zero valent iron. Anal. Methods 2016, 8, 1239–1248. [Google Scholar] [CrossRef]
- Li, J.; Dou, X.; Qin, H.; Sun, Y.; Yin, D.; Guan, X. Characterization methods of zerovalent iron for water treatment and remediation. Water Res. 2019, 148, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Alamilla, J.L.; Espinosa-Medina, M.A.; Sosa, E. Modelling steel corrosion damage in soil environment. Corros. Sci. 2009, 51, 2628–2638. [Google Scholar] [CrossRef]
- Noubactep, C. Predicting the hydraulic conductivity of metallic iron filters: Modeling gone astray. Water 2016, 8, 162. [Google Scholar] [CrossRef]
- Heimann, S.; Ndé-Tchoupé, A.I.; Hu, R.; Licha, T.; Noubactep, C. Investigating the suitability of Fe0 packed-beds for water defluoridation. Chemosphere 2018, 209, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Brenner, S. Sequences and consequences. Philos. Trans. R. Soc. Lond. B 2010, 365, 207–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miehr, R.; Tratnyek, G.P.; Bandstra, Z.J.; Scherer, M.M.; Alowitz, J.M.; Bylaska, J.E. Diversity of contaminant reduction reactions by zerovalent iron: Role of the reductate. Environ. Sci. Technol. 2004, 38, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Noubactep, C.; Meinrath, G.; Dietrich, P.; Sauter, M.; Merkel, B. Testing the suitability of zerovalent iron materials for reactive Walls. Environ. Chem. 2005, 2, 71–76. [Google Scholar] [CrossRef]
- Mwakabona, H.T.; Ndé-Tchoupé, A.I.; Njau, K.N.; Noubactep, C.; Wydra, K.D. Metallic iron for safe drinking water provision: Considering a lost knowledge. Water Res. 2017, 117, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Ndé-Tchoupé, A.I.; Nanseu-Njiki, C.P.; Hu, R.; Nassi, A.; Noubactep, C.; Licha, T. Characterizing the reactivity of metallic iron for water defluoridation in batch studies. Chemosphere 2018, in press. [Google Scholar]
- Hu, R.; Noubactep, C. Iron corrosion: Scientific heritage in jeopardy. Sustainability 2018, 10, 4138. [Google Scholar] [CrossRef]
- Btatkeu-K, B.D.; Miyajima, K.; Noubactep, C.; Caré, S. Testing the suitability of metallic iron for environmental remediation: Discoloration of methylene blue in column studies. Chem. Eng. J. 2013, 215–216, 959–968. [Google Scholar] [CrossRef]
- Banerji, T.; Chaudhari, S. A cost-effective technology for arsenic removal: Case study of zerovalent iron-based IIT Bombay arsenic filter in West Bengal. In Water and Sanitation in the New Millennium; Nath, K., Sharma, V., Eds.; Springer: New Delhi, India, 2017. [Google Scholar]
- Hildebrant, B. Characterizing the reactivity of commercial steel wool for water treatment. Freib. Online Geosci. 2018, 53, 1–60. [Google Scholar]
- Lauderdale, R.A.; Emmons, A.H. A method for decontaminating small volumes of radioactive water. J. Am. Water Works Assoc. 1951, 43, 327–331. [Google Scholar] [CrossRef]
- Tseng, C.L.; Yang, M.H.; Lin, C.C. Rapid determination of cobalt-60 in sea water with steel wool adsorption. J. Radioanal. Nucl. Chem. Lett. 1984, 85, 253–260. [Google Scholar] [CrossRef]
- Oldright, G.L.; Keyes, H.E.; Miller, V.; Sloan, W.A. Precipitation of Lead and Copper from Solution on Sponge Iron. 1928. Available online: http://digital.library.unt.edu/ark:/67531/metadc12459 (accessed on 27 May 2018).
- Lung, T.N. The history of copper cementation on iron—The world's first hydrometallurgical process from medieval China. Hydrometallurgy 1986, 17, 113–129. [Google Scholar] [CrossRef]
- Habashi, F. A short history of hydrometallurgy. Hydrometallurgy 2005, 79, 15–22. [Google Scholar] [CrossRef]
- Baker, M.N. Sketch of the history of water treatment. J. Am. Water Works Assoc. 1934, 26, 902–938. [Google Scholar] [CrossRef]
- Devonshire, E. The purification of water by means of metallic iron. J. Frankl. Inst. 1890, 129, 449–461. [Google Scholar] [CrossRef]
- Bischof, G. On putrescent organic matter in potable water. Proc. R. Soc. London 1877, 26, 258–261. [Google Scholar] [CrossRef]
- Anderson, W. The purification of water by means of iron on the large scale. J. Soc. Arts 1884, 32, 963–964. [Google Scholar]
- Anderson, W. The purification of water by means of iron on the large scale. Minutes Proc. Inst. Civ. Eng. 1885, 81, 279–284. [Google Scholar]
- Werner, J. Amination by Reduction. Ind. Eng. Chem. 1951, 43, 1917–1919. [Google Scholar] [CrossRef]
- Werner, J. Amination by Reduction. Ind. Eng. Chem. 1952, 44, 1980–1982. [Google Scholar] [CrossRef]
- Werner, J. Amination by Reduction. Ind. Eng. Chem. 1953, 45, 1912–1914. [Google Scholar] [CrossRef]
- Werner, J. Amination by Reduction. Ind. Eng. Chem. 1954, 46, 1800–1801. [Google Scholar] [CrossRef]
- Werner, J. Amination by Reduction. Ind. Eng. Chem. 1956, 48, 1563–1565. [Google Scholar] [CrossRef]
- Werner, J. Amination by Reduction. Ind. Eng. Chem. 1959, 51, 1065–1066. [Google Scholar] [CrossRef]
- Werner, J. Amination by Reduction. Unit Processes Review. Ind. Eng. Chem. 1961, 53, 77–78. [Google Scholar] [CrossRef]
- Case, O.P.; Jones, R.B.L. Connecticut Research Commission. Treatment of Brass Mill Effluents; RSA-68-34; Anaconda American Brass Co.: Waterbury, CT, USA, 1969. [Google Scholar]
- Harza Environmental Services. Fundamental Aspects of Selenium Removal by Harza Process; Technical Report Prepared under Contract for the Federal-State San Joaquin Valley Drainage Program; Harza Environmental Services: Sacramento, CA, USA, 1989. [Google Scholar]
- Khan, A.H.; Rasul, S.B.; Munir, A.K.M.; Habibuddowla, M.; Alauddin, M.; Newaz, S.S.; Hussam, A. Appraisal of a simple arsenic removal method for groundwater of bangladesh. J. Environ. Sci. Health A 2000, 35, 1021–1041. [Google Scholar] [CrossRef]
- Leupin, O.X.; Hug, S.J.; Badruzzaman, A.B.M. Arsenic removal from Bangladesh tube well water with filter columns containing zerovalent iron filings and sand. Environ. Sci. Technol. 2005, 39, 8032–8037. [Google Scholar] [CrossRef] [PubMed]
- Hussam, A. Contending with a Development Disaster: SONO Filters Remove Arsenic from Well Water in Bangladesh. Innovations 2009, 4, 89–102. [Google Scholar] [CrossRef]
- Neumann, A.; Kaegi, R.; Voegelin, A.; Hussam, A.; Munir, A.K.M.; Hug, S.J. Arsenic removal with composite iron matrix filters in Bangladesh: A field and laboratory study. Environ. Sci. Technol. 2013, 47, 4544–4554. [Google Scholar] [CrossRef] [PubMed]
- Marwa, J.; Lufingo, M.; Noubactep, C.; Machunda, R. Defeating fluorosis in the East African Rift Valley: Transforming the Kilimanjaro into a rainwater harvesting park. Sustainability 2018, 10, 4194. [Google Scholar] [CrossRef]
- Tratnyek, P.G.; Salter, A.J.; Nurmi, J.T.; Sarathy, V. Environmental applications of zerovalent metals: Iron vs. Zinc. ACS Symp. Ser. 2010, 1045, 165–178. [Google Scholar]
- DeVor, R.; Carvalho-Knighton, K.; Aitken, B.; Maloney, P.; Holland, E.; Talalaj, L.; Elsheimer, S.; Clausen, C.A.; Geiger, C.L. Mechanism of the degradation of individual PCB congeners using mechanically alloyed Mg/Pd in methanol. Chemosphere 2009, 76, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Al-Abed, S.R.; Dionysiou, D.D. Enhanced corrosion-based Pd/Mg bimetallic systems for dechlorination of PCBs. Environ. Sci. Technol. 2007, 41, 3722–3727. [Google Scholar] [CrossRef] [PubMed]
- Bigg, T.; Judd, S.J. Zero-valent iron for water treatment. Environ. Technol. 2000, 21, 661–670. [Google Scholar] [CrossRef]
- Scherer, M.M.; Balko, B.A.; Tratnyek, P.G. The Role of Oxides in Reduction Reactions at the Metal-Water Interface. ACS Symp. Ser. 1998, 715, 301–322. [Google Scholar]
- Roberts, A.L.; Totten, L.A.; Arnold, W.A.; Burris, D.R.; Campbell, T.J. Reductive elimination of chlorinated ethylenes by zero-valent metals. Environ. Sci. Technol. 1996, 30, 2654–2659. [Google Scholar] [CrossRef]
- Khudenko, B.M. Mechanism and kinetics of cementation processes. Water Sci. Technol. 1985, 17, 719–731. [Google Scholar] [CrossRef]
- Gould, J.P.; Escovar, I.B.; Khudenko, B.M. Examination of the zinc cementation on cadmium in aqueous solutions. Water Sci. Technol. 1987, 19, 333–344. [Google Scholar]
- Khudenko, B.M. Mathematical models of cementaion process. J. Environ. Eng. 1987, 113, 681–702. [Google Scholar]

| Location | Processes | Comments |
|---|---|---|
| Bulk Fe0 | dealloying, electron transport | + + + |
| Fe0/H2O interface | Fe dissolution, complexation, precipitation | + + |
| Fe0/Oxides | oxide precipitation | + |
| Oxide scale | migration of species, oxide recrystallization | + |
| Oxides/H2O | oxide dissolution/precipitation, Fe complexation | + |
| H2O | mass transfer (advection and diffusion) | + + + |
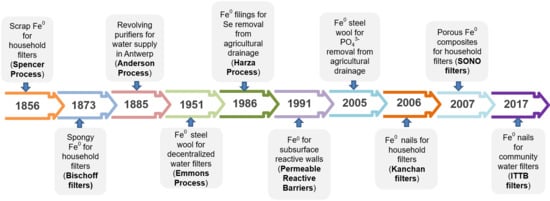
| Year | Event |
|---|---|
| <1850 | The cementation reaction is used for winning metals from ores [155,156,157] |
| <1850 | Iron shavings are used to treat drinking water [147,158,159] |
| 1854 | Béchamp synthesized of aniline from nitrobenzene and Fe0 (iron and organic acid) [97] |
| 1865 | Bekelov suggested that all cementation reactions are electrochemical in nature [155,157] |
| 1873 | Bischoff established the spongy iron filter for household [147,159,160] |
| 1881 | Spongy iron filters are tested at large scale in Antwerp (Belgium) [147,158,160] |
| 1883 | Spongy iron filters secured water supply in Antwerp (Belgium) [147,160,161,162] |
| 1885 | Revolving purifiers are installed in Antwerp (Belgium) [147,161,162] |
| 1888 | Muspratt rationalized the successful use of HCl in the Béchamp reduction [98] |
| 1914 | Holt used scrap iron instead of coarse scrap iron for the cementation of PbII [98,155,157] |
| 1923 | Lueg showed that aniline and other substances inhibit iron corrosion [98,155,157] |
| 1928 | Oldright and co-workers showed that only thin Fe0 beds are long-term sustainable [155] |
| 1928 | Knowlton reported that the rate of iron corrosion is higher in NaCl solutions [60] |
| 1928 | Knowlton reported that the used Fe0 type determines the extent of reduction [60] |
| 1951 | Lauderdale and Emmons used steel wool to remove radioactive species from water [153] |
| 1951–1961 | Werner published almost yearly review articles on “Amination by Reduction” [163,164,165,166,167,168,169] |
| 1951–1961 | The Béchamp reduction is extended to other groups of compounds [163,164,165,166,167,168,169] |
| 1969 | Case and Jones treated CrVI- and CuII-containing brass mill effluents with scrap iron [170] |
| 1984 | Tseng et al. used steel wool to concentrate 60Co from nuclear effluent [154] |
| 1986 | Harza Environmental Services patented Se(VI) removal in Fe0 beds [171] |
| 1991 | Khudenko established the cementation based reductive degradation of organics [67] |
| 1990 | Reynolds and co-authors observed dechlorination of RCl in Fe0-based vessels [11] |
| 1994 | Fe0 is established as an efficient material for subsurface reactive walls [1,10,113] |
| Year | Journal | Type | Section | Reference |
|---|---|---|---|---|
| 2015 | Water Research | Review | Introduction | [16] |
| 2014 | Environmental Science & Technology Letters | Research | Discussion | [75] |
| 2010 | ACS Symposium Series | Review | Introduction | [177] |
| 2009 | Chemosphere | Research | Discussion | [178] |
| 2007 | Environmental Science & Technology | Research | Introduction | [179] |
| 2004 | Environmental Science & Technology | Research | Discussion | [145] |
| 2000 | Environmental Technology | Review | Introduction/discussion | [180] |
| 1998 | ACS Symposium Series | Review | Discussion | [181] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, R.; Cui, X.; Gwenzi, W.; Wu, S.; Noubactep, C. Fe0/H2O Systems for Environmental Remediation: The Scientific History and Future Research Directions. Water 2018, 10, 1739. https://doi.org/10.3390/w10121739
Hu R, Cui X, Gwenzi W, Wu S, Noubactep C. Fe0/H2O Systems for Environmental Remediation: The Scientific History and Future Research Directions. Water. 2018; 10(12):1739. https://doi.org/10.3390/w10121739
Chicago/Turabian StyleHu, Rui, Xuesong Cui, Willis Gwenzi, Shuanghong Wu, and Chicgoua Noubactep. 2018. "Fe0/H2O Systems for Environmental Remediation: The Scientific History and Future Research Directions" Water 10, no. 12: 1739. https://doi.org/10.3390/w10121739
APA StyleHu, R., Cui, X., Gwenzi, W., Wu, S., & Noubactep, C. (2018). Fe0/H2O Systems for Environmental Remediation: The Scientific History and Future Research Directions. Water, 10(12), 1739. https://doi.org/10.3390/w10121739