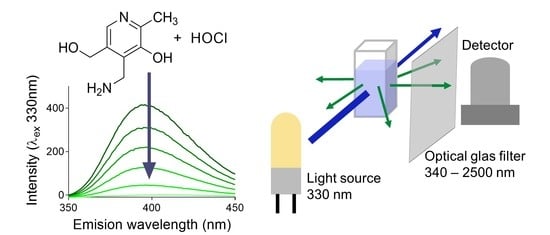
Quantification of Hypochlorite in Water Using the Nutritional Food Additive Pyridoxamine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. UV-Vis and Fluorescence Spectroscopy
2.3. Method Development
2.3.1. Linearity
2.3.2. pH Effects
2.3.3. Stability
2.3.4. Measuring Range
2.3.5. Combined Chlorine
2.3.6. Method Application in Field Samples
3. Results
3.1. UV-Vis Absorption and Fluorescence Spectroscopy
3.1.1. Effect of pH
3.2. Method Optimization and Validation
3.2.1. Quantification Range
3.2.2. Determination of LOD and LOQ
3.3. Quantification of Chloramines
3.4. Quantification of Free Chlorine in Different Water Matrices
3.4.1. Membrane Clean-in-Place Monitoring
3.4.2. Public Swimming Pool Chlorine Concentration Verification
3.4.3. Chlorination of Drinking Water
3.5. Construction of a Free Chlorine Meter
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bergendahl, J.A.; Stevens, L. Oxidation reduction potential as a measure of disinfection effectiveness for chlorination of wastewater. Environ. Prog. 2005, 24, 214–222. [Google Scholar] [CrossRef]
- Cheema, W.A.; Manasfi, T.; Kaarsholm, K.M.; Andersen, H.R.; Boudenne, J.-L. Effect of medium-pressure UV-lamp treatment on disinfection by-products in chlorinated seawater swimming pool waters. Sci. Total Environ. 2017, 599–600, 910–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbas, S.; Hashmi, I.; Rehman, M.S.U.; Qazi, I.A.; Awan, M.A.; Nasir, H. Monitoring of chlorination disinfection by-products and their associated health risks in drinking water of Pakistan. J. Water Health 2015, 13, 270–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tchobanoglous, G.; Burton, F.L.; Stensel, H.D. Wastewater Engineering, Treatment and Reuse, 4th ed.; Metcalf & Eddy Inc.: Boston, MA, USA; McGraw Hill: New York, NY, USA, 2003. [Google Scholar]
- Harp, D.L. Current Technology of Chlorine Analysis for Water and Wastewater; Hach Company: Loveland, CO, USA, 2002. [Google Scholar]
- Ibanez, J.G.; Balderas-Hernandez, P.; Garcia-Pintor, E.; Espinosa-Marvan, L.; Ruiz-Martin, R.M.; Arrieta, J.J.; Ramirez-Silva, M.T.; Casillas, N. Microscale environmental chemistry. Part 8: Properties, preparation and characterization of chloramines. Chem. Educ. 2006, 11, 402–405. [Google Scholar]
- Kaydos-Daniels, S.C.; Beach, M.J.; Shwe, T.; Magri, J.; Bixler, D. Health effects associated with indoor swimming pools: A suspected toxic chloramine exposure. Public Health 2008, 122, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Boorman, G.A. Drinking water disinfection byproducts: Review and approach to toxicity evaluation. Environ. Health Perspect. 1999, 107, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Malkov, V.B.; Zachman, B.; Gordon, S. Comparison of On-line Chlorine Analysis Methods and Instrumentation Built on Amperometric and Colorimetric Technologies. In Proceedings of the AWWA Water Quality Conference and Exposition, Seattle, WA, USA, 15–19 November 2009; pp. 1–22. [Google Scholar]
- Pinkernell, U. Methods for the photometric determination of reactive bromine and chlorine species with ABTS. Water Res. 2000, 34, 4343–4350. [Google Scholar] [CrossRef]
- Moberg, L.; Karlberg, B. An improved N,N′-diethyl-p-phenylenediamine (DPD) method for the determination of free chlorine based on multiple wavelength detection. Anal. Chim. Acta 2000, 407, 127–133. [Google Scholar] [CrossRef]
- Voziyan, P.A.; Hudson, B. Pyridoxamine as a multifunctional pharmaceutical: Targeting pathogenic glycation and oxidative damage. Cell. Mol. Life Sci. 2005, 62, 1671–1681. [Google Scholar] [CrossRef] [PubMed]
- Daumer, K.M.; Khan, A.U.; Steinbeck, M.J. Chlorination of Pyridinium Compounds. J. Biol. Chem. 2000, 275, 34681–34692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- APHA; AWWA; WEF. Standard Methods for the Examination of Water and Waste Water, 22nd ed.; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Bueno, C.; Encinas, M.V. Photophysical and Photochemical Studies of Pyridoxamine. Helvetica Chim. Acta 2003, 86, 3363–3375. [Google Scholar] [CrossRef]
- Skoog, D.A.; West, D.M.; Holler, F.J. Fundamentals of Analytical Chemistry, 7th ed.; Harcourt, Inc.: San Diego, CA, USA, 1996. [Google Scholar]
- Awtrey, A.D.; Connick, R.E. The Absorption Spectra of I2, I3-, I-, IO3-, S4O6= and S2O3=. Heat of the Reaction I3- = I2 + I-. J. Am. Chem. Soc. 1951, 73, 1842–1843. [Google Scholar] [CrossRef]
- World Health Organisation. Guidelines for Safe Recreational Water Environments. In Swimming Pools and Similar Environments; WHO Press: Geneva, Switzerland, 2006; Volume 2. [Google Scholar]
- Pool Water Treatment Advisory Group. Swimming Pool Water: Treatment and Quality Standards for Pools and Spas, 2nd ed.; Countrywide Publications: Suffolk, UK, 2009. [Google Scholar]
- Hambly, A.C.; Henderson, R.K.; Baker, A.; Stuetz, R.M.; Khan, S.J. Cross-connection detection in Australian dual reticulation systems by monitoring inherent fluorescent organic matter. Environ. Technol. Rev. 2012, 1, 67–80. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaarsholm, K.M.S.; Kokkoli, A.; Keliri, E.; Mines, P.D.; Antoniou, M.G.; Jakobsen, M.H.; Andersen, H.R. Quantification of Hypochlorite in Water Using the Nutritional Food Additive Pyridoxamine. Water 2021, 13, 3616. https://doi.org/10.3390/w13243616
Kaarsholm KMS, Kokkoli A, Keliri E, Mines PD, Antoniou MG, Jakobsen MH, Andersen HR. Quantification of Hypochlorite in Water Using the Nutritional Food Additive Pyridoxamine. Water. 2021; 13(24):3616. https://doi.org/10.3390/w13243616
Chicago/Turabian StyleKaarsholm, Kamilla M. S., Argyro Kokkoli, Eleni Keliri, Paul D. Mines, Maria G. Antoniou, Mogens Havsteen Jakobsen, and Henrik R. Andersen. 2021. "Quantification of Hypochlorite in Water Using the Nutritional Food Additive Pyridoxamine" Water 13, no. 24: 3616. https://doi.org/10.3390/w13243616
APA StyleKaarsholm, K. M. S., Kokkoli, A., Keliri, E., Mines, P. D., Antoniou, M. G., Jakobsen, M. H., & Andersen, H. R. (2021). Quantification of Hypochlorite in Water Using the Nutritional Food Additive Pyridoxamine. Water, 13(24), 3616. https://doi.org/10.3390/w13243616