Predicting Potential Spawning Habitat by Ensemble Species Distribution Models: The Case Study of European Anchovy (Engraulis encrasicolus) in the Strait of Sicily
Abstract
:1. Introduction
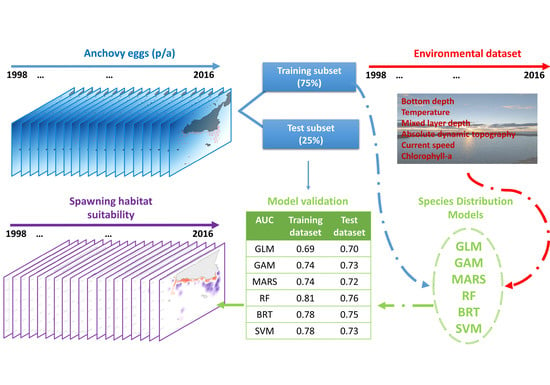
2. Materials and Methods
2.1. The Biological Dataset
2.2. The Environmental Dataset
2.3. Species Distribution Modelling
2.3.1. Regression-Based Models
2.3.2. Machine-Learning Models
2.3.3. Model Validation and Mapping
3. Results
3.1. Regression-Based Outputs
3.2. Machine-Learning Outputs
3.3. Evaluation of Model Results
3.4. Displaying the Anchovy Spawning Habitat
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Franklin:, J. Mapping Species Distributions: Spatial Inference and Prediction; Cambride University Press: Cambride, UK, 2010. [Google Scholar]
- Peterson, A.T.; Soberon, J.; Pearson, R.G.; Anderson, R.P.; Martinez-Meyer, E.; Nakamura, M.; Araujo, M.B. Ecological Niches and Geographic Distributions; Princeton University Press: Princeton, NJ, USA, 2011. [Google Scholar]
- Guisan, A.; Thuiller, W. Predicting Species Distribution: Offering more than simple habitat models. Ecol. Lett. 2005, 8, 993–1009. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W.; Zimmermann, N.E. Habitat Suitability and Distribution Models with Applications in R; Cambride University Press: Cambride, UK, 2017. [Google Scholar]
- Guisan, A.; Zimmermann, N.E. Predictive habitat distribution models in ecology. Ecol. Modell. 2000, 135, 147–186. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evolut. System. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Tzanatos, E.; Raitsos, D.E.; Triantafyllou, G.; Somarakis, S.; Tsonis, A.A. Indications of a climate effect on Mediterranean fisheries. Clim. Change 2014, 122, 41–54. [Google Scholar] [CrossRef]
- Corrales, X.; Coll, M.; Ofir, E.; Heymans, J.J.; Steenbeek, J.; Goren, M.; Edelist, D.; Gal, G. Future scenarios of marine resources and ecosystem conditions in the Eastern Mediterranean under the impacts of fishing, alien species and sea warming. Sci. Rep. 2018, 8, 14284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patti, B.; Torri, M.; Cuttitta, A. General surface circulation controls the interannual fluctuations of anchovy stock biomass in the Central Mediterranean Sea. Sci. Rep. 2020, 10, 1554. [Google Scholar] [CrossRef] [PubMed]
- Schickele, A.; Goberville, E.; Leroy, B.; Beaugrand, G.; Hattab, T.; Francour, P.; Raybaud, V. European small pelagic fish distribution under global change scenarios. Fish Fish. 2021, 22, 212–225. [Google Scholar] [CrossRef]
- Patti, B.; Guisande, C.; Bonanno, A.; Basilone, G.; Cuttitta, A.; Mazzola, S. Role of physical forcings and nutrient availability on the control of satellite-based chlorophyll-a concentration in the coastal upwelling area of the Sicilian Channel. Sci. Mar. 2010, 74, 577–588. [Google Scholar] [CrossRef] [Green Version]
- Linnæus, C. Systema naturæ per regna tria naturæ, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Tomus I. Editio Decima Reformata 1758, 1–4, 1–824. [Google Scholar]
- Cuttitta, A.; Quinci, E.M.; Patti, B.; Bonomo, S.; Bonanno, A.; Musco, M.; Torri, M.; Placenti, F.; Basilone, B.; Genovese, S.; et al. Different key roles of mesoscale oceanographic structures and ocean bathymetry in shaping larval fish distribution pattern: A case study in Sicilian waters in summer 2009. J. Sea Res. 2016, 115, 6–17. [Google Scholar] [CrossRef]
- Haii, S.J. The Effects of Fishing on Marine Ecosystems and Communities; Blackwell Sciences: Oxford, UK, 1999; 274p. [Google Scholar]
- Duarte, O. Trophic role of small pelagic fishes in a tropical upwelling ecosystem. Ecol. Modell. 2004, 172, 323–338. [Google Scholar] [CrossRef]
- Freon, P.; Cury, P.; Shannon, L.; Roy, C. Sustainable exploitation of small pelagic fish stocks challenged by environmental and ecosystem changes: A review. Bull. Mater. Sci. 2005, 76, 385–462. [Google Scholar]
- Alheit, J.; Roy, C.; Kifani, S. Decadal-scale variability in populations. In Climate Change and Small Pelagic Fish; Checkley, D., Alheit, J., Oozeki, Y., Roy, C., Eds.; Cambridge University Press: Cambridge, UK, 2009; pp. 67–87. [Google Scholar]
- Basilone, G.; Guisande, C.; Patti, B.; Mazzola, S.; Cuttitta, A.; Bonanno, A.; Vergara, A.R.; Maneiro, I. Effect of habitat conditions on reproduction of the European anchovy ((Engraulis encrasicolus) in the Strait of Sicily. Fish. Oceanogr. 2006, 15, 271–280. [Google Scholar] [CrossRef]
- Olivar, M.P.; Salat, J.; Palomera, I. A comparative study of spatial distribution patterns of the early stages of anchovy and pilchard in the NW Mediterranean Sea. Mar. Ecol. Prog. Ser. 2001, 217, 111–120. [Google Scholar] [CrossRef] [Green Version]
- Guraslan, C.; Fac, B.B.; Oguz, T. Modeling the impact of climate variability on Black Sea anchovy recruitment and production. Fish. Oceanog. 2014, 23, 436–457. [Google Scholar] [CrossRef]
- Ospina-Alvarez, A.; Catalan, I.A.; Bernal, M.; Roos, D.; Palomera, I. From egg production to recruits: Connectivity and inter-annual variability in the recruitment patterns of European anchovy in the northwestern Mediterranean. Prog. Oceanogr. 2015, 138, 431–447. [Google Scholar] [CrossRef] [Green Version]
- Politikos, D.; Somarakis, S.; Tsiaras, K.P.; Giannoulaki, M.; Petihakis, G.; Machias, A.; Triantafyllou, G. Simulating anchovy’s full life cycle in the northern Aegean Sea (eastern Mediterranean): A coupled hydro-biogeochemical-IBM model. Prog. Oceanogr. 2015, 138, 399–416. [Google Scholar] [CrossRef]
- Fernandez-Corredor, E.; Albo-Puigserver, M.; Pennino, M.G.; Bellido, J.M.; Coll, M. Influence of environmental factors on different life stages of European anchovy ((Engraulis encrasicolus) and European sardine (Sardina pilchardus) from the Mediterranean Sea: A literature review. Reg. Stud. Mar. Sci. 2021, 41, 101606. [Google Scholar] [CrossRef]
- Palomera, I.; Sabates, A. Co-occurrence of (Engraulis encrasicolus and Sardinella aurita eggs and larvae in the western Mediterranean. Sci. Mar. 1990, 54, 51–67. [Google Scholar]
- Palomera, I. Spawning of anchovy (Engraulis encrasicolus in the North Western Mediterranean relative to hydrographic features in the region. Mar. Ecol. Prog. Ser. 1992, 79, 215–223. [Google Scholar] [CrossRef]
- Palomera, I.; Olivar, M.P. Nearshore ichthyoplankton off the Costa Brava (Northwest Mediterranean). Bol. Inst. Esp. Oceanogr. 1996, 22, 71–76. [Google Scholar]
- Ibaibarriaga, L.; Irigoien, X.; Santos, M.; Motos, L.; Fives, J.M.; Franco, C.; Lago De Lanzos, A.; Acevedo, S.M.; Bernal, M.; Bez, N.; et al. Egg and larvae distribution of seven fish species in the northeast Atlantic waters. Fish. Oceanogr. 2007, 16, 284–293. [Google Scholar] [CrossRef]
- Somarakis, S.; Nikolioudakis, N. Oceanographic habitat, growth and mortality of larval anchovy ((Engraulis encrasicolus) in the northern Aegean Sea (eastern Mediterranean). Mar. Biol. 2007, 152, 1143–1158. [Google Scholar] [CrossRef]
- Basilone, G.; Bonanno, A.; Patti, B.; Mazzola, S.; Barra, M.; Cuttitta, A.; McBrige, R. Spawning site selection by European anchovy ((Engraulis encrasicolus) in relation to oceanographic conditions in the Strait of Sicily. Fish. Oceanogr. 2013, 22, 309–323. [Google Scholar] [CrossRef]
- Basilone, G.; Ferreri, R.; Barra, M.; Bonanno, A.; Pulizzi, M.; Gargano, A.; Fontana, I.; Giacalone, G.; Rumolo, P.; Mazzola, S.; et al. Spawning ecology of the European anchovy ((Engraulis encrasicolus) in the Strait of Sicily: Linking variations of zooplankton prey, fish density, growth, and reproduction in an upwelling system. Progr. Oceanogr. 2020, 184, 102330. [Google Scholar] [CrossRef]
- Giannoulaki, M.; Iglesias, M.; Tugores, M.P.; Bonanno, A.; Patti, B.; De Felice, A.; Leonori, I.; Bigot, L.; Ticina, V.; Pyrounaki, M.M.; et al. Characterizing the potential habitat of European anchovy (Engraulis encrasicolus in the Mediterranean Sea, at different life stages. Fish. Oceanogr. 2013, 22, 69–89. [Google Scholar] [CrossRef]
- Bonanno, A.; Giannoulaki, M.; Barra, M.; Basilone, G.; Machias, A.; Genovese, S.; Goncharov, S.; Popov, S.; Rumolo, P.; Bitetto, M.D.; et al. Habitat selection response of small pelagic fish in different environments. Two examples from the oligotrophic Mediterranean Sea. PLoS ONE 2014, 9, e101498. [Google Scholar] [CrossRef]
- Bonanno, A.; Barra, M.; Basilone, G.; Genovese, S.; Rumolo, P.; Goncharov, S.; Popov, S.; Buongiorno Nardelli, B.; Iudicone, D.; Procaccini, G.; et al. Environmental processes driving anchovy and sardine distribution in a highly variable environment: The role of the coastal structure and riverine input. Fish. Oceanogr. 2016, 25, 471–490. [Google Scholar] [CrossRef]
- García Lafuente, J.; García, A.; Mazzola, S.; Qyuintanilla, L.; Delgado, J.; Cuttitta, A.; Patti, B. Hydrographic phenomena influencing early life stages of the Sicilian Channel anchovy. Fish. Oceanogr. 2002, 11, 31–44. [Google Scholar] [CrossRef] [Green Version]
- Patti, B.; Bonanno, A.; Basilone, G.; Goncharov, S.; Mazzola, S.; Buscaino, G.; Cuttitta, A.; García Lafuente, J.; García, A.; Palumbo, V.; et al. Interannual fluctuations in acoustic biomass estimates and in landings of small pelagic fish populations in relation to hydrology in the strait of Sicily. Chem. Ecol. 2004, 20, 365–375. [Google Scholar] [CrossRef]
- Bonanno, A.; Zgosi, S.; Cuttitta, A.; El Turki, A.; Di Nieri, A.; Ghmati, H.; Basilone, G.; Aronica, S.; Hamza, M.; Barra, M.; et al. Influence of environmental variability on anchovy early life stages ((Engraulis encrasicolus) in two different areas of the Central Mediterranean Sea. Hydrobiologia 2013, 1, 273–287. [Google Scholar] [CrossRef]
- Falcini, F.; Palatella, L.; Cuttitta, A.; Buongiorno Nardelli, B.; Lacorata, G.; Lanotte, A.S.; Patti, B.; Santoleri, R. The role of hydrodynamic processes on anchovy eggs and larvae distribution in the Sicily channel (Mediterranean Sea): A case study for the 2004 data set. PLoS ONE 2015, 10, 0123213. [Google Scholar] [CrossRef] [PubMed]
- Lemursiaux, P.F.J. Estimation and study of mesoscale variability in the Strait of Sicily. Dynam. Atmos. Oceans 1999, 29, 255–303. [Google Scholar]
- Robinson, A.R.; Sellschopp, J.; Warn-Varnas, A.; Leslie, W.G.; Lozano, C.J.; Haley, P.J.; Anderson, L.A.; Lermusiaux, P.F.J. The Atlantic Ionian Stream. J. Mar. Syst. 1999, 20, 129–156. [Google Scholar] [CrossRef]
- Lemursiaux, P.F.J.; Robinson, A.R. Features of dominant mesoscale variability, circulation patterns and dynamics in the Strait of Sicily. Deep-Sea Res. I 2001, 48, 1953–1997. [Google Scholar]
- Béranger, K.; Mortier, L.; Gasparini, G.P.; Gervasio, L.; Astraldi, M.; Crepon, M. The dynamics of the Strait of Sicily: A comprehensive study from observations and models. Deep-Sea Res. 2004, 51, 411–440. [Google Scholar]
- Gasparini, G.P.; Smeed, D.A.; Alderson, S.; Sparnocchia, S.; Vetrano, A.; Mazzola, S. Tidal and subtidal currents in the Strait of Sicily. J. Geophys. Res. 2004, 109, C02011. [Google Scholar] [CrossRef]
- Cuttitta, A.; Arigò, A.; Basilone, G.; Bonanno, A.; Buscaino, G.; Rollandi, L.; Garcìa Lafuente, J.; Garcìa, A.; Mazzola, S.; Patti, B. Mesopelagic fish larvae species in the Strait of Sicily and their relationships to main oceanographic events. Hydrobiologia 2004, 527, 177–182. [Google Scholar] [CrossRef]
- Cuttitta, A.; Torri, M.; Zarrad, R.; Zgozi, S.; Jarboui, O.; Quinci, E.M.; Hamza, M.; Abdulfatah, E.; Haddoud, D.; El Turki, A.; et al. Linking surface hydrodynamics to planktonic ecosystem: The case study of the ichthyoplanktonic assemblages in the Central Mediterranean Sea. Hydrobiologia 2018, 821, 191–214. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H. Do they? How do they? Why do they differ? On finding reasons for differing performances of species distribution models. Ecography 2009, 32, 66–77. [Google Scholar] [CrossRef]
- Araújo, M.B.; New, M. Ensemble forecasting of species distributions. Trends Ecol. Evolut. 2007, 22, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Marmion, M.; Parviainen, M.; Luoto, M.; Heikkinen, R.K.; Thuiller, W. Evaluation of consensus methods in predictive species distribution modelling. Divers. Distrib. 2009, 15, 59–69. [Google Scholar] [CrossRef]
- Thuiller, W.; Lafourcade, B.; Engler, R.; Araújo, M.B. BIOMOD—A platform for ensemble forecasting of species distributions. Ecography 2009, 32, 369–373. [Google Scholar] [CrossRef]
- Brankart, J.M. Technical Report; University of Liège: Liège, Belgium, 1994; p. 5. [Google Scholar]
- Palomera, I. Vertical distribution of eggs and larvae of (Engraulis encrasicolus in stratified waters of the Western Mediterranean. Mar. Biol. 1991, 111, 37–44. [Google Scholar] [CrossRef]
- Mazzola, S.; Garcia, A.; Garcia-Lafuente, J. Distribution, Biology and Biomass Estimates of the Sicilian Channel Anchovy. 2000 Final Report, DG XIV, MED 96–052, 151p. Available online: https://www.researchgate.net/publication/259254780_Distribution_biology_and_biomass_estimates_of_the_Sicilian_Channel_anchovy_DGXIV_MED_96052_Final_Report (accessed on 29 March 2022).
- Kara, A.B.; Rochford, P.A.; Hurlburt, H.E. An optimal definition for ocean mixed layer depth. J. Geophys. Res. 2000, 105, 16803–16821. [Google Scholar] [CrossRef]
- Planque, B.; Bellier, E.; Lazure, P. Modelling potential spawning habitat of sardine (Sardina pilchardus) and anchovy ((Engraulis encrasicolus) in the Bay of Biscay. Fish. Oceanogr. 2007, 16, 16–30. [Google Scholar] [CrossRef]
- Torri, M.; Corrado, R.; Falcini, F.; Cuttitta, A.; Palatella, L.; Lacorata, G.; Patti, B.; Arculeo, M.; Mifsud, R.; Mazzola, S.; et al. Planktonic stages of small pelagic fishes (Sardinella aurita and (Engraulis encrasicolus) in the central Mediterranean Sea: The key role of physical forcings and implications for fisheries management. Prog. Oceanogr. 2018, 162, 25–39. [Google Scholar] [CrossRef]
- Cuttitta, A.; Patti, B.; Maggio, T.; Quinci, E.M.; Pappalardo, A.M.; Ferrito, V.; De Pinto, V.; Torri, M.; Falco, F.; Nicosia, A.; et al. Larval population structure of (Engraulis encrasicolus in the Strait of Sicily as revealed by morphometric and genetic analysis. Fish. Oceanogr. 2015, 24, 135–149. [Google Scholar] [CrossRef]
- Pujol, I.; Larnicol, G. Mediterranean sea eddy kinetic energy variability from 11 years of altimetric data. J. Marine Sys. 2005, 58, 121–142. [Google Scholar] [CrossRef]
- Joint, I.; Groom, S. Estimation of phytoplankton production from space: Current status and future potential of satellite remote sensing. J. Exp. Mar. Biol. Ecol. 2000, 250, 233–255. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 30 September 2021).
- Naimi, B.; Araujo, M.B. sdm: A reproducible and extensible R platform for species distribution modelling. Ecography 2016, 39, 368–375. [Google Scholar] [CrossRef] [Green Version]
- Elith, J.; Ferrier, S.; Huettmann, F.; Leathwick, J.R. The evaluation strip: A new and robust method for plotting predicted responses from species distribution models. Ecol. Modell. 2005, 186, 280–289. [Google Scholar] [CrossRef]
- McCullagh, P.; Nelder, J.A. Generalized Linear Models; Chapman and Hall/CRC: London, UK, 1989; 532p. [Google Scholar]
- Hastie, T.; Tibshirani, R. Generalised Additive Models; Chapman and Hall/CRC: London, UK, 1990; 352p. [Google Scholar]
- Friedman, J.H. Multivariate adaptive regression splines. Ann. Stat. 1991, 19, 1–67. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Friedman, J.H. Greedy function approximation: A gradient boosting machine. Ann. Stat. 2001, 29, 1189–1232. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R.; Friedman, J.H. The Elements of Statistical Learning: Data Mining, Inference, and Prediction; Springer: New York, NY, USA, 2001. [Google Scholar]
- Vapnik, V. The Support Vector Method of Function Estimation. In Nonlinear Modeling; Suykens, J.A.K., Vandewalle, J., Eds.; Springer: Boston, MA, USA, 1998. [Google Scholar]
- Pearce, J.; Ferner, S. Evaluating the predictive performance of habitat models developed using logistic regression. Ecol. Model. 2000, 133, 225–245. [Google Scholar] [CrossRef] [Green Version]
- Fielding, A.H.; Bell, J.F. A review of methods for the measurement of prediction errors in conservation presence/absence models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Mason, S.J.; Graham, N.E. Areas beneath the relative operating characteristics (ROC) and relative operating levels (ROL) curves statistical significance and interpretation. Q. J. R. Meteorol. Soc. 2002, 128, 2145–2166. [Google Scholar] [CrossRef]
- Hosmer, D.W., Jr.; Lemeshow, S.; Sturdivant, R.X. Applied Logistic Regression, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Rodríguez, J.P.; Brotons, L.; Bustamante, J.; Seoane, J. The application of predictive modelling of species distribution to biodiversity conservation. Divers. Distrib. 2007, 13, 243–251. [Google Scholar] [CrossRef]
- Bonanno, A.; Placenti, F.; Basilone, G.; Mifsud, R.; Genovese, S.; Patti, B.; Di Bitetto, M.; Aronica, S.; Barra, M.; Giacalone, G.; et al. Variability of water mass properties in the Strait of Sicily in summer period of 1998–2013. Ocean Sci. 2014, 10, 759–770. [Google Scholar] [CrossRef] [Green Version]
- Basilone, G.; Guisande, C.; Patti, B.; Mazzola, S.; Cuttitta, A.; Bonanno, A.; Kallianiotis, A. Linking habitat conditions and growth in the European anchovy ((Engraulis encrasicolus). Fish. Res. 2004, 68, 9–19. [Google Scholar] [CrossRef]
- Bucklin, D.B.; Basille, M.; Benscoter, A.M.; Brandt, L.A.; Mazzotti, F.J.; Romanach, S.S.; Speroterra, C.; Watling, J.I. Comparing species distribution models constructed with different subsets of environmental predictors. Divers. Distrib. 2015, 21, 23–35. [Google Scholar] [CrossRef]
- Janowski, L.; Wroblewski, R.; Dworniczak, J.; Kolakowski, M.; Rogowska, K.; Wojcik, M.; Gajewski, J. Offshore benthic habitat mapping based on object-based image analysis and geomorphometric approach. A case study from the Slupsk Bank, Southern Baltic Sea. Sci Total Environ. 2021, 801, 149712. [Google Scholar] [CrossRef] [PubMed]
- Vezza, P.; Muñoz-Mas, R.; Martinez-Capel, F.; Mouton, A. Random forests to evaluate biotic interactions in fish distribution models. Environ. Model. Softw. 2015, 67, 173–183. [Google Scholar] [CrossRef]
- Dormann, C.F.; Purschke, O.; Marquez, J.R.G.; Lautenbach, S.; Schröder, B. Components of uncertainty in species distribution analysis: A case study of the Great Grey Shrike. Ecology 2008, 89, 3371–3386. [Google Scholar] [CrossRef]
- Cutler, D.R.; Edwards, T.C., Jr.; Beard, K.H.; Cutler, A.; Hess, K.T.; Gibson, J.; Lawler, J.J. Random forests for classification in ecology. Ecology 2007, 88, 2783–2792. [Google Scholar] [CrossRef]
- Ahmed, N.; Atzberger, C.; Zewdie, W. Species Distribution Modelling performance and its implication for Sentinel-2-based prediction of invasive Prosopis juliflora in lower Awash River basin, Ethiopia. Ecol. Proces. 2021, 10, 18. [Google Scholar] [CrossRef]
- Janowski, L.; Wroblewski, R.; Rucinska, M.; Kubowicz-Grajewska, A.; Tysiac, P. Automatic classification and mapping of the seabed using airborne LiDAR bathymetry. Eng. Geol. 2022, 301, 106615. [Google Scholar] [CrossRef]
- Ready, J.; Kaschner, K.; South, A.; Eastwood, P.D.; Rees, T.; Rius, J.; Agbayani, E.; Kullander, S.; Froese, R. Predicting the distributions of marine organisms at the global scale. Ecolog. Model. 2010, 221, 467–478. [Google Scholar] [CrossRef]
- Cuttitta, A.; Bonomo, S.; Zgozi, S.; Bonanno, A.; Patti, B.; Quinci, E.M.; Torri, M.; Hamza, M.; Fatah, A.; Haddoud, D.; et al. The influence of physical and biological processes on the ichthyoplankton communities in the Gulf of Sirte (Southern Mediterranean Sea). Mar. Ecol. 2016, 37, 831–844. [Google Scholar] [CrossRef]
- Agostini, V.; Bakun, A. ‘Ocean triads’ in the Mediterranean Sea: Physical mechanisms potentially structuring reproductive habitat suitability (with example application to European anchovy, (Engraulis encrasicolus). Fish. Oceanog. 2002, 11, 129–142. [Google Scholar] [CrossRef]
- Palomera, I.; Olivar, M.P.; Salat, J.; Sabatés, A.; Coll, M.; García, A.; Morales-Nin, B. Small pelagic fish in the NW Mediterranean Sea: An ecological review. Progr. Oceanogr. 2007, 74, 377–396. [Google Scholar] [CrossRef]
- Tudela, S.; Palomera, I.; Quílez, G. Feeding of anchovy (Engraulis encrasicolus larvae in the north-west Mediterranean. J. Mar. Biol. Assoc. United Kingd. 2002, 82, 349–350. [Google Scholar] [CrossRef]
- Morote, E.; Olivar, M.P.; Villate, F.; Uriarte, I. Diet of round sardinella, Sardinella aurita, larvae in relation to plankton availability in the NW Mediterranean. J. Plankt. Res. 2008, 30, 807–816. [Google Scholar] [CrossRef]
- Patti, B.; Zarrad, R.; Jarboui, O.; Cuttitta, A.; Basilone, G.; Aronica, S.; Placenti, F.; Tranchida, G.; Armeri, G.M.; Buffa, G.; et al. Anchovy ((Engraulis encrasicolus) early life stages in the Central Mediterranean Sea: Connectivity issues emerging among adjacent sub-areas across the Strait of Sicily. Hydrobiologia 2018, 821, 25–40. [Google Scholar] [CrossRef]
- Falcini, F.; Corrado, R.; Torri, M.; Mangano, M.C.; Zarrad, R.; Di Cintio, A.; Palatella, L.; Jarboui, O.; Missaoui, H.; Cuttitta, A.; et al. Seascape connectivity of European anchovy in the Central Mediterranean Sea revealed by weighted Lagrangian backtracking and bio-energetic modelling. Sci. Rep. 2020, 10, 18630. [Google Scholar] [CrossRef]
- Coetzee, B.W.T.; Robertson, M.P.; Erasmus, B.F.N.; van Rensburg, B.J.; Thuiller, W. Ensemble models predict Important Bird Areas in southern Africa will become less effective for conserving endemic birds under climate change. Glob. Ecol. Biogeogr. 2009, 18, 701–710. [Google Scholar] [CrossRef]
- Jones-Farrand, D.T.; Fearer, T.M.; Thogmartin, W.E.; Iii, F.R.T.; Nelson, M.D.; Tirpak, J.M. Comparison of statistical and theoretical habitat models for conservation planning: The benefit of ensemble prediction. Ecolog. Applic. 2011, 21, 2269–2282. [Google Scholar] [CrossRef] [Green Version]
- FAO 2022. GFCM (Mediterranean and Black Sea) Capture Production. Fisheries and Aquaculture Division [Online]. Rome. Available online: https://www.fao.org/fishery/statistics-query/en/gfcm_capture (accessed on 29 March 2022).







| Year | Survey | Period | Number of Stations |
|---|---|---|---|
| 1998 | BANSIC98 | 25 June–11 July 1998 | 73 |
| 1999 | BANSIC99B | 19 June–25 June 1999 | 53 |
| 2000 | BANSIC2000 | 24 June–8 July 2000 | 74 |
| 2001 | ANSIC2001 | 7 July–25 July 2001 | 77 |
| 2002 | ANSIC2002 | 11 July–31 July 2002 | 181 |
| 2003 | ANSIC2003 | 11 July–2 August 2003 | 129 |
| 2004 | ANSIC2004 | 18 June–7 July 2004 | 131 |
| 2005 | BANSIC2005 | 7 July–24 July 2005 | 97 |
| 2006 | BANSIC2006 | 30 July–10 August 2006 | 74 |
| 2007 | BANSIC2007 | 28 June–17 July 2007 | 106 |
| 2008 | BANSIC2008 | 25 June–14 July 2008 | 119 |
| 2009 | BANSIC2009 | 3 July–22 July 2009 | 96 |
| 2010 | BANSIC2010 | 25 June–14 July 2010 | 118 |
| 2011 | BANSIC2011 | 8 July–26 July 2011 | 77 |
| 2012 | BANSIC2012 | 4 July–23 July 2012 | 86 |
| 2013 | BANSIC2013 | 26 June–16 July 2013 | 134 |
| 2014 | BANSIC2014 | 22 July–9 August 2014 | 103 |
| 2015 | BANSIC2015 | 16 July–3 August 2015 | 103 |
| 2016 | BANSIC2016 | 30 June–14 July 2016 | 115 |
| Training Dataset | GLM | GAM | MARS | RF | BRT | SVM |
|---|---|---|---|---|---|---|
| Threshold-dependent measures | ||||||
| Threshold | 0.42 | 0.42 | 0.40 | 0.52 | 0.42 | 0.41 |
| Sensitivity | 0.69 | 0.76 | 0.76 | 0.82 | 0.79 | 0.77 |
| Specificity | 0.63 | 0.63 | 0.64 | 0.76 | 0.64 | 0.66 |
| Threshold-independent measures | ||||||
| AUC | 0.69 | 0.74 | 0.74 | 0.81 | 0.78 | 0.78 |
| Test Dataset | GLM | GAM | MARS | RF | BRT | SVM |
|---|---|---|---|---|---|---|
| Threshold-dependent measures | ||||||
| Threshold | 0.41 | 0.51 | 0.45 | 0.40 | 0.43 | 0.42 |
| Sensitivity | 0.77 | 0.66 | 0.68 | 0.79 | 0.76 | 0.71 |
| Specificity | 0.59 | 0.72 | 0.66 | 0.60 | 0.64 | 0.66 |
| Threshold-independent measures | ||||||
| AUC | 0.70 | 0.73 | 0.72 | 0.76 | 0.75 | 0.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quinci, E.M.; Torri, M.; Cuttitta, A.; Patti, B. Predicting Potential Spawning Habitat by Ensemble Species Distribution Models: The Case Study of European Anchovy (Engraulis encrasicolus) in the Strait of Sicily. Water 2022, 14, 1400. https://doi.org/10.3390/w14091400
Quinci EM, Torri M, Cuttitta A, Patti B. Predicting Potential Spawning Habitat by Ensemble Species Distribution Models: The Case Study of European Anchovy (Engraulis encrasicolus) in the Strait of Sicily. Water. 2022; 14(9):1400. https://doi.org/10.3390/w14091400
Chicago/Turabian StyleQuinci, Enza Maria, Marco Torri, Angela Cuttitta, and Bernardo Patti. 2022. "Predicting Potential Spawning Habitat by Ensemble Species Distribution Models: The Case Study of European Anchovy (Engraulis encrasicolus) in the Strait of Sicily" Water 14, no. 9: 1400. https://doi.org/10.3390/w14091400
APA StyleQuinci, E. M., Torri, M., Cuttitta, A., & Patti, B. (2022). Predicting Potential Spawning Habitat by Ensemble Species Distribution Models: The Case Study of European Anchovy (Engraulis encrasicolus) in the Strait of Sicily. Water, 14(9), 1400. https://doi.org/10.3390/w14091400