Performance, Modeling, and Cost Analysis of Chemical Coagulation-Assisted Solar Powered Electrocoagulation Treatment System for Pharmaceutical Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pharmaceutical Wastewater
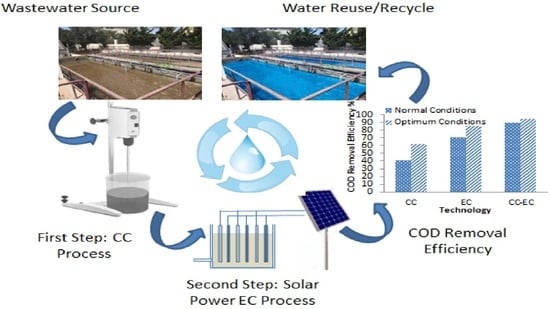
2.2. The Combined Treatment System
2.2.1. Chemical Coagulation (CC)
2.2.2. Solar-Powered Electrocoagulation (SPEC)
Factors Influencing the EC Process Efficiency in COD Removal
2.3. Analytical
3. Results
3.1. Chemical Coagulation Pretreatment Process
3.2. Process Performance of SPEC
3.2.1. The Effect of the Electrode Number
3.2.2. Effect of Distance between Electrodes
3.2.3. Effect of Electrode Configuration
3.2.4. Effects of Reaction Time and Current Density
3.2.5. Kinetic Study
3.3. Comparison of CC, EC, and Combined CC-EC
4. Operational Cost (OPC) Analysis for Solar Photovoltaic Electrocoagulation
5. Conclusions
- The COD removal efficiency is increased by decreasing the current density, number of electrodes, and distance between electrodes. Meanwhile, it increased with the alum dose and reaction time.
- First- and second-order kinetic models were investigated on the EC. The first-order kinetic model was shown to be more suitable than the second-order kinetic model, with (higher R2) values.
- Photovoltaic energy sources have shown to be more efficient and thus more economically feasible than conventional energy sources.
- Finally, the study results showed that a combination of EC and CC processes in pharmaceutical wastewater treatment proved effective for the removal of COD.
6. Recommendations
- The results of the combined CC and EC processes in this research may motivate researchers to adopt combination treatment methods since they show that such systems can produce water that is suitable for reuse in agriculture and irrigation.
- An important parameter in the EC process is the type of electrodes used. This issue needs more investigation. The most commonly used types are Al and Fe electrodes. Al electrodes have shown higher removal efficiencies than Fe. However, it is more expensive, and it produces sludge that needs special management.
- More studies should be conducted to investigate and optimize the most efficient electrode arrangement.
- The use of kinetic models to describe the treatment processes in these combined systems is still very limited. For this reason, it is necessary to develop suitable models for these new systems. If these models precisely describe the experimental results, they can be used in the scaling up of these systems [59].
- According to our findings, the combined system had a removal effectiveness of 94.4%. This encourages researchers to apply this integrated system to more contaminated industrial wastewater.
- H2 production and conversion into electrical energy to reduce overall energy consumption.
- The application of a sustainable treatment process in which the recovery of valuable materials in the wastewater should be performed before or after the treatment process [81].
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ait-Mouheb, N.; Mayaux, P.L.; Mateo-Sagasta, J.; Hartani, T.; Molle, B. Water reuse: A resource for Mediterranean agriculture. In Water Resources in the Mediterranean Region; Elsevier: Amsterdam, The Netherlands, 2020; pp. 107–136. [Google Scholar] [CrossRef]
- Bani-Melhem, K. Development of a Novel Submerged Membrane Electro-Bioreactor for Wastewater Treatment. Ph.D. Thesis, Concordia University, Montreal, QC, Canada, 2008. [Google Scholar]
- UNICEF. Water, Sanitation and Hygiene-Access to Safe Water and Sanitation for Every Child. Available online: https://www.unicef.org/wash (accessed on 8 February 2023).
- Al-Weshah, R.; Saidan, M.; Al-Omari, A. Environmental ethics as a tool for sustainable water resource management. J.-Am. Water Work. Assoc. 2016, 108, 175–181. [Google Scholar] [CrossRef]
- Jordan’s Water Strategy 2016–2025; Ministry of Water and Irrigation (MWI): Amman, Jordan, 2016.
- Karthikeyan, K.T.; Nithya, A.; Jothivenkatachalam, K. Photocatalytic and antimicrobial activities of chitosan-TiO2 nanocomposite. Int. J. Biol. Macromol. 2017, 104, 1762–1773. [Google Scholar] [CrossRef] [PubMed]
- Khalid, N.R.; Majid, A.; Tahir, M.B.; Niaz, N.A.; Khalid, S. Carbonaceous-TiO2 nanomaterials for photocatalytic degradation of pollutants: A review. Ceram. Int. 2017, 43, 14552–14571. [Google Scholar] [CrossRef]
- Sin, J.-C.; Lam, S.-M.; Zeng, H.; Lin, H.; Li, H.; Tham, K.-O.; Mohamed, A.R.; Lim, J.-W.; Qin, Z. Magnetic NiFe2O4 nanoparticles decorated on N-doped BiOBr nanosheets for expeditious visible light photocatalytic phenol degradation and hexavalent chromium reduction via a Z-scheme heterojunction mechanism. Appl. Surf. Sci. 2021, 559, 149966. [Google Scholar] [CrossRef]
- Graça, N.S.; Rodrigues, A.E. The Combined Implementation of Electrocoagulation and Adsorption Processes for the Treatment of Wastewaters. Clean Technol. 2022, 4, 1020–1053. [Google Scholar] [CrossRef]
- Chen, X.; Xue, S.; Lv, M.; Wang, R. Pharmaceutical industry in China: Policy, market and IP. In Innovation, Economic Development, and Intellectual Property in India and China; Springer: Singapore, 2019; pp. 215–250. [Google Scholar] [CrossRef] [Green Version]
- Maniakova, G.; Kowalska, K.; Murgolo, S.; Mascolo, G.; Libralato, G.; Lofrano, G.; Rizzo, L. Comparison between heterogeneous and homogeneous solar driven advanced oxidation processes for urban wastewater treatment: Pharmaceuticals removal and toxicity. Sep. Purif. Technol. 2020, 236, 116249. [Google Scholar] [CrossRef]
- Zhao, F.; Ju, F.; Huang, K.; Mao, Y.; Zhang, X.X.; Ren, H.; Zhang, T. Comprehensive insights into the key components of bacterial assemblages in pharmaceutical wastewater treatment plants. Sci. Total Environ. 2019, 651, 2148–2157. [Google Scholar] [CrossRef] [PubMed]
- Singal, N.; Kaur, S. Review on physico-chemical analysis of various pharmaceutical industrial effluents and its impact on the environment. Int. J. Sci. Appl. Res. 2018, 5, 7–11. [Google Scholar]
- Sher, F.; Hanif, K.; Iqbal, S.Z.; Imran, M. Implications of advanced wastewater treatment: Electrocoagulation and electroflocculation of effluent discharged from a wastewater treatment plant. J. Water Process Eng. 2020, 33, 101101. [Google Scholar] [CrossRef]
- Al-Zboon, K.; Matalqah, W.; Al Qodah, Z. Health Risk Assessment of Desalination Plant Using AERMOD Dispersion Model. Jordan J. Civ. Eng. 2022, 16, 518–530. [Google Scholar]
- Mousazadeh, M.; Naghdali, Z.; Rahimian, N.; Hashemi, M.; Paital, B.; Al-Qodah, Z.; Mukhtar, A.; Karri, R.R.; Mahmoud, A.E.D.; Sillanpää, M.; et al. Management of environmental health to prevent an outbreak of COVID-19: A review. In Environmental and Health Management of Novel Coronavirus Disease (COVID-19); Elsevier: Amsterdam, The Netherlands, 2021; p. 235. [Google Scholar] [CrossRef]
- Padmaja, K.; Cherukuri, J.; Reddy, M.A. A comparative study of the efficiency of chemical coagulation and electrocoagulation methods in the treatment of pharmaceutical effluent. J. Water Process Eng. 2020, 34, 101153. [Google Scholar] [CrossRef]
- Li, H.; Cai, Y.; Gu, Z.; Yang, Y.L.; Zhang, S.; Yang, X.L.; Song, H.L. Accumulation of sulfonamide resistance genes and bacterial community function prediction in microbial fuel cell-constructed wetland treating pharmaceutical wastewater. Chemosphere 2020, 248, 126014. [Google Scholar] [CrossRef] [PubMed]
- Akhoundi, A.; Nazif, S. Sustainability assessment of wastewater reuse alternatives using the evidential reasoning approach. J. Clean. Prod. 2018, 195, 1350–1376. [Google Scholar] [CrossRef]
- La Jeunesse, I.; Cirelli, C.; Aubin, D.; Larrue, C.; Sellami, H.; Afifi, S.; Bellin, A.; Benabdallah, S.; Bird, D.; Deidda, R.; et al. Is climate change a threat for water uses in the Mediterranean region? Results from a survey at local scale. Sci. Total Environ. 2016, 543, 981–996. [Google Scholar] [CrossRef] [PubMed]
- Husnain, T.; Liu, Y.; Riffat, R.; Mi, B. Integration of forward osmosis and membrane distillation for sustainable wastewater reuse. Sep. Purif. Technol. 2015, 156, 424–431. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, S.W.; Welter, J.B.; Albornoz, L.L.; Heberle, A.N.A.; Ferreira, J.Z.; Bernardes, A.M. Advanced electrochemical oxidation processes in the treatment of pharmaceutical containing water and wastewater: A review. Curr. Pollut. Rep. 2021, 7, 146–159. [Google Scholar] [CrossRef]
- Qu, J.G.; Li, N.N.; Liu, B.J.; He, J.X. Preparation of BiVO4/bentonite catalysts and their photocatalytic properties under simulated solar irradiation. Mater. Sci. Semicond. Process. 2013, 16, 99–105. [Google Scholar] [CrossRef]
- Ochando-Pulido, J.M.; Vellido-Pérez, J.A.; González-Hernández, R.; Martínez-Férez, A. Optimization and modeling of two-phase olive-oil washing wastewater integral treatment and phenolic compounds recovery by novel weak-base ion exchange resins. Sep. Purif. Technol. 2020, 249, 117084. [Google Scholar] [CrossRef]
- Mousazadeh, M.; Alizadeh, S.; Frontistis, Z.; Kabdaşlı, I.; Niaragh, E.K.; Al Qodah, Z.; Naghdali, Z.; Mahmoud, A.; Sandoval, M.; Butler, E.; et al. Electrocoagulation as a Promising defluoridation technology from water: A review of state of the art of removal mechanisms and performance trends. Water 2021, 13, 656. [Google Scholar] [CrossRef]
- Mariam, T.; Nghiem, L.D. Landfill leachate treatment using hybrid coagulation-nanofiltration processes. Desalination 2010, 250, 677–681. [Google Scholar] [CrossRef]
- Jamrah, A.I.; Abu-Ghunmi, L.N. One independent variable rate equation describing utilization of biodegradable organic matter in activated sludge processes. Environ. Model. Assess. 2005, 10, 21–31. [Google Scholar] [CrossRef]
- Wang, Z.P.; Zhang, Z.; Lin, Y.J.; Deng, N.S.; Tao, T.; Zhuo, K. Landfill leachate treatment by a coagulation–photooxidation process. J. Hazard. Mater. 2002, 95, 153–159. [Google Scholar] [CrossRef]
- Al-Qodah, Z.; Al-Qudah, Y.; Omar, W. On the performance of electrocoagulation-assisted biological treatment processes: A review on the state of the art. Environ. Sci. Pollut. Res. 2019, 26, 28689–28713. [Google Scholar] [CrossRef] [PubMed]
- Abdelhay, A.; Al Bsoul, A.; Al-Othman, A.; Al-Ananzeh, N.M.; Jum’h, I.; Al-Taani, A.A. Kinetic and thermodynamic study of phosphate removal from water by adsorption onto (Arundo donax) reeds. Adsorpt. Sci. Technol. 2018, 36, 46–61. [Google Scholar] [CrossRef] [Green Version]
- Al-Qodah, Z.; Al-Qudah, Y.; Assirey, E. Combined biological wastewater treatment with electrocoagulation as a post-polishing process: A review. Sep. Sci. Technol. 2020, 55, 2334–2352. [Google Scholar] [CrossRef]
- Bagchi, S.; Behera, M. Pharmaceutical wastewater treatment in microbial fuel cell. In Integrated Microbial Fuel Cells for Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2020; pp. 135–155. [Google Scholar] [CrossRef]
- Chandra, S.; Dohare, D.; Kotiya, A. Study of Electrocoagulation Process for Removal of Heavy Metals from Industrial Wastewater. A Review. Int. J. Eng. Res. Technol. 2020, 9, 993–999. [Google Scholar]
- Khandegar, V.; Acharya, S.; Jain, A.K. Data on treatment of sewage wastewater by electrocoagulation using punched aluminum electrode and characterization of generated sludge. Data Brief 2018, 18, 1229–1238. [Google Scholar] [CrossRef]
- Haldar, D.; Manna, M.S.; Sen, D.; Bhowmick, T.K.; Gayen, K. Microbial fuel cell for the treatment of wastewater. Microb. Fuel Cells Mater. Appl. 2019, 46, 289–306. [Google Scholar] [CrossRef]
- Tang, H.; Sha, J.P.; Liu, G.Z.; Ou, Y.L. Advanced Treatment of Biologically Pretreated Coking Wastewater by Electro-Coagulation: Degradation Behavior and Mechanism. Pol. J. Environ. Stud. 2015, 24, 1355–1362. [Google Scholar]
- Tahreen, A.; Jami, M.S.; Ali, F. Role of electrocoagulation in wastewater treatment: A developmental review. J. Water Process Eng. 2020, 37, 101440. [Google Scholar] [CrossRef]
- Khandegar, V.; Saroha, A.K. Electrocoagulation for the treatment of textile industry effluent–a review. J. Environ. Manag. 2013, 128, 949–963. [Google Scholar] [CrossRef] [PubMed]
- Siringi, D.O.; Home, P.; Joseph, S.; Enno, K. Is Electrocoagulation (EC) A solution to the treatment of wastewater and providing clean water for daily use. ARPN J. Eng. Appl. Sci. 2012, 7, 197–204. [Google Scholar]
- Kadier, A.; Al-Qodah, Z.; Akkaya, G.K.; Song, D.; Peralta-Hernández, J.M.; Wang, J.-Y.; Phalakornkule, C.; Bajpai, M.; Niza, N.M.; Gilhotra, V.; et al. A state-of-the-art review on electrocoagulation (EC): An efficient, emerging, and green technology for oil elimination from oil and gas industrial wastewater streams. Case Stud. Chem. Environ. Eng. 2022, 6, 100274. [Google Scholar] [CrossRef]
- Hussin, F.; Aroua, M.K.; Szlachta, M. Combined solar electrocoagulation and adsorption processes for Pb(II) removal from aqueous solution. Chem. Eng. Process. Process Intensif. 2019, 143, 107619. [Google Scholar] [CrossRef]
- Hussin, F.; Abnisa, F.; Issabayeva, G.; Aroua, M.K. Removal of lead by solar-photovoltaic electrocoagulation using novel perforated zinc electrode. J. Clean. Prod. 2017, 147, 206–216. [Google Scholar] [CrossRef]
- Valero, D.; Ortiz, J.M.; Exposito, E.; Montiel, V.; Aldaz, A. Electrocoagulation of a synthetic textile effluent powered by photovoltaic energy without batteries: Direct connection behaviour. Sol. Energy Mater. Sol. Cells 2008, 92, 291–297. [Google Scholar] [CrossRef]
- Colacicco, A.; Zacchei, E. Optimization of energy consumptions of oxidation tanks in urban wastewater treatment plants with solar photovoltaic systems. J. Environ. Manag. 2020, 276, 111353. [Google Scholar] [CrossRef] [PubMed]
- Al-Qodah, Z.; Al-Zoubi, H.; Hudaib, B.; Omar, W.; Soleimani, M.; Abu-Romman, S.; Frontistis, Z. Sustainable vs. Conventional Approach for Olive Oil Wastewater Management: A Review of the State of the Art. Water 2022, 14, 1695. [Google Scholar] [CrossRef]
- GilPavas, E.; Dobrosz-Gómez, I.; Miguel-Ángel Gómez-García, M.A. Optimization and toxicity assessment of a combined electrocoagulation, H2O2/Fe2+/UV and activated carbon adsorption for textile wastewater treatment. Sci. Total Environ. 2019, 651, 551–560. [Google Scholar] [CrossRef]
- Shamaei, L.; Khorshidi, B.; Perdicakis, B.; Sadrzadeh, M. Treatment of oil sands produced water using combined electrocoagulation and chemical coagulation techniques. Sci. Total Environ. 2018, 645, 560–572. [Google Scholar] [CrossRef]
- Tejedor-Sanz, S.; Ortiz, J.M.; Esteve-Núñez, A. Merging microbial electrochemical systems with electrocoagulation pretreatment for achieving a complete treatment of brewery wastewater. Chem. Eng. J. 2017, 330, 1068–1074. [Google Scholar] [CrossRef]
- Swain, K.; Abbassi, B.; Kinsley, C. Combined electrocoagulation and chemical coagulation in treating brewery wastewater. Water 2020, 12, 726. [Google Scholar] [CrossRef] [Green Version]
- Ezechi, E.H.; Isa, M.H.; Kutty, S.R.M. Removal of boron from produced water by electrocoagulation. In Proceedings of the 10th WSEAS International Conference on Environment, Ecosystems and Development, Montreux, Switzerland, 29–31 December 2012; pp. 29–31. [Google Scholar]
- Maleki, A.; Zazouli, M.A.; Izanloo, H.; Rezaee, R. Composting plant leachate treatment by coagulation-flocculation process. Am. Eurasian J. Agric. Environ. Sci. 2009, 5, 638–643. [Google Scholar]
- Malakoutian, M.; Fatehizadeh, A. Color removal from water by coagulation/caustic soda and lime. Iran. J. Environ. Health Sci. Eng. 2010, 7, 267–272. [Google Scholar]
- Nurul Hanira, M.L.; Hasfalina, C.M.; Sani, A.; Rashid, M. Pre-treatment ammonia removal of scheduled waste leachate with hydrated lime and caustic soda. J. Teknol. 2017, 79, 107–118. [Google Scholar]
- Precious Sibiya, N.; Rathilal, S.; Kweinor Tetteh, E. Coagulation treatment of wastewater: Kinetics and natural coagulant evaluation. Molecules 2021, 26, 698. [Google Scholar] [CrossRef]
- Wei, H.; Gao, B.; Ren, J.; Li, A.; Yand, H. Coagulation/flocculation in dewatering of sludge: A review. Water Res. 2018, 143, 608–631. [Google Scholar] [CrossRef]
- Bouchareb, R.; Derbal, K.; Özay, Y.; Bilici, Z.; Dizge, N. Combined natural/chemical coagulation and membrane filtration for wood processing wastewater treatment. J. Water Process Eng. 2020, 37, 101521. [Google Scholar] [CrossRef]
- Cheung, K.C.; Chu, L.M.; Wong, M.H. Ammonia stripping as a pretreatment for landfill leachate. Water Air Soil Pollut. 1997, 94, 209–221. [Google Scholar] [CrossRef]
- Thakur, L.S.; Mondal, P. Techno-economic evaluation of simultaneous arsenic and fluoride removal from synthetic groundwater by electrocoagulation process: Optimization through response surface methodology. Desalination Water Treat. 2016, 57, 28847–28863. [Google Scholar] [CrossRef]
- Hakizimana, J.N.; Gourich, B.; Chafi, M.; Stiriba, Y.; Vial, C.; Drogui, P.; Naja, J. Electrocoagulation process in water treatment: A review of electrocoagulation modeling approaches. Desalination 2017, 404, 1–21. [Google Scholar] [CrossRef]
- Gatsios, E.; Hahladakis, J.N.; Gidarakos, E. Optimization of electrocoagulation (EC) process for the purification of a real industrial wastewater from toxic metals. J. Environ. Manag. 2015, 154, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Salih Muharam, S.M.; Rahmah, C.I.; Yuningsih, L.M. Simultaneous combination of electrocoagulation and chemical coagulation methods for medical wastewater treatment. Makara J. Sci. 2017, 21, 113–118. [Google Scholar]
- Janpoor, F.; Torabian, A.; Khatibikamal, V. Treatment of laundry waste-water by electrocoagulation. J. Chem. Technol. Biotechnol. 2011, 86, 1113–1120. [Google Scholar] [CrossRef]
- Nasrullah, M.; Siddique, M.N.I.; Zularisam, A.W. Effect of high current density in electrocoagulation process for sewage treatment. Asian J. Chem. 2014, 26, 4281. [Google Scholar] [CrossRef]
- Bhagawan, D.; Poodari, S.; Chaitanya, N.; Ravi, S.; Rani, Y.M.; Himabindu, V.; Vidyavathi, S. Industrial solid waste landfill leachate treatment using electrocoagulation and biological methods. Desalin Water Treat 2017, 68, 137–142. [Google Scholar] [CrossRef]
- Kobya, M.; Ulu, F.; Gebologlu, U.; Demirbas, E.; Oncel, M.S. Treatment of potable water containing low concentration of arsenic with electrocoagulation: Different connection modes and Fe–Al electrodes. Sep. Purif. Technol. 2011, 77, 283–293. [Google Scholar] [CrossRef]
- Naje, A.S.; Chelliapan, S.; Zakaria, Z.; Abbas, S.A. Treatment performance of textile wastewater using electrocoagulation (EC) process under combined electrical connection of electrodes. Int. J. Electrochem. Sci. 2015, 10, 5924–5941. [Google Scholar]
- Reynolds, T.D.; Richards, P.A.C. Unit operations and processes in environmental engineering. In Unit Operations and Processes in Environmental Engineering; PWS Publishing Company: Boston, MA, USA, 1995. [Google Scholar]
- Ahmadian, M.; Yousefi, N.; Van Ginkel, S.W.; Zare, M.R.; Rahimi, S.; Fatehizadeh, A. Kinetic study of slaughterhouse wastewater treatment by electrocoagulation using Fe electrodes. Water Sci. Technol. 2012, 66, 754–760. [Google Scholar] [CrossRef]
- Can, O.T.; Gengec, E.; Kobya, M. TOC and COD removal from instant coffee and coffee products production wastewater by chemical coagulation assisted electrooxidation. J. Water Process Eng. 2019, 28, 28–35. [Google Scholar] [CrossRef]
- Al-Qodah, Z.; Tawalbeh, M.; Al-Shannag, M.; Al-Anber, Z.; Bani-Melhem, K. Combined electrocoagulation processes as a novel approach for enhanced pollutants removal: A state-of-the-art review. Sci. Total Environ. 2020, 744, 140806. [Google Scholar] [CrossRef]
- Dindaş, G.B.; Çalışkan, Y.; Celebi, E.E.; Tekbaş, M.; Bektaş, N.; Yatmaz, H.C. Treatment of pharmaceutical wastewater by combination of electrocoagulation, electro-fenton and photocatalytic oxidation processes. J. Environ. Chem. Eng. 2020, 8, 103777. [Google Scholar] [CrossRef]
- Changotra, R.; Rajput, H.; Dhir, A. Treatment of real pharmaceutical wastewater using combined approach of Fenton applications and aerobic biological treatment. J. Photochem. Photobiol. A Chem. 2019, 376, 175–184. [Google Scholar] [CrossRef]
- Patel, S.; Mondal, S.; Majumder, S.K.; Das, P.; Ghosh, P. Treatment of a pharmaceutical industrial effluent by a hybrid process of advanced oxidation and adsorption. ACS Omega 2020, 5, 32305–32317. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, A.M.; Satyanarayan, S.; Ramakant, S. Treatment of high-strength pharmaceutical wastewater by electrocoagulation combined with anaerobic process. Water Sci. Technol. 2010, 61, 463–472. [Google Scholar] [CrossRef]
- Changotra, R.; Rajput, H.; Guin, J.P.; Varshney, L.; Dhir, A. Hybrid coagulation, gamma irradiation and biological treatment of real pharmaceutical wastewater. Chem. Eng. J. 2019, 370, 595–605. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Brillas, E. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: A general review. Appl. Catal. B Environ. 2009, 87, 105–145. [Google Scholar] [CrossRef]
- Kobya, M.; Bayramoglu, M.; Eyvaz, M. Techno-economical evaluation of electrocoagulation for the textile wastewater using different electrode connections. J. Hazard. Mater. 2007, 148, 311–318. [Google Scholar] [CrossRef]
- Ilhan, F.; Kurt, U.; Apaydin, O.; Gonullu, M.T. Treatment of leachate by electrocoagulation using aluminum and iron electrodes. J. Hazard. Mater. 2008, 154, 381–389. [Google Scholar] [CrossRef]
- Kobya, M.; Delipinar, S. Treatment of the baker’s yeast wastewater by electrocoagulation. J. Hazard. Mater. 2008, 154, 1133–1140. [Google Scholar] [CrossRef]
- Al Qedra, B. Boron Removal from Seawater by Hybrid Solar Photovoltaic Electrocoagulation (SPEC) Process. Master’s Dissertation, The Islamic University, Gaza, Palestine, 2015. [Google Scholar]
- Al-Qodah, Z.; Al-Shannag, M. Heavy metal ions removal from wastewater using electrocoagulation processes: A comprehensive review. Sep. Sci. Technol. 2017, 52, 2649–2676. [Google Scholar] [CrossRef]












| Parameter | Value |
|---|---|
| pH | 6.32 |
| Conductivity (mS/cm) | 8.31 |
| T (°C) | 29 |
| Initial COD (mg/L) | 3447.9 |
| BOD5 (mg/L) | 930.9 |
| Coagulant Dosage (mg/L) | COD Removal (%) | |
|---|---|---|
| Caustic Soda (NaOH) | Alum (Al2(SO4)3·18H2O) | |
| 0 | 0 | 0 |
| 250 | 16.2 | 41 |
| 500 | 18.8 | 61.5 |
| 750 | 26 | 62.3 |
| 1000 | 30.7 | 63.6 |
| Operating Parameters | COD Removal (%) | ||
|---|---|---|---|
| EC | CC-EC | ||
| Distance between Electrodes (cm) | 2 | 81.60 | 92.92 |
| 3 | 77.83 | 91.46 | |
| 4 | 70.53 | 88.65 | |
| Number of Electrodes | 2 | 68.28 | 87.79 |
| 4 | 69.96 | 88.43 | |
| 6 | 70.53 | 88.65 | |
| Electrode Arrangement | MP-S | 85.42 | 94.4 |
| BP-S | 77.51 | 90.5 | |
| MP-P | 70.53 | 88.65 | |
| COD Removal (%) | |||
|---|---|---|---|
| Time (min) | CD (mA/cm2) | EC | CC-EC |
| 1.553 | 72.52 | 89.42 | |
| 20 | 3.105 | 70.53 | 88.65 |
| 4.658 | 69.49 | 88.25 | |
| 1.553 | 72.50 | 89.41 | |
| 30 | 3.105 | 70.32 | 88.57 |
| 4.658 | 68.74 | 87.96 | |
| 1.553 | 72.20 | 89.30 | |
| 40 | 3.105 | 70.02 | 88.46 |
| 4.658 | 68.59 | 87.91 | |
| 1.553 | 71.98 | 89.21 | |
| 50 | 3.105 | 69.87 | 88.40 |
| 4.658 | 68.36 | 87.82 | |
| 1.553 | 80.41 | 92.46 | |
| 60 | 3.105 | 78.49 | 91.72 |
| 4.658 | 77.32 | 91.27 | |
| Parameters | CD (mA/cm2) | First-Order Kinetic Model | R2 (−) | Second-Order Kinetic Model | R2 (−) |
|---|---|---|---|---|---|
| k1 (min−1) | k2 (L/mg/min) | ||||
| COD | 1.553 | 8.22 × 10−3 | 0.9122 | 2.65 × 10−5 | 0.8854 |
| 3.105 | 7.66 × 10−3 | 0.9052 | 2.25 × 10−5 | 0.8792 | |
| 4.658 | 7.06 × 10−3 | 0.8943 | 2.01 × 10−5 | 0.8681 |
| Combined Treatment | Abbreviation | Operating Conditions | Removal Efficiency | Reference |
|---|---|---|---|---|
| Combination of electro-coagulation (EC), electro-Fenton (EF) and photocatalytic oxidation (PcO) | EC + EF | 1 h EF, 5 mA/cm2 | 64% TOC | [71] |
| EC + PcO | 4 h PcO, Fe:H2O2 molar ratio as 1:10 | 70.2% COD | ||
| EF + PcO | 1.5 g/L TiO2 10 mM H2O2 | 97.8% BOD5 | ||
| Solar-driven photo-Fenton (PF) followed by subsequent biological treatment | PF + biological | pH hydrogen peroxide dosage iron concentration applied voltage | 84% of COD for LSW 82% of COD for HSW | [72] |
| Ozone-based advanced oxidation and adsorption | AO-Ad | pH (5–11), 3 h | 75–88.5% COD | [73] |
| activated char for adsorption | 85.4–92.7% COD | |||
| Combined electrocoagulation followed by anaerobic fixed film bed reactor (AFFBR) | EC-AFFBR | pH 7.2 80 A/m2 of CD 25 min | 24% COD 35% BOD 70.25 of color removal | [74] |
| Hybrid coagulation, gamma irradiation, and biological treatment | CC-GI | coagulants: Ca(OH)2, FeCl3 and Al2(SO4)3 oxidants: gamma-rays, H2O2 and S2O7−2 | (92.7% ± 2.3%) of COD for LSW | [75] |
| (90.2% ± 2.9%) of COD for HSW |
| Item | Unit | Current Density (mA/cm2) | ||
|---|---|---|---|---|
| 1.553 | 3.105 | 4.658 | ||
| Energy Consumption | kWh/m3 | 0.6 | 1.533 | 3.4 |
| Electrode Consumption | Fe kg/m3 | 0.3481 | 0.696 | 1.045 |
| Chemicals | kg/m3 | 0.0005 | 0.0005 | 0.0005 |
| Energy Cost | 0.13 $/kWh | 0.078 | 0.1993 | 0.442 |
| Electrode Cost | 0.89 $/kg | 0.31 | 0.619 | 0.93 |
| Chemical Cost | 20 $/kg | 0.01 | 0.01 | 0.01 |
| Total Conventional EC | $/m3 | 0.398 | 0.829 | 1.382 |
| Total Solar EC | $/m3 | 0.31 | 0.619 | 0.93 |
| Time | V = 1.8 (V) | V = 2.3 (V) | V = 3.4 (V) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| (min) | CD = 1.553 (mA/cm2) | CD = 3.105 (mA/cm2) | CD = 4.658 (mA/cm2) | ||||||
| ENC | ELC | OPC | ENC | ELC | OPC | ENC | ELC | OPC | |
| kWh/m3 | kg/m3 | $/m3 | kWh/m3 | kg/m3 | $/m3 | kWh/m3 | kg/m3 | $/m3 | |
| 5 | 0.05 | 0.029 | 0.042 | 0.128 | 0.058 | 0.078 | 0.283 | 0.087 | 0.124 |
| 10 | 0.1 | 0.058 | 0.075 | 0.256 | 0.116 | 0.147 | 0.567 | 0.174 | 0.239 |
| 20 | 0.2 | 0.116 | 0.139 | 0.511 | 0.232 | 0.283 | 1.133 | 0.348 | 0.467 |
| 30 | 0.3 | 0.174 | 0.204 | 0.767 | 0.348 | 0.420 | 1.7 | 0.522 | 0.696 |
| 40 | 0.4 | 0.232 | 0.269 | 1.022 | 0.464 | 0.556 | 2.267 | 0.696 | 0.924 |
| 50 | 0.5 | 0.290 | 0.333 | 1.278 | 0.580 | 0.693 | 2.833 | 0.870 | 1.153 |
| 60 | 0.6 | 0.348 | 0.398 | 1.533 | 0.696 | 0.829 | 3.4 | 1.045 | 1.382 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Zghoul, T.M.; Al-Qodah, Z.; Al-Jamrah, A. Performance, Modeling, and Cost Analysis of Chemical Coagulation-Assisted Solar Powered Electrocoagulation Treatment System for Pharmaceutical Wastewater. Water 2023, 15, 980. https://doi.org/10.3390/w15050980
Al-Zghoul TM, Al-Qodah Z, Al-Jamrah A. Performance, Modeling, and Cost Analysis of Chemical Coagulation-Assisted Solar Powered Electrocoagulation Treatment System for Pharmaceutical Wastewater. Water. 2023; 15(5):980. https://doi.org/10.3390/w15050980
Chicago/Turabian StyleAl-Zghoul, Tharaa M., Zakaria Al-Qodah, and Ahmad Al-Jamrah. 2023. "Performance, Modeling, and Cost Analysis of Chemical Coagulation-Assisted Solar Powered Electrocoagulation Treatment System for Pharmaceutical Wastewater" Water 15, no. 5: 980. https://doi.org/10.3390/w15050980
APA StyleAl-Zghoul, T. M., Al-Qodah, Z., & Al-Jamrah, A. (2023). Performance, Modeling, and Cost Analysis of Chemical Coagulation-Assisted Solar Powered Electrocoagulation Treatment System for Pharmaceutical Wastewater. Water, 15(5), 980. https://doi.org/10.3390/w15050980