Obligations of Researchers and Managers to Respect Wetlands: Practical Solutions to Minimizing Field Monitoring Impacts
Abstract
:1. Call to Action for Wetland Researchers
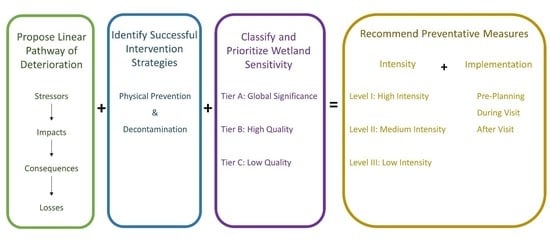
2. Linear Pathway of Deterioration
3. Identify Successful Intervention Strategies
4. Classify and Prioritize Ecosystem Sensitivity
- A Tier: Global and Regional Significance
- B Tier: High Quality Wetlands
- C Tier: Low Quality Wetlands
5. Recommended Preventative Measures
6. Conclusions: What’s at Stake?
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilcox, D.A. History of Wetland Science: A Perspective from Wetland Leaders; Wilcox, D.A., Ed.; Amazon Print-on-Demand: Middletown, DE, USA, 2020. [Google Scholar]
- Mitsch, W.J.; Gosselink, J.G. Wetlands, 4th ed.; John Wiley & Sons Inc: Hoboken, NJ, USA, 2007; Volume 99, pp. 15–16. [Google Scholar]
- Kadlec, R.H.; Kadlec, J.A. Wetlands and Water Quality. Wetland Functions and Values: The State of Our Understanding; Greeson, P.E., Clark, J.R., Clark, J.E., Eds.; American Water Resources Association: Minneapolis, MN, USA, 1979; pp. 436–456. [Google Scholar]
- Whigham, D.F.; Chitterling, C.; Palmer, B. Impacts of Freshwater Wetlands on Water Quality: A Landscape Perspective. J. Environ. Manag. 1988, 12, 663–671. [Google Scholar] [CrossRef]
- Acreman, M.; Holden, J. How Wetlands Affect Floods. Wetlands 2013, 33, 773–786. [Google Scholar] [CrossRef] [Green Version]
- Davies, G.T.; Finlayson, C.M.; Pritchard, D.E.; Davidson, N.C.; Gardner, R.C.; Moomaw, W.R.; Okuno, E.; Whitacre, J.C. Towards a Universal Declaration of the Rights of Wetlands. Mar. Freshwater Res. 2021, 72, 593. [Google Scholar] [CrossRef]
- Taillardat, P.J.; Thompson, B.S.; Garneau, M.; Trottier, K.; Friess, D.A. Climate change mitigation potential of wetlands and the cost-effectiveness of their restoration. Interface Focus 2020, 10, 20190129. [Google Scholar] [CrossRef] [PubMed]
- Sinthumule, N.I. An analysis of communities’ attitudes towards wetlands and implications for sustainability. Glob. Ecol. Conserv. 2021, 27, e01604. [Google Scholar] [CrossRef]
- Crozier, G.K.D.; Schulte-Hostedde, A.I. Towards Improving the Ethics of Ecological Research. Sci. Eng. Ethics 2015, 21, 577–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farmer, M.C. Setting Up an Ethics of Ecosystem Research Structure Based on the Precautionary Principle. ILAR J. 2013, 54, 58–62. [Google Scholar] [CrossRef] [Green Version]
- Farnsworth, E. Guidelines for Ethical Field Research on Rare Plant Species; New England Wild Flower Society: Framingham, MA, USA, 2005. [Google Scholar]
- Costello, M.J.; Beard, K.H.; Corlett, R.T.; Cumming, G.S.; Devictor, V.; Loyola, R.; Maas, B.; Miller-Rushing, A.J.; Pakeman, R.; Primack, R.B. Field Work Ethics in Biological Research. Biol. Conserv. 2016, 203, 268–271. [Google Scholar] [CrossRef]
- Minteer, B.A.; Collins, J.P. From Environmental to Ecological Ethics: Toward a Practical Ethics for Ecologists and Conservationists. Sci. Eng. Ethics 2008, 14, 483–501. [Google Scholar] [CrossRef]
- Parris, K.M.; McCall, S.C.; McCarthy, M.A.; Minteer, B.A.; Steele, K.; Bekessy, S.; Medvecky, F. Assessing Ethical Trade-offs in Ecological Field Studies. J. Appl. Ecol. 2010, 47, 227–234. [Google Scholar] [CrossRef]
- Wallace, M.C.; Curzer, H.J. Moral Problems and Perspectives for Ecological Field Research. ILAR J. 2013, 54, 3–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Chen, M.; Yang, G.; Jiang, B.; Zhang, J. Wetland Ecosystem Services Research: A Critical Review. Glob. Ecol. Conserv. 2020, 22, e01027. [Google Scholar] [CrossRef]
- Ramsar Convention. The 4th Strategic Plan 2016–2024; Ramsar Convention Secretariat: Gland, Switzerland, 2016; Available online: https://www.ramsar.org/document/the-fourth-ramsar-strategic-plan-2016-2024 (accessed on 17 March 2022).
- Koning, C.O. Vegetation Patterns Resulting from Spatial and Temporal Variability in Hydrology, Soils, and Trampling in an Isolated Basin Marsh, New Hampshire, USA. Wetlands 2005, 25, 239–251. [Google Scholar] [CrossRef]
- Ross, P.M. Macrofaunal Loss and Microhabitat Destruction: The Impact of Trampling in a Temperate Mangrove Forest, NSW Australia. Wetl. Ecol. Manag. 2006, 14, 167–184. [Google Scholar] [CrossRef]
- Hill, R.; Pickering, C. Differences in Resistance of Three Subtropical Vegetation Types to Experimental Trampling. J. Environ. Manag. 2009, 90, 1305–1312. [Google Scholar] [CrossRef] [Green Version]
- Rees, J.; Tivy, J. Recreational Impact on Scottish Lochshore Wetlands. J. Biogeogr. 1978, 5, 93. [Google Scholar] [CrossRef]
- Taylor, B.R.; Raney, S. Correlation Between ATV Tracks and Density of a Rare Plant (Drosera filiformis) in a Nova Scotia Bog. Rhodora 2013, 115, 158–169. [Google Scholar] [CrossRef]
- Sagerman, J.; Hansen, J.P.; Wikström, S.A. Effects of boat traffic and mooring infrastructure on aquatic vegetation: A systematic review and meta-analysis. Ambio 2020, 49, 517–530. [Google Scholar] [CrossRef] [Green Version]
- Arnesen, T. Vegetation Dynamics Following Trampling in Rich Fen at Sølendet, Central Norway; a 15 Year Study of Recovery. Nord. J. Bot. 1999, 19, 313–327. [Google Scholar] [CrossRef]
- Hsu, C.; Chen, C.; Hsieh, H. Effects of sediment compaction on macroinfauna in a protected coastal wetland in Taiwan. Mar. Ecol. Prog. Ser. 2009, 375, 73–83. [Google Scholar] [CrossRef] [Green Version]
- Pescott, O.L.; Stewart, G.B. Assessing the Impact of Human Trampling on Vegetation: A Systematic Review and Meta-Analysis of Experimental Evidence. PeerJ 2014, 2, e360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kettenring, K.M.; Whigham, D.F.; Hazelton, E.L.G.; Gallagher, S.K.; Weiner, H.M. Biotic Resistance, Disturbance, and Mode of Colonization Impact the Invasion of a Widespread, Introduced Wetland Grass. Ecol. Appl. 2015, 25, 466–480. [Google Scholar] [CrossRef] [PubMed]
- Stockton, K.A.; Moffitt, C.M. Disinfection of Three Wading Boot Surfaces Infested with New Zealand Mudsnails. N. Am. J. Fish. Manag. 2013, 33, 529–538. [Google Scholar] [CrossRef]
- Mohit, S.; Johnson, T.; Arnott, S. Recreational Watercraft Decontamination: Can Current Recommendations Reduce Aquatic Invasive Species Spread? Manag. Biol. Invasions 2021, 12, 148–164. [Google Scholar] [CrossRef]
- Banha, F.; Marques, M.; Anastácio, P.M. Dispersal of Two Freshwater Invasive Macroinvertebrates, Procambarus clarkii and Physella acuta, by off-Road Vehicles: Dispersal of Invasive Macroinvertebrates by off-Road Vehicles. Aquat. Conserv. Mar. Freshw. Ecosyst. 2014, 24, 582–591. [Google Scholar] [CrossRef]
- Stinca, A.; Musarella, C.M.; Rosati, L.; Laface, V.L.A.; Licht, W.; Fanfarillo, E.; Wagensommer, R.P.; Galasso, G.; Fascetti, S.; Esposito, A.; et al. Italian Vascular Flora: New Findings, Updates and Exploration of Floristic Similarities between Regions. Diversity 2021, 13, 600. [Google Scholar] [CrossRef]
- Sinks, I.A.; Borde, A.B.; Diefenderfer, H.L.; Karnezis, J.P. Assessment of methods to control invasive reed canarygrass (Phalaris arundinacea) in tidal freshwater wetlands. Nat. Areas J. 2021, 41, 172–185. [Google Scholar] [CrossRef]
- Hazelton, E.L.; Downard, R.; Kettenring, K.M.; McCormick, M.K.; Whigham, D.F. Spatial and temporal variation in brackish wetland seed banks: Implications for wetland restoration following Phragmites control. Estuaries Coast. 2018, 41, 68–84. [Google Scholar] [CrossRef]
- Reinhart, K.O.; Callaway, R.M. Soil biota and invasive plants. New Phytol. 2006, 170, 445–457. [Google Scholar] [CrossRef]
- Whitt, M.B.; Prince, H.H.; Cox, R.R., Jr. Avian use of purple loosestrife dominated habitat relative to other vegetation types in a Lake Huron wetland complex. Wilson Bull. 1999, 111, 105–114. [Google Scholar]
- Spyreas, G.; Wilm, B.W.; Plocher, A.E.; Ketzner, D.M.; Matthews, J.W.; Ellis, J.L.; Heske, E.J. Biological consequences of invasion by reed canary grass (Phalaris arundinacea). Biol. Invasions 2010, 12, 1253–1267. [Google Scholar] [CrossRef]
- Nagy, C.; Aschen, S.; Christie, R.; Weckel, M. Japanese stilt grass (Microstegium vimineum), a nonnative invasive grass, provides alternative habitat for native frogs in a suburban forest. Urban Habitats 2011, 6, 1–10. [Google Scholar]
- Bezemer, T.M.; Harvey, J.A.; Cronin, J.T. Response of native insect communities to invasive plants. Annu. Rev. Entomol. 2014, 59, 119–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lysne, S.; Koetsier, P. Comparison of desert valvata snail growth at three densities of the invasive New Zealand mudsnail. West. N. Am. Nat. 2008, 68, 103–106. [Google Scholar] [CrossRef]
- Hall, R.O.; Dybdahl, M.F.; VanderLoop, M.C. Extremely high secondary production of introduced snails in rivers. Ecol. Appl. 2006, 16, 1121–1131. [Google Scholar] [CrossRef]
- Dick, J.T.A.; Platvoet, D. Invading predatory crustacean Dikerogammarus villosus eliminates both native and exotic species. Proc. Royal Soc. B. 2000, 267, 977–983. [Google Scholar] [CrossRef] [Green Version]
- Warren, D.A.; Bradbeer, S.J.; Dunn, A.M. Superior predatory ability and abundance predicts potential ecological impact towards early-stage anurans by invasive ‘Killer Shrimp’ (Dikerogammarus villosus). Sci. Rep. 2021, 11, 4570. [Google Scholar] [CrossRef]
- Thomas, M.; Samuel, K.A.; Kurian, P. Rodentborne Fungal Pathogens in Wetland Agroecosystem. Braz. J. Microbiol. 2012, 43, 247–252. [Google Scholar] [CrossRef] [Green Version]
- Shelley, V.; Kaiser, S.; Shelley, E.; Williams, T.; Kramer, M.; Haman, K.; Keel, K.; Barton, H. Evaluation of Strategies for the Decontamination of Equipment for Geomyces destructans, the Causative Agent of the White-Nose Syndrome (WNS). J. Cave Karst Stud. 2013, 75, 1–10. [Google Scholar] [CrossRef]
- Ossiboff, R.J.; Towe, A.E.; Brown, M.A.; Longo, A.V.; Lips, K.R.; Miller, D.L.; Carter, E.D.; Gray, M.J.; Frasca, S. Differentiating Batrachochytrium dendrobatidis and B. salamandrivorans in Amphibian Chytridiomycosis Using RNAScope® in Situ Hybridization. Front. Vet. Sci. 2019, 6, 304. [Google Scholar] [CrossRef]
- Fisher, M.C.; Garner, T.W.J.; Walker, S.F. Global Emergence of Batrachochytrium dendrobatidis and Amphibian Chytridiomycosis in Space, Time, and Host. Annu. Rev. Microbiol. 2009, 63, 291–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolby, J.E.; Ramirez, S.D.; Berger, L.; Griffin, D.W.; Jocque, M.; Skerratt, L.F. Presence of Amphibian Chytrid Fungus (Batrachochytrium dendrobatidis) in Rainwater Suggests Aerial Dispersal Is Possible. Aerobiologia 2015, 31, 411–419. [Google Scholar] [CrossRef]
- Feldmeier, S.; Schefczyk, L.; Wagner, N.; Heinemann, G.; Veith, M.; Lötters, S. Exploring the Distribution of the Spreading Lethal Salamander Chytrid Fungus in Its Invasive Range in Europe—A Macroecological Approach. PLoS ONE 2016, 11, e0165682. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.J.; Chinchar, V.G. (Eds.) . Ranaviruses; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Harp, E.M.; Petranka, J.W. Ranavirus in wood frogs (Rana sylvatica): Potential sources of transmission within and between ponds. J. Wildl. Dis. 2006, 42, 307–318. [Google Scholar] [CrossRef] [Green Version]
- Schmeller, D.S.; Courchamp, F.; Killeen, G. Biodiversity Loss, Emerging Pathogens and Human Health Risks. Biodivers. Conserv. 2020, 29, 3095–3102. [Google Scholar] [CrossRef]
- Brunner, J.; Olson, A.; Rice, J.; Meiners, S.; Le Sage, M.; Cundiff, J.; Goldberg, C.; Pessier, A. Ranavirus Infection Dynamics and Shedding in American Bullfrogs: Consequences for Spread and Detection in Trade. Dis. Aquat. Org. 2019, 135, 135–150. [Google Scholar] [CrossRef]
- Bryan, L.; Baldwin, C.; Gray, M.; Miller, D. Efficacy of Select Disinfectants at Inactivating Ranavirus. Dis. Aquat. Org. 2009, 84, 89–94. [Google Scholar] [CrossRef]
- De Stasio, B.T.; Acy, C.N.; Frankel, K.E.; Fritz, G.M.; Lawhun, S.D. Test of disinfection methods for invasive snails and zooplankton: Effects of treatment methods and contaminated materials. Lake Reserv. Manag. 2019, 35, 156–166. [Google Scholar] [CrossRef]
- Root, S.; O’Reilly, C.M. Didymo Control: Increasing the Effectiveness of Decontamination Strategies and Reducing Spread. Fisheries 2012, 37, 440–448. [Google Scholar] [CrossRef]
- Van Rooji, P.; Pasmans, F.; Coen, Y.; Martel, A. Efficacy of chemical disinfectants for the containment of the salamander chytrid fungus Batrachochytrium salamandrivorans. PLoS ONE 2017, 12, e0186269. [Google Scholar] [CrossRef] [Green Version]
- Johnson, M.L.; Berger, L.; Phillips, L.; Speare, R. Fungicidal effects of chemical disinfectants, UV light, desiccation and heat on the amphibian chytrid Batrachochytrium dendrobatidis. Dis. Aquat. Org. 2003, 57, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.G.; Dunn, A.M.; Rosewarne, P.J.; Stebbing, P.D. Invaders in Hot Water: A Simple Decontamination Method to Prevent the Accidental Spread of Aquatic Invasive Non-Native Species. Biol. Invasions 2015, 17, 2287–2297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradbeer, S.J.; Coughlan, N.E.; Cuthbert, R.N.; Crane, K.; Dick, J.T.A.; Caffrey, J.M.; Lucy, F.E.; Renals, T.; Davis, E.; Warren, D.A.; et al. The Effectiveness of Disinfectant and Steam Exposure Treatments to Prevent the Spread of the Highly Invasive Killer Shrimp, Dikerogammarus villosus. Sci. Rep. 2020, 10, 1919. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, N.E.; O’Hara, S.; Crane, K.; Dick, J.T.A.; MacIsaac, H.J.; Cuthbert, R.N. Touch Too Much: Aquatic Disinfectant and Steam Exposure Treatments Can Inhibit Further Spread of Invasive Bloody-Red Mysid Shrimp Hemimysis anomala. Wetl. Ecol. Manag. 2020, 28, 397–402. [Google Scholar] [CrossRef] [Green Version]
- Elzinga, C.L.; Salzer, D.W.; Willoughby, J.W. Measuring & Monitoring Plant Populations; US Department of the Interior, Bureau of Land Management: Washington, DC, USA, 1998; pp. 25–38. [Google Scholar]
- Phillott, A.; Speare, R.; Hines, H.; Skerratt, L.; Meyer, E.; McDonald, K.; Cashins, S.; Mendez, D.; Berger, L. Minimising Exposure of Amphibians to Pathogens during Field Studies. Dis. Aquat. Org. 2010, 92, 175–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gottschalk, S.D.; Karol, K.G. Survivability of Starry Stonewort Bulbils Using Commonly Available Decontamination Strategies. J. Aquat. Plant Manag. 2020, 58, 19–25. [Google Scholar]
- Gold, K.; Reed, P.; Bemis, D.; Miller, D.; Gray, M.; Souza, M. Efficacy of Common Disinfectants and Terbinafine in Inactivating the Growth of Batrachochytrium Dendrobatidis in Culture. Dis. Aquat. Org. 2013, 107, 77–81. [Google Scholar] [CrossRef] [Green Version]




| Disease | Aquatic Invertebrates | Invasive Vegetation |
|---|---|---|
| (Chytrid Fungus (C), Ranavirus (R), Snake Fungal Disease (S)) | (Aquatic (A), Seeds (S)) | |
| Air dry C, R | Air dry | Air dry A |
| Alcohol C | Alcohol | Alcohol A |
| Biocidal C | Bleach and water | Bleach and water A, S |
| Bleach and water C, R, S | Chlorine bleach | Chlorine bleach A |
| Chloramine-T C | Freezing | Freezing A, S |
| Chlorine bleach C, R | Hot water bath | Hot water bath A, S |
| Dettol medical C | Rinse/power wash | Rinse/power wash A |
| Disolol C | Steam | Steam A, S |
| F10 C | Virasure | |
| Hibiscrub C | Virkon Aquatic | |
| Hot water bath C, R | Virkon S® | |
| Kickstart C | ||
| Nolvasan® C, R | ||
| Potassium permanganate solution C | ||
| QUAT-128 C | ||
| Safe4 C | ||
| Sodium Chloride C | ||
| UV light R |
| Ranking Criteria and Definition | A Tier | B Tier | C Tier |
|---|---|---|---|
| Rank of T&E Species: The presence and rank of threatened and endangered species, considering both global and state ranks. | Globally significant | Regional | Not present (to our knowledge) |
| Biodiversity: Natural assemblages of species that exist in a stable state and support ecosystem functions. | High | Moderate | Low |
| Ecosystem Services: Assess the functions of the wetland at their small- and large-scale roles. | Significant and unique | Moderate and multiple | Minimal or singular |
| Availability of Management Actions: Ownership factors influencing current and long-term management strategies such as grazing, as well as the availability of conservation resources and investments. | International, national, or regional | Regional or private | Private |
| Current Quality: Describes the wetland on a spectrum of natural/pristine to degraded/destroyed. | Large and/or intact | Intact or threatened | Low and/or degraded |
| Immediacy/Extent of Threats: Assess the scale and intensity of anthropogenic impact. Scale describes the distribution and extent of threats, and intensity describes their severity. | Minimal | Minimal and threatened | Present and extensive |
| Public Interest: Refers to how much the public is involved, interested, and aware of the wetland. | High | High or moderate | Low |
| Recovery Potential: Recognizes the disturbed and degraded state and approximates the investment of resources needed for the wetland to recover. | Low (not much to recover) | Medium (could benefit from some recovery) | High |
| Monitoring Difficulty: Characteristics that describe the accessibility and feasibility of access, as well as potential temporal and spatial variability difficulties. | Difficult | Difficult or moderate | Moderate or low |
| Tier | Preventative Measures | ||
|---|---|---|---|
| Before Visit | During Visit | After Visit | |
| A: Global and Regional Significance |
|
|
|
| B: High Quality Wetlands |
|
|
|
| C: Low Quality Wetlands |
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bryzek, J.A.; Noe, K.L.; De Silva, S.; MacKenzie, A.; Von Haugg, C.L.; Hartman, D.; McCall, J.E.; Veselka, W., IV; Anderson, J.T. Obligations of Researchers and Managers to Respect Wetlands: Practical Solutions to Minimizing Field Monitoring Impacts. Land 2022, 11, 481. https://doi.org/10.3390/land11040481
Bryzek JA, Noe KL, De Silva S, MacKenzie A, Von Haugg CL, Hartman D, McCall JE, Veselka W IV, Anderson JT. Obligations of Researchers and Managers to Respect Wetlands: Practical Solutions to Minimizing Field Monitoring Impacts. Land. 2022; 11(4):481. https://doi.org/10.3390/land11040481
Chicago/Turabian StyleBryzek, Jessica A., Krista L. Noe, Sindupa De Silva, Andrew MacKenzie, Cindy L. Von Haugg, Donna Hartman, Jordan E. McCall, Walter Veselka, IV, and James T. Anderson. 2022. "Obligations of Researchers and Managers to Respect Wetlands: Practical Solutions to Minimizing Field Monitoring Impacts" Land 11, no. 4: 481. https://doi.org/10.3390/land11040481
APA StyleBryzek, J. A., Noe, K. L., De Silva, S., MacKenzie, A., Von Haugg, C. L., Hartman, D., McCall, J. E., Veselka, W., IV, & Anderson, J. T. (2022). Obligations of Researchers and Managers to Respect Wetlands: Practical Solutions to Minimizing Field Monitoring Impacts. Land, 11(4), 481. https://doi.org/10.3390/land11040481