Sorption of Cu2+ Ions by Bentonite Modified with Al Keggin Cations and Humic Acid in Solutions with pH 4.5
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Al-Pillared Bentonite
2.2. Experiments on the Sorption of Humic Acid by Na- and Al13-bentonite
2.3. Experiment on the Sorption of the Cu2+ on the HA-Al13-Bentonites
2.4. Measurements
3. Results
3.1. Mineral Composition and Properties of Various Bentonite Forms
3.2. Kinetics of Cu2+ Adsorption
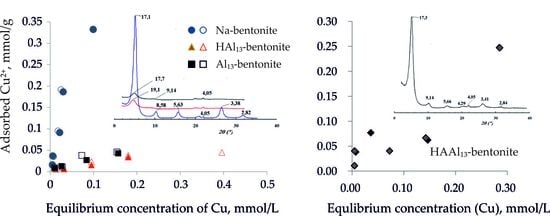
3.3. Sorption of Cu2+ Depending on the Concentration of the Initial Solution
3.4. Sorption of Humic Acid by Na- and Al13-bentonites
3.5. Sorption of Cu2+ Ions by HAAl13-bentonite
4. The Discussion of the Results
4.1. Regularities of Sorption of Cu2+ by Different Bentonite Forms
4.2. Regularities of HA Sorption on Na- and Al13-bentonites
4.3. Sorption of Cu2+ Ions by HAAl13-bentonite
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Churchman, G.J.; Gates, W.P.; Theng, B.K.G.; Yuan, G. Clays and Clay Minerals for Pollution Control. In Handbook of Clay Science; Bergaya, F., Theng, B.K.G., Lagaly, G., Eds.; Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2006; Volume 1, pp. 625–675. [Google Scholar]
- Semenkova, A.S.; Evsiunina, M.V.; Verma, P.K.; Mohapatra, P.K.; Petrov, V.G.; Seregina, I.F.; Bolshov, M.A.; Krupskaya, V.V.; Romanchuk, A.Y.; Kalmykov, S.N. Cs+ sorption onto Kutch clays: Influence of competing ions. Appl. Clay Sci. 2018, 166, 88–93. [Google Scholar] [CrossRef]
- Belousov, P.; Semenkova, A.; Egorova, T.; Romanchuk, A.; Zakusin, S.; Dorzhieva, O.; Tyupina, E.; Izosimova, Y.; Tolpeshta, I.; Chernov, M.; et al. Cesium Sorption and Desorption on Glauconite, Bentonite, Zeolite, and Diatomite. Minerals 2019, 9, 625. [Google Scholar] [CrossRef] [Green Version]
- Semenkova, A.S.; Romanchuk, A.Y.; Krupskaya, V.V.; Pokidko, B.V.; Dorzhieva, O.V.; Sobolev, A.V.; Presnyakov, I.A.; Verma, P.K.; Mohapatra, P.K.; Kalmykov, S.N. Np(V) uptake by various clays. Appl. Geochem. 2018, 92, 1–8. [Google Scholar] [CrossRef]
- Verma, P.K.; Semenkova, A.S.; Krupskaya, V.V.; Zakusin, S.V.; Mohapatra, P.K.; Yu, A.; Kalmykov, S.N. Eu (III) sorption onto various montmorillonites: Experiments and modeling. Appl. Clay Sci. 2019, 175, 22–29. [Google Scholar] [CrossRef]
- Krupskaya, V.V.; Zakusin, S.V.; Tyupina, E.A.; Dorzhieva, O.V.; Zhukhlistov, A.P.; Belousov, P.E.; Timofeeva, M.N. Experimental Study of Montmorillonite Structure and Transformation of its Properties under the Treatment of Inorganic Acid Solutions. Minerals 2017, 7, 49. [Google Scholar] [CrossRef]
- Krupskaya, V.; Novikova, L.; Tyupina, E.; Belousov, P.; Dorzhieva, O.; Zakusin, S.; Kim, K.; Roessner, F.; Badetti, E.; Brunelli, A.; et al. The influence of acid modification on the structure of montmorillonites and surface properties of bentonites. Appl. Clay Sci. 2019. [Google Scholar] [CrossRef]
- Babel, S.; Kurniawan, T.A. Low-cost adsorbents for heavy metals uptake from contaminated water: A review. J. Hazard. Mater. 2003, 97, 219–243. [Google Scholar] [CrossRef]
- Zhao, G.; Wu, X.; Tan, X.; Wang, X. Sorption of Heavy Metal Ions from Aqueous Solutions: A Review. Open Colloid Sci. J. 2011, 4, 19–31. [Google Scholar] [CrossRef]
- Usman, A.R.A.; Kuzyakov, Y.; Stahr, K. Effect of Clay Minerals on Extractability of Heavy Metals and Sewage Sludge Mineralization in Soil. Chem. Ecol. Mon. 2004, 20, 1–13. [Google Scholar] [CrossRef]
- Veli, S.; Alyuz, B. Adsorption of copper and zinc from aqueous solutions by using natural clay. J. Hazard. Mater. 2007, 149, 226–233. [Google Scholar] [CrossRef]
- Stylianou, M.A.; Inglezakis, V.J.; Loizidou, M.D.; Agapiou, A.; Itskos, G. Equilibrium ion exchange studies of Zn2+, Cr3+, and Mn2+ on natural Bentonite. Desalin. Water Treat. 2016, 57, 1–11. [Google Scholar] [CrossRef]
- Aljlil, S.A.; Alsewailem, F.D. Adsorption of Cu & Ni on Bentonite Clay from Waste Water. Athens J. Nat. Form. Sci. 2014, 1, 21–30. [Google Scholar]
- Bourliva, A.; Michailidis, K.; Sikalidis, C.; Filippidis, A.; Betsiou, M. Adsorption of Cd(II), Cu(II), Ni(II) and Pb(II) onto natural bentonite: Study in mono- and multi-metal systems. Environ. Earth Sci. 2015, 73, 5435–5444. [Google Scholar] [CrossRef] [Green Version]
- Budsaereechai, S.; Kamwialisak, K.; Ngernyen, Y. Adsorption of lead, cadmium and copper on natural and acid activated bentonite clay. KKU Res. J. 2012, 17, 800–810. [Google Scholar]
- Melichova, Z.; Hromada, L. Adsorption of Pb2+ and Cu2+ ions from aqueous solutions on natural bentonite. Pol. J. Environ. Stud. 2013, 22, 457–464. [Google Scholar]
- Matthes, W.; Madsen, F.W.; Kahr, G. Sorption of Heavy-Metal Cations by A1 and Zr-Hydroxyintercalated and Pillared Bentonite. Clays Clay Miner. 1999, 47, 617–629. [Google Scholar] [CrossRef]
- Bergaya, F.; Aouad, A.; Mandalia, T. Pillared Clays and Clay Minerals. In Handbook of Clay Science; Bergaya, F., Theng, B.K.G., Lagaly, G., Eds.; Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2006; Volume 1, pp. 393–421. [Google Scholar]
- Karamanis, D.; Assimakopoulos, P.A. Efficiency of aluminum-pillared montmorillonite on the removal of cesium and copper from aqueous solutions. Water Res. 2007, 11, 1897–1906. [Google Scholar] [CrossRef]
- Humelnicu, D.; Ignat, M.; Suchea, M. Evaluation of Adsorption Capacity of Montmorillonite and Aluminium-pillared Clay for Pb2+, Cu2+ and Zn2+. Acta Chim. Slov. 2015, 62, 947–957. [Google Scholar] [CrossRef] [Green Version]
- Wu, P.; Zhang, Q.; Dai, Y.; Zhu, N.; Dang, Z.; Li, P.; Wu, J.; Wang, X. Adsorption of Cu(II), Cd(II) and Cr(III) ions from aqueous solutions on humic acid modified Ca-montmorillonite. Geoderma 2011, 164, 215–219. [Google Scholar] [CrossRef]
- Baik, M.H.; Lee, S.Y. Colloidal stability of bentonite clay considering surface charge properties as a function of pH and ionic strength. J. Ind. Eng. Chem. 2010, 16, 837–841. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, Z.; Han, F. Effects of pH on the gel properties of montmorillonite, palygorskite and montmorillonite-palygorskite composite clay. Appl. Clay Sci. 2020, 190, 105543. [Google Scholar] [CrossRef]
- Timofeeva, M.N.; Panchenko, V.N.; Gil, A.; Chesalov, Y.A.; Sorokina, T.P.; Likholobov, V.A. Synthesis of propylene glycol methyl ether from methanol and propylene oxide over alumina-pillared clays. Appl. Catal. B Environ. 2011, 102, 433–440. [Google Scholar] [CrossRef]
- Christl, I.; Kretzshmar, R. Interaction of copper and fulvic acid at the hematite—Water interface. Geochim. Cosmochim. Acta 2001, 65, 3435–3442. [Google Scholar] [CrossRef]
- Mullassery, M.D.; Fernandez, N.B.; Anirudhan, T.S. Humic Acid removal from Aqueous solution using Aluminium Pillared Bentonite clay and its Recovery. Res. J. Recent Sci. 2016, 5, 71–78. [Google Scholar]
- Xu, J.; Tan, W.; Xiong, J.; Wang, M.; Fang, L.; Koopal, L.K. Copper binding to soil fulvic and humic acids: NICA-Donnan modeling and conditional affinity spectra. J. Colloid Interface Sci. 2016, 473, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Vorobyova, L.A. (Ed.) Theory and Practice Chemical Analysis of Soils; GEOS: Moscow, Russia, 2006; 400p. (In Russian) [Google Scholar]
- Moore, D.M.; Reynolds, C. X-ray Diffraction and the Identification and Analysis of Clay Minerals; Oxford University Press: Oxford, UK, 1989; p. 3321. [Google Scholar]
- Caglar, B.; Afsin, B.; Tabak, A.; Eren, E. Characterization of the cation-exchanged bentonites by XRPD, ATR, DTA/TG analyses and BET measurement. Chem. Eng. J. 2009, 149, 242–248. [Google Scholar] [CrossRef]
- Krupskaya, V.V.; Zakusin, S.V.; Tyupina, E.A.; Dorzhieva, O.V.; Chernov, M.S.; Bychkove, Y.V. Transformation of Structure and Adsorption Properties of Montmorillonite under Thermochemical Treatment. Geochem. Int. 2019, 57, 314–330. [Google Scholar] [CrossRef]
- Osipov, V.I.; Sokolov, V.N. Clays and their Properties. In Composition, Structure, and Formation of Properties; GEOS: Moscow, Russia, 2013; 576p. (In Russian) [Google Scholar]
- Bradl, H.B. Adsorption of heavy metal ions on soils and soils constituents. J. Colloid Interface Sci. 2004, 277, 1–18. [Google Scholar] [CrossRef]
- Liu, X.; Lu, X.; Sprik, M.; Cheng, J.; Meijer, E.J.; Wang, R. Acidity of edge surface sites of montmorillonite and kaolinite. Geochim. Cosmochim. Acta 2013, 117, 180–190. [Google Scholar] [CrossRef] [Green Version]
- Morton, J.D.; Semrau, J.D.; Hayes, K.F. An X-ray absorption spectroscopy study of the structure and reversibility of copper adsorbed to montmorillonite clay. Geochim. Cosmochim. Acta 2001, 65, 2709–2722. [Google Scholar] [CrossRef]
- Stadler, M.; Schindler, P.W. Modeling of H+ and Cu2+ Adsorption on Calcium-Montmorillonite. Clays Clay Miner. 1993, 41, 288–296. [Google Scholar] [CrossRef]
- Carrado, K.A.; Thiyagarajan, P.; Winans, R.E.; Song, K. Anomalous small angle x-ray scattering studies of heavy metal ion solvation behavior in clay minerals. Chem. Mater. 1998, 10, 1130–1134. [Google Scholar] [CrossRef]
- Ijagbemi, C.O.; Baek, M.-H.; Kim, D.-S. Montmorillonite surface properties and sorption characteristics for heavy metal removal from aqueous solutions. J. Hazard. Mater. 2009, 166, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Evans, L.J.; Barabash, S.J. Modeling the adsorption of Cd (II), Cu (II), Ni (II), Pb (II) and Zn (II) onto montmorillonite. Geochim. Cosmochim. Acta 2010, 74, 5718–5728. [Google Scholar] [CrossRef]
- Eren, E.; Afsin, B. An investigation of Cu(II) adsorption by raw and acid-activated bentonite: A combined potentiometric, thermodynamic, XRD, IR, DTA study. J. Hazard. Mater. 2008, 151, 682–691. [Google Scholar] [CrossRef]
- Almeida Neto, A.F.; Vieira, M.G.A.; Silva, M.G.C. Cu(II) Adsorption on Modified Bentonitic Clays: Different Isotherm Behaviors in Static and Dynamic Systems. Mater. Res. 2012, 15, 114–124. [Google Scholar] [CrossRef] [Green Version]
- Tombacz, E.; Szekeres, M. Colloidal behavior of aqueous montmorillonite suspensions: The specific role of pH in the presence of indifferent electrolytes. Appl. Clay Sci. 2004, 27, 75–94. [Google Scholar] [CrossRef]
- Bringle, C.D.; Shibi, I.G.; Vinod, V.P.; Anirudhan, T.S. Sorption on humic acid from aqueous solutions by lanthana-alumina mixed oxide pillared bentonite. J. Sci. Ind. Res. 2005, 46, 782–788. [Google Scholar]
- Zhang, L.; Luo, L.; Zhang, S. Integrated investigations on the adsorption mechanisms of fulvic and humic acids on three clay minerals. Colloids Surfaces A Physicochem. Eng. Aspects 2012, 406, 84–90. [Google Scholar] [CrossRef]
- Kleber, M.; Eusterhues, K.; Keiluweit, M.; Mikutta, C.; Mikutta, R.; Nico, P.S. Mineral–Organic Associations: Formation, Properties, and Relevance in Soil Environments. Edv. Agron. 2015, 130, 1–140. [Google Scholar]
- Wang, S.; Hu, J.; Li, J.; Dong, Y. Influence of pH, soil humic/fulvic acid, ionic strength, foreign ions and addition sequences on adsorption of Pb(II) onto GMZ bentonite. J. Hazard. Mater. 2009, 167, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Koopal, L.K.; Weng, L.; Wang, M.; Tan, W. Effect of soil fulvic and humic acid on binding of Pb to goethite–water interface: Linear additivity and volume fractions of HS in the Stern layer. J. Colloid Interface Sci. 2015, 457, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Elzinga, E.J.; Sparks, D.L. Reaction Condition Effects on Nickel Sorption Mechanisms in Illite–Water Suspensions. Soil Sci. Soc. Am. J. 2001, 65, 94–101. [Google Scholar] [CrossRef] [Green Version]
- Strawn, D.G.; Palmer, N.E.; Furnare, L.J.; Goodell, C.; Amonette, J.E.; Kukkadapu, R.K. Copper Sorption Mechanisms on Smectites. Clays Clay Miner. 2004, 52, 321–333. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, H.; Fan, Q.; Ren, X.; Li, J.; Chen, Y.; Wang, X. Sorption of copper(II) onto super-adsorbent of bentonite–polyacrylamide Composites. J. Hazard. Mater. 2010, 173, 661–668. [Google Scholar] [CrossRef]
- Undabeytia, T.; Nir, S.; Rytwo, G.; Serban, C.; Morillo, E.; Maqueda, C. Modeling Adsorption-Desorption Processes of Cu on Edge and Planar Sites of Montmorillonite. Environ. Sci. Technol. 2002, 36, 2677–2683. [Google Scholar] [CrossRef] [Green Version]










| Bentonite | CEC, cmol(+)/kg | Sorbate | k | n | R2 |
|---|---|---|---|---|---|
| Na | 104 | Cu2+ | 4.9 | 1.0 | 0.90 |
| HA | 0.2 | 1.0 | 0.95 | ||
| HAl13 | 29 | Cu2+ | 0.1 | 0.6 | 0.94 |
| Al13 | 39 | Cu2+ | 0.2 | 0.8 | 0.97 |
| HA | 0.002 | 1.0 | 0.93 | ||
| HAAl13 | Undefined | Cu2+ | 0.3 | 0.5 | 0.76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izosimova, Y.; Tolpeshta, I.; Gurova, I.; Karpukhin, M.; Zakusin, S.; Krupskaya, V. Sorption of Cu2+ Ions by Bentonite Modified with Al Keggin Cations and Humic Acid in Solutions with pH 4.5. Minerals 2020, 10, 1121. https://doi.org/10.3390/min10121121
Izosimova Y, Tolpeshta I, Gurova I, Karpukhin M, Zakusin S, Krupskaya V. Sorption of Cu2+ Ions by Bentonite Modified with Al Keggin Cations and Humic Acid in Solutions with pH 4.5. Minerals. 2020; 10(12):1121. https://doi.org/10.3390/min10121121
Chicago/Turabian StyleIzosimova, Yulia, Inna Tolpeshta, Irina Gurova, Michail Karpukhin, Sergey Zakusin, and Victoria Krupskaya. 2020. "Sorption of Cu2+ Ions by Bentonite Modified with Al Keggin Cations and Humic Acid in Solutions with pH 4.5" Minerals 10, no. 12: 1121. https://doi.org/10.3390/min10121121
APA StyleIzosimova, Y., Tolpeshta, I., Gurova, I., Karpukhin, M., Zakusin, S., & Krupskaya, V. (2020). Sorption of Cu2+ Ions by Bentonite Modified with Al Keggin Cations and Humic Acid in Solutions with pH 4.5. Minerals, 10(12), 1121. https://doi.org/10.3390/min10121121