X-ray Laue Microdiffraction and Raman Spectroscopic Investigation of Natural Silicon and Moissanite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Description
2.2. Data Collection
3. Results
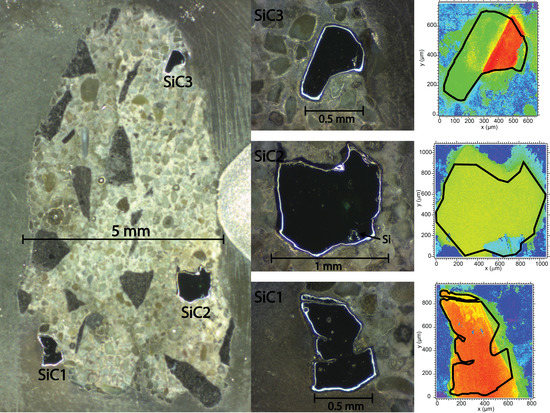
3.1. SiC1
3.2. SiC2
3.3. SiC3
4. Discussion
4.1. SiC Formation—Geological Context
4.2. Stress/Strain Relationships and Crystal Grain Formation
4.3. SiC/Si Relationship
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cheung, R. Silicon Carbide Microelectromechanical Systems for Harsh Environments; Imperial College Press: London, UK, 2006; ISBN 978-1-86094-624-0. [Google Scholar]
- Ramsdell, L.S. Studies on silicon carbide. Am. Mineral. 1947, 32, 64–82. [Google Scholar]
- Jepps, N.W.; Page, T.F. Polytypic transformations in silicon carbide. Prog. Cryst. Growth Charact. 1983, 7, 259–307. [Google Scholar] [CrossRef]
- Powell, J.A.; Will, H.A. Low-temperature solid-state phase transformations in 2H silicon carbide. J. Appl. Phys. 1972, 43, 1400–1408. [Google Scholar] [CrossRef] [Green Version]
- Jagodzinski, H. Polytypism in SiC crystals. Acta Cryst. 1954, 7, 300. [Google Scholar] [CrossRef]
- Haase, V.; Kirschstein, G.; List, H.; Ruprecht, S.; Sangster, R.; Schröder, F.; Töpper, W.; Vanecek, H.; Heit, W.; Schlichting, J.; et al. Si Silicon: System Si-C. SiC: Natural Occurrence. Preparation and Manufacturing Chemistry. Special Forms. Manufacture. Electrochemical Properties. Chemical Reactions. Applications. Ternary and Higher Systems with Si and C; Gmelin Handbook of Inorganic Chemistry; Springer-Verlag Berlin Heidelberg: Berlin, Germany, 1985; Volume B3, ISBN 978-3-662-06994-3. [Google Scholar]
- Burdick, C.L.; Owen, E.A. The structure of carborundum determined by X-rays. J. Am. Chem. Soc. 1918, 40, 1749–1759. [Google Scholar] [CrossRef]
- Thibault, N.W. Morphological and structural crystallography and optical properties of silicon carbide (SiC). Am. Mineral. 1944, 29, 327–362. [Google Scholar]
- Moissan, H. Nouvelles recherches sur la météorité de Cañon Diablo. Comptes rendus 1904, 139, 773–786. [Google Scholar]
- Milton, C.; Vitaliano, D.B. Moissanite SiC, a geological aberration. In Proceedings of the 98th Annual Meeting of the Geological Society of America, Orlando, FL, USA, 14 October 1985; p. 665. [Google Scholar]
- Abderrazak, H.; Hmida, E.S.B.H. Silicon Carbide: Synthesis and Properties. In Properties and Applications of Silicon Carbide; InTech: Rijeka, Croatia, 2011; p. 361. [Google Scholar]
- Lyakhovich, V.V. Origin of accessory moissanite. Int. Geol. Rev. 1980, 22, 961–970. [Google Scholar] [CrossRef]
- Marshintsev, V.K. Nature of silicon carbide in kimberlite rocks of Yakutiya. Mineral. Zhurnal 1990, 12, 17–26. [Google Scholar]
- Leung, I.; Guo, W.; Friedman, I.; Gleason, J. Natural occurrence of silicon carbide in a diamondiferous kimberlite from Fuxian. Nature 1990, 346, 352. [Google Scholar] [CrossRef]
- Qi, X.; Yang, J.; Xu, Z.; Bai, W.; Zhang, Z.; Fang, Q. Discovery of moissanite in retrogressive eclogite from the Pre-pilot Hole of the Chinese Continental Scientific Drilling Project (CCSD-PP2) and its geological implication. Acta Petrol. Sin. 2007, 23, 3207–3214. [Google Scholar]
- Lee, J.-S.; Yu, S.-C.; Tung, S.-F.; Bai, W.-J.; Yang, J.-S.; Fang, Q.-S.; Zhang, Z. The crystal structure of natural 33R moissanite from Tibet. Z. für Krist.–Cryst. Mater. 2009, 221, 213–217. [Google Scholar] [CrossRef]
- Kaminsky, F. Mineralogy of the lower mantle: A review of ‘super-deep’ mineral inclusions in diamond. Earth-Sci. Rev. 2012, 110, 127–147. [Google Scholar] [CrossRef]
- Shiryaev, A.A.; Griffin, W.L.; Stoyanov, E. Moissanite (SiC) from kimberlites: Polytypes, trace elements, inclusions and speculations on origin. Lithos 2011, 122, 152–164. [Google Scholar] [CrossRef]
- Ballhaus, C.; Wirth, R.; Fonseca, R.O.C.; Blanchard, H.; Pröll, W.; Bragagni, A.; Nagel, T.; Schreiber, A.; Dittrich, S.; Thome, V.; et al. Ultra-high pressure and ultra-reduced minerals in ophiolites may form by lightning strikes. Geochem. Perspect. Lett. 2017, 5, 42–46. [Google Scholar] [CrossRef]
- Ballhaus, C.; Blanchard, H.; Fonseca, R.O.C.; Bragagni, A. Reply 2 to Comment on “Ultra-high pressure and ultra-reduced minerals in ophiolites may form by lightning strikes”. Geochem. Perspect. Lett. 2018, 8, 8–10. [Google Scholar] [CrossRef]
- Mathez, E.A.; Fogel, R.A.; Hutcheon, I.D.; Marshintsev, V.K. Carbon isotopic composition and origin of SiC from kimberlites of Yakutia, Russia. Geochim. Cosmochim. Ac. 1995, 59, 781–791. [Google Scholar] [CrossRef]
- Golubkova, A.; Schmidt, M.W.; Connolly, J.A.D. Ultra-reducing conditions in average mantle peridotites and in podiform chromitites: a thermodynamic model for moissanite (SiC) formation. Contrib. Mineral. Petrol. 2016, 171, 41. [Google Scholar] [CrossRef]
- Ohrenschall, R.D.; Milton, C. The occurrence of moissanite (silicon carbide) in sediments. J. Sediment. Res. 1931, 1, 96–99. [Google Scholar]
- Kaminskiy, F.V.; Bukin, V.J.; Potapov, S.V.; Arkus, N.G.; Ivanova, V.G. Discoveries of silicon carbide under natural conditions and their genetic importance. Int. Geol. Rev. 1969, 11, 561–569. [Google Scholar] [CrossRef]
- Ross, J. Kimberlites and Related Rocks; John Wiley & Sons: Hoboken, NJ, USA, 1989; ISBN 978-0-86793-384-0. [Google Scholar]
- Leung, I.S. Silicon carbide cluster entrapped in a diamond from Fuxian, China. Am. Mineral. 1990, 75, 1110–1119. [Google Scholar]
- Wilding, M.C.; Harte, B.; Harris, J.W. Evidence for a deep origin of São Luis diamonds. In CPRM Special Publication 2/91, Proceedings of the Extended Abstracts 5th International Kimberlite Conference; CPRM: Brasília, Brazil, 1991; pp. 456–458. [Google Scholar]
- Svisero, D.P. Distribution and origin of diamonds in Brazil: An overview. J. Geodyn. 1995, 20, 493–514. [Google Scholar] [CrossRef]
- Di Pierro, S.; Gnos, E.; Grobety, B.H.; Armbruster, T.; Bernasconi, S.M.; Ulmer, P. Letters. Rock-forming moissanite (natural α-silicon carbide). Am. Mineral. 2003, 88, 1817–1821. [Google Scholar] [CrossRef]
- Zhang, Z. Native gold and native copper grains enclosed by olivine phenocrysts in a picrite lava of the Emeishan large igneous province, SW China. Am. Mineral. 2006, 91, 1178–1183. [Google Scholar] [CrossRef]
- Klein-BenDavid, O.; Wirth, R.; Navon, O. Micrometer-scale cavities in fibrous and cloudy diamonds—A glance into diamond dissolution events. Earth Planet Sc. Lett. 2007, 264, 89–103. [Google Scholar] [CrossRef]
- Xu, S.; Wu, W.; Xiao, W.; Yang, J.; Chen, J.; Ji, S.; Liu, Y. Moissanite in serpentinite from the Dabie Mountains in China. Mineral. Mag. 2008, 72, 899–908. [Google Scholar] [CrossRef]
- Trumbull, R.B.; Yang, J.-S.; Robinson, P.T.; Di Pierro, S.; Vennemann, T.; Wiedenbeck, M. The carbon isotope composition of natural SiC (moissanite) from the Earth’s mantle: New discoveries from ophiolites. Lithos 2009, 113, 612–620. [Google Scholar] [CrossRef] [Green Version]
- Yusupov, R.G.; Stanley, C.J.; Welch, M.D.; Spratt, J.; Cressey, G.; Rumsey, M.S.; Seltmann, R.; Igamberdiev, E. Mavlyanovite, Mn5Si3: A new mineral species from a lamproite diatreme, Chatkal Ridge, Uzbekistan. Mineral. Mag. 2009, 73, 43–50. [Google Scholar] [CrossRef]
- Fritsch, E.; Toledo, V.; Matlins, A. Record-size natural moissanite crystals discovered in Israel. Gems Gemol. 2014, 50, 160–161. [Google Scholar]
- Liang, F.; Xu, Z.; Zhao, J. In-situ moissanite in dunite: deep mantle origin of mantle peridotite in Luobusa ophiolite, Tibet. Acta Geol. Sin. Engl. Ed. 2014, 88, 517–529. [Google Scholar] [CrossRef]
- Liu, Y.; He, D.; Gao, C.; Foley, S.; Gao, S.; Hu, Z.; Zong, K.; Chen, H. First direct evidence of sedimentary carbonate recycling in subduction-related xenoliths. Sci. Rep. 2015, 5, 11547. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yang, J.; Robinson, P.T.; Xiong, F.; Ba, D.; Guo, G. Origin of ultrahigh pressure and highly reduced minerals in podiform chromitites and associated mantle peridotites of the Luobusa ophiolite, Tibet. Gondwana Res. 2015, 27, 686–700. [Google Scholar] [CrossRef]
- Yang, J.; Meng, F.; Xu, X.; Robinson, P.T.; Dilek, Y.; Makeyev, A.B.; Wirth, R.; Wiedenbeck, M.; Cliff, J. Diamonds, native elements and metal alloys from chromitites of the Ray-Iz ophiolite of the Polar Urals. Gondwana Res. 2015, 27, 459–485. [Google Scholar] [CrossRef]
- Di Pierro, S.; Gnos, E. Ca-Al-silicate inclusions in natural moissanite (SiC). Am. Mineral. 2016, 101, 71–81. [Google Scholar] [CrossRef]
- Dobrzhinetskaya, L.; Mukhin, P.; Wang, Q.; Wirth, R.; O’Bannon, E.; Zhao, W.; Eppelbaum, L.; Sokhonchuk, T. Moissanite (SiC) with metal-silicide and silicon inclusions from tuff of Israel: Raman spectroscopy and electron microscope studies. Lithos 2018, 310–311, 355–368. [Google Scholar] [CrossRef]
- Machev, P.; O’Bannon, E.F.; Bozhilov, K.N.; Wang, Q.; Dobrzhinetskaya, L. Not all moissanites are created equal: New constraints on moissanite from metamorphic rocks of Bulgaria. Earth Planet. Sci. Lett. 2018, 498, 387–396. [Google Scholar] [CrossRef]
- Nazzareni, S.; Nestola, F.; Zanon, V.; Bindi, L.; Scricciolo, E.; Petrelli, M.; Zanatta, M.; Mariotto, G.; Giuli, G. Discovery of moissanite in a peralkaline syenite from the Azores Islands. Lithos 2019, 324–325, 68–73. [Google Scholar] [CrossRef]
- Baer, G.; Aharon, L.; Heimann, A.; Shaliv, G.; Agnon, A. The Nahal Tavor vent: Interplay of Miocene tectonics, dikes, and volcanism in the Lower Galilee, Israel. Isr. J. Earth Sci. 2006, 55, 1–16. [Google Scholar] [CrossRef]
- Kunz, M.; Tamura, N.; Chen, K.; MacDowell, A.A.; Celestre, R.S.; Church, M.M.; Fakra, S.; Domning, E.E.; Glossinger, J.M.; Kirschman, J.L.; et al. A dedicated superbend X-ray microdiffraction beamline for materials, geo-, and environmental sciences at the advanced light source. Rev. Sci. Instrum. 2009, 80, 035108. [Google Scholar] [CrossRef] [Green Version]
- Stan, C.V.; Tamura, N. Synchrotron X-ray Microdiffraction and Fluorescence Imaging of Mineral and Rock Samples. JoVE 2018, in press. [Google Scholar] [CrossRef] [Green Version]
- Tamura, N.; MacDowell, A.A.; Spolenak, R.; Valek, B.C.; Bravman, J.C.; Brown, W.L.; Celestre, R.S.; Padmore, H.A.; Batterman, B.W.; Patel, J.R. Scanning X-ray microdiffraction with submicrometer white beam for strain/stress and orientation mapping in thin films. J. Synchrotron Radiat. 2003, 10, 137–143. [Google Scholar] [CrossRef]
- Merlen, A.; Sangar, A.; Torchio, P.; Kallepalli, L.N.D.; Grojo, D.; Utéza, O.; Delaporte, P. Multi-wavelength enhancement of silicon Raman scattering by nanoscale laser surface ablation. Appl. Surf. Sci. 2013, 284, 545–548. [Google Scholar] [CrossRef]
- Russell, J.P. Raman scattering in silicon. Appl. Phys. Lett. 1965, 6, 223–224. [Google Scholar] [CrossRef]
- Parker, J.H.; Feldman, D.W.; Ashkin, M. Raman Scattering by Silicon and Germanium. Phys. Rev. 1967, 155, 712–714. [Google Scholar] [CrossRef]
- Uchinokura, K.; Sekine, T.; Matsuura, E. Raman scattering by silicon. Solid State Commun. 1972, 11, 47–49. [Google Scholar] [CrossRef]
- Weinstein, B.A.; Piermarini, G.J. Raman scattering and phonon dispersion in Si and GaP at very high pressure. Phys. Rev. B 1975, 12, 1172–1186. [Google Scholar] [CrossRef]
- Poborchii, V.; Tada, T.; Kanayama, T. Study of stress in a shallow-trench-isolated Si structure using polarized confocal near-UV Raman microscopy of its cross section. Appl. Phys. Lett. 2007, 91, 241902. [Google Scholar] [CrossRef]
- Zhang, C.; Duan, Z. A model for C–O–H fluid in the Earth’s mantle. Geochim. et Cosmochim. Acta 2009, 73, 2089–2102. [Google Scholar] [CrossRef]
- Li, Z.; Bradt, R.C. Thermal Expansion of the Hexagonal (6H) Polytype of Silicon Carbide. J. Am. Ceram. Soc. 1986, 69, 863–866. [Google Scholar] [CrossRef]
- Watanabe, H.; Yamada, N.; Okaji, M. Linear Thermal Expansion Coefficient of Silicon from 293 to 1000 K. Int. J. Thermophys. 2004, 25, 221–236. [Google Scholar] [CrossRef]
- Arsenault, R.J.; Shi, N. Dislocation generation due to differences between the coefficients of thermal expansion. Mater. Sci. Eng. 1986, 81, 175–187. [Google Scholar] [CrossRef]
- Vogelsang, M.; Arsenault, R.J.; Fisher, R.M. An in situ HVEM study of dislocation generation at Al/SiC interfaces in metal matrix composites. MTA 1986, 17, 379–389. [Google Scholar] [CrossRef] [Green Version]
- Anastassakis, E.; Pinczuk, A.; Burstein, E.; Pollak, F.H.; Cardona, M. Effect of static uniaxial stress on the Raman spectrum of silicon. Solid State Commun. 1970, 8, 133–138. [Google Scholar] [CrossRef]
- Hart, T.R.; Aggarwal, R.L.; Lax, B. Temperature Dependence of Raman Scattering in Silicon. Phys. Rev. B 1970, 1, 638–642. [Google Scholar] [CrossRef]
- Cerdeira, F.; Buchenauer, C.J.; Pollak, F.H.; Cardona, M. Stress-Induced Shifts of First-Order Raman Frequencies of Diamond- and Zinc-Blende-Type Semiconductors. Phys. Rev. B 1972, 5, 580–593. [Google Scholar] [CrossRef]
- Campbell, I.H.; Fauchet, P.M. The effects of microcrystal size and shape on the one phonon Raman spectra of crystalline semiconductors. Solid State Commun. 1986, 58, 739–741. [Google Scholar] [CrossRef]
- Kouteva-Arguirova, S.; Arguirov, T.; Wolfframm, D.; Reif, J. Influence of local heating on micro-Raman spectroscopy of silicon. J. Appl. Phys. 2003, 94, 4946–4949. [Google Scholar] [CrossRef]
- Georgi, C.; Hecker, M.; Zschech, E. Effects of laser-induced heating on Raman stress measurements of silicon and silicon-germanium structures. J. Appl. Phys. 2007, 101, 123104. [Google Scholar] [CrossRef]
- Yang, Y.; Munck, K.D.; Teixeira, R.C.; Swinnen, B.; Verlinden, B.; Wolf, I.D. Process induced sub-surface damage in mechanically ground silicon wafers. Semicond. Sci. Technol. 2008, 23, 075038. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, D.; Gao, W.; Kang, R. Residual stress analysis on silicon wafer surface layers induced by ultra-precision grinding. Rare Met. 2011, 30, 278–281. [Google Scholar] [CrossRef]
- Xu, Z.; He, Z.; Song, Y.; Fu, X.; Rommel, M.; Luo, X.; Hartmaier, A.; Zhang, J.; Fang, F. Topic Review: Application of Raman Spectroscopy Characterization in Micro/Nano-Machining. Micromachines 2018, 9, 361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, P.T.; Bai, W.-J.; Malpas, J.; Yang, J.-S.; Zhou, M.-F.; Fang, Q.-S.; Hu, X.-F.; Cameron, S.; Staudigel, H. Ultra-high pressure minerals in the Luobusa Ophiolite, Tibet, and their tectonic implications. Geol. Soc. Lond. Spec. Publ. 2004, 226, 247–271. [Google Scholar] [CrossRef]
- Zarudi, I.; Zhang, L. Subsurface damage in single-crystal silicon due to grinding and polishing. J. Mater Sci. Lett. 1996, 15, 586–587. [Google Scholar] [CrossRef]
- Amelinckx, S.; Strumane, G.; Webb, W.W. Dislocations in Silicon Carbide. J. Appl. Phys. 1960, 31, 1359–1370. [Google Scholar] [CrossRef]
- Dobrzhinetskaya, L.F.; Green, H.W.; Bozhilov, K.N.; Mitchell, T.E.; Dickerson, R.M. Crystallization environment of Kazakhstan microdiamond: evidence from nanometric inclusions and mineral associations. J. Metamorph. Geol. 2003, 21, 425–437. [Google Scholar] [CrossRef]
- Kvasnytsya, V.M.; Wirth, R. Nanoinclusions in microdiamonds from Neogenic sands of the Ukraine (Samotkan’ placer): A TEM study. Lithos 2009, 113, 454–464. [Google Scholar] [CrossRef]











| Grain | Map Dimensions (μm2) | Pixel Dimension (μm) |
|---|---|---|
| SiC1 | 798 × 972 | 6 |
| SiC2 | 1064 × 1080 | 8 |
| SiC2 a | 382 × 202 | 2 |
| SiC3 | 665 × 755 | 5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stan, C.V.; O’Bannon, E.F., III; Mukhin, P.; Tamura, N.; Dobrzhinetskaya, L. X-ray Laue Microdiffraction and Raman Spectroscopic Investigation of Natural Silicon and Moissanite. Minerals 2020, 10, 204. https://doi.org/10.3390/min10030204
Stan CV, O’Bannon EF III, Mukhin P, Tamura N, Dobrzhinetskaya L. X-ray Laue Microdiffraction and Raman Spectroscopic Investigation of Natural Silicon and Moissanite. Minerals. 2020; 10(3):204. https://doi.org/10.3390/min10030204
Chicago/Turabian StyleStan, Camelia Veronica, Earl Francis O’Bannon, III, Pavel Mukhin, Nobumichi Tamura, and Larissa Dobrzhinetskaya. 2020. "X-ray Laue Microdiffraction and Raman Spectroscopic Investigation of Natural Silicon and Moissanite" Minerals 10, no. 3: 204. https://doi.org/10.3390/min10030204
APA StyleStan, C. V., O’Bannon, E. F., III, Mukhin, P., Tamura, N., & Dobrzhinetskaya, L. (2020). X-ray Laue Microdiffraction and Raman Spectroscopic Investigation of Natural Silicon and Moissanite. Minerals, 10(3), 204. https://doi.org/10.3390/min10030204