Vibrational Investigation of Pressure-Induced Phase Transitions of Hydroxycarbonate Malachite Cu2(CO3)(OH)2
Abstract
:1. Introduction
2. Samples and Experiments
3. Results
3.1. Vibrational Characteristics of Malachite at Atmospheric Pressure and Room Temperature
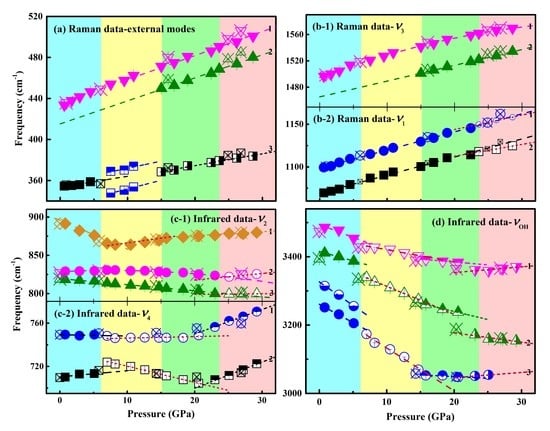
3.2. Vibrational Changes of Malachite under Pressure
4. Discussion
4.1. Structural Transitions of Malachite under Pressure
4.2. Expectations on Hydroxycarbonates in the Earth’s Mantle
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ardia, P.; Hirschmann, M.M.; Withers, A.C.; Tenner, T.J. H2O storage capacity of olivine at 5–8 GPa and consequences for dehydration partial melting of the upper mantle. Earth Planet Sci. Lett. 2012, 345, 104–116. [Google Scholar] [CrossRef]
- Revenaugh, J.; Sipkin, S.A. Seismic evidence for silicate melt atop the 410-km mantle discontinuity. Nature 1994, 369, 474–476. [Google Scholar] [CrossRef]
- Lawrence, J.F.; Wysession, M.E. Seismic evidence for subduction-transported water in the lower mantle. Earth’s Deep Water Cycle. AGU 2013, 168, 251–261. [Google Scholar]
- Frost, D.J. The stability of dense hydrous magnesium silicates in Earth’s transition zone and lower mantle. Mantle Petrology: Field observations and high pressure experimentation: A tribute to francis R.(Joe) Boyd. Geochem. Soc. 1999, 6, 283–296. [Google Scholar]
- Nishi, M.; Irifune, T.; Tsuchiya, J.; Tange, Y.; Nishihara, Y.; Fujino, K.; Higo, Y. Stability of hydrous silicate at high pressures and water transport to the deep lower mantle. Nat. Geosci. 2014, 7, 224–227. [Google Scholar] [CrossRef]
- Ohtani, E.; Livasov, A.K.; Haosoya, T.; Kubo, T.; Kondo, T. Water transport into the deep mantle and formation of a hydrous transition zone. Phys. Earth Planet. Int. 2004, 143, 255–269. [Google Scholar] [CrossRef]
- Ringwood, A.E.; Major, A. High-pressure reconnaissance investigations in the system Mg2SiO4–MgO–H2O. Earth Planet. Sci. Lett. 1967, 2, 130–133. [Google Scholar] [CrossRef]
- Ghosh, S.; Schmidt, M.W. Melting of phase D in the lower mantle and implications for recycling and storage of H2O in the deep mantle. Geochim. Cosmochim. Acta 2014, 145, 72–88. [Google Scholar] [CrossRef]
- Ohtani, E.; Litasov, K.; Suzuki, A.; Kondo, T. Stability field of new hydrous phase, δ-AlOOH, with implications for water transport into the deep mantle. Geophys. Res. Lett. 2001, 28, 3991–3993. [Google Scholar] [CrossRef]
- Ohira, I.; Ohtani, E.; Sakai, T.; Miyahara, M.; Hirao, N.; Ohishi, Y.; Nishijima, M. Stability of a hydrous δ-phase, AlOOH-MgSiO2(OH)2, and a mechanism for water transport into the base of lower mantle. Earth Planet. Sci. Lett. 2014, 401, 12–17. [Google Scholar] [CrossRef]
- Huang, X.G.; Xu, Y.S.; Karato, S.I. Water content in the transition zone from electrical conductivity of wadsleyite and ringwoodite. Nature 2005, 434, 746–749. [Google Scholar] [CrossRef] [PubMed]
- Karato, S. Water distribution across the mantle transition zone and its implications for global material circulation. Earth Planet. Sci. Lett. 2011, 301, 413–423. [Google Scholar] [CrossRef]
- Pearson, D.G.; Brenker, F.E.; Nestola, F.; McNeill, J.; Nasdala, L.; Hutchison, M.T.; Matveev, S.; Mather, K.; Silversmit, G.; Schmitz, S.; et al. Hydrous mantle transition zone indicated by ring-woodite included within diamond. Nature 2014, 507, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.A. Oxide Zone Geochemistry; Ellis Horwood Ltd.: Chichester, UK, 1990. [Google Scholar]
- Schmidt, M.; Lutz, H.D. Hydrogen bonding in basic copper salts: A spectroscopic study of malachite, Cu2(OH)2CO3, and brochantite, Cu4(OH)6SO4. Phys. Chem. Miner. 1993, 20, 27–32. [Google Scholar] [CrossRef]
- Zigan, F.; Joswig, W.; Schuster, H.U.; Mason, S.A. Verfeinerung der Struktur von Malachit, Cu2(OH)2CO3, durch Neutronenbeugung. Z. Kristallogr. 1977, 145, 412–426. [Google Scholar] [CrossRef]
- Kiseleva, I.A.; Ogorodova, L.P.; Melchakova, L.V.; Bisengalieva, M.R.; Becturganov, N.S. Thermodynamic properties of copper carbonates-malachite Cu2(CO3)(OH)2 and azurite Cu3(CO3)2(OH)2. Phys. Chem. Miner. 1992, 19, 322–333. [Google Scholar] [CrossRef]
- Brown, I.W.M.; Mackenzie, K.J.D.; Gainsford, G.J. Thermal decomposition of the basic copper carbonates malachite and azurite. Thermochim. Acta 1984, 74, 23–32. [Google Scholar] [CrossRef]
- Lee, Y.; Vogt, T.; Hriljac, J.A.; Parise, J.B.; Hanson, J.C.; Kimk, S.J. Non-framework cation migration and irreversible pressure-induced hydration in a zeolite. Nature 2002, 420, 485–489. [Google Scholar] [CrossRef]
- Merlini, M.; Perchiazzi, N.; Hanfland, M.; Bossak, A. Phase transition at high pressure in Cu2CO3(OH)2 related to the reduction of the Jahn-Teller effect. Acta Crystallogr. B 2012, 68, 266–274. [Google Scholar] [CrossRef]
- Lebernegg, S.; Tsirlin, A.A.; Janson, O.; Rosner, H. The spin gap in malachite Cu2(OH)2CO3 and its evolution under pressure. Phys. Rev. B 2013, 256070369. [Google Scholar]
- Mao, H.K.; Shen, G.; Hemley, G.J.; Duffy, T.S. X-ray diffraction with a double hot-plate laser-heated diamond cell. In Properties of the Earth and Planetary Materials at High Pressure and Temperature, American Geophysical Union; Manghnani, M.H., Yagi, T., Eds.; American Geophysical Union: Washington, DC, USA, 1998; pp. 27–34. [Google Scholar]
- Mao, H.K.; Xu, J.A.; Bell, P.M. Calibration of the ruby pressure gauge to 800 kbar. J. Geophys. Res. 1986, 91, 4673–4676. [Google Scholar] [CrossRef]
- Bouchard, M.; Smith, D.C. Catalogue of 45 reference Raman spectra of minerals concerning research in art history or archaeology, especially on corroded metals and coloured glass. Spectrochim. Acta A 2003, 59, 2247–2266. [Google Scholar] [CrossRef]
- Frost, R.L.; Martens, W.N.; Rintoul, L.; Mahmutagic, E.; Kloprogge, J.T. Raman spectroscopic study of azurite and malachite at 298 and 77 K. J. Raman Spectrosc. 2002, 33, 252–259. [Google Scholar] [CrossRef] [Green Version]
- Buzgar, N.; Apopei, A.I. The Raman study of certain carbonates. Analele Stiintifice Ale Universitatii AL I Cuza Iasi. Geologie 2009, 55, 97–112. [Google Scholar]
- Yu, B.S.; Fang, J.N.; Huang, E.P. Characteristics of the Raman spectra of archaeological Malachites. J. Raman Spectrosc. 2013, 44, 630–636. [Google Scholar] [CrossRef]
- Goldsmith, J.A.; Ross, S.D. The infra-red spectra of azurite and malachite. Spectrochim. Acta A 1968, 24, 2131–2137. [Google Scholar] [CrossRef]
- Stoilova, D.; Koleva, V.; Vassileva, V. Infrared study of some synthetic phases of malachite (Cu2(OH)2CO3)-hydrozincite (Zn5(OH)6(CO3)2) series. Spectrochim. Acta A 2002, 58, 2051–2059. [Google Scholar] [CrossRef]
- Efthimiopoulos, I.; Jahn, S.; Kuras, A.; Schade, U.; Koch-Müller, M. Combined high-pressure and high-temperature vibrational studies of dolomite: Phase diagram and evidence of a new distorted modification. Phys. Chem. Miner. 2017, 44, 465–476. [Google Scholar] [CrossRef]
- Gao, J.; Wu, X.; Qin, S.; Li, Y.C. Pressure-induced phase transformations of PbCO3 by x-ray diffraction and Raman spectroscopy. High Pressure Res. 2016, 1, 1–15. [Google Scholar] [CrossRef]
- Gillet, P.; Biellmann, C.; Reynard, B.; McMillan, P. Raman spectroscopic studies of carbonates; part I, high-pressure and high-temperature behavior of calcite, magnesite, dolomite and aragonite. Phys. Chem. Miner. 1993, 20, 1–18. [Google Scholar] [CrossRef]
- Kraft, S.; Knittle, E.; Williams, Q. Carbonate stability in the Earth’s mantle: A vibrational spectroscopic study of aragonite and dolomite at high pressures and temperatures. J. Geophys. Res. 1991, 96, 17997–18009. [Google Scholar] [CrossRef]
- Catalli, K.; Williams, Q. A high-pressure phase transition of calcite-III. Am. Mineral. 2005, 90, 437–444. [Google Scholar] [CrossRef]
- Liu, Z.X.; Lager, G.A.; Hemley, R.J.; Ross, N.L. Synchrotron infrared spectroscopy of OH-chondrodite and OH-clinohumite at high pressure. Am. Mineral. 2003, 88, 1412–1415. [Google Scholar] [CrossRef]
- Mattei, E.; De Vivo, G.; De Santis, A.; Gaetani, C.; Pelosi, C.; Santamaria, U. Raman spectroscopic analysis of azurite blackening. J. Raman Spectrosc. 2008, 39, 302–306. [Google Scholar] [CrossRef]
- White, W.B. The carbonate minerals. In The Infrared Spectra of Minerals; Farmer, V.C., Ed.; Mineralogical Society Monograph: London, UK, 1974; pp. 227–279. [Google Scholar]
- Fiquet, G.; Guyot, F.; Kunz, M.; Matas, J.; Andrault, D.; Hanfland, M. Structural refinements of magnesite at very high pressure. Am. Mineral. 2002, 87, 1261–1265. [Google Scholar] [CrossRef]
- Santillan, J.; Catalli, K.; Williams, Q. An infrared study of carbon-oxygen bond distance in magnesite from 0 to 60 GPa. Am. Mineral. 2005, 90, 1669–1673. [Google Scholar] [CrossRef]
- Williams, Q.; Jeanloz, R. Static amorphization of anorthite at 300K and comparison with diaplectic glass. Nature 1989, 338, 413–415. [Google Scholar] [CrossRef]
- Williams, Q.; Iratittle, E.; Reichtin, R.; Martin, S.; Jeanloz, R. Structural and electronic properties of Fe2SiO4-fayalite at ultra-high pressures: Amorphization and gapclosure. J. Geophys. Res. 1990, 95, 21549–21563. [Google Scholar] [CrossRef]
- Rundle, R.E.; Parasol, M. O–H stretching frequencies in very short and possibly symmetrical hydrogen Bonds. J. Chem. Phys. 1952, 20, 1487–1488. [Google Scholar] [CrossRef]
- Libowitzky, E. Correlation of O–H stretching frequencies and O–H–O hydrogen bond lengths in minerals. Monatsh. Chem. 1999, 130, 1047–1059. [Google Scholar]
- Hofmeister, A.M.; Cynn, H.; Burnley, P.C.; Meade, C. Vibrational spectra of dense, hydrous magnesium silicates at high pressure: Importance of the hydrogen bond angle. Am. Mineral. 1999, 84, 454–464. [Google Scholar] [CrossRef]
- Kleppe, A.K.; Jephcoat, A.P.; Welch, M.D. The effect of pressure upon hydrogen bonding in chlorite: A Raman spectroscopic study of clinochlore to 26.5 GPa. Am. Mineral. 2003, 88, 567–573. [Google Scholar] [CrossRef]
- Liu, L.G.; Lin, C.C.; Mernagh, T.P. Raman spectra of norbergite at various pressures and temperatures. Eur. J. Mineral. 1999, 11, 1011–1021. [Google Scholar] [CrossRef] [Green Version]
- Lutz, H.D. Hydroxide ions in condensed materials-correlation of spectroscopic and structural data. Struct. Bond. 1995, 82, 86–103. [Google Scholar]
- Wirth, R.; Vollmer, C.; Brenker, F.; Matsyuk, S.; Kaminsky, F. Nanocrystalline hydrous aluminium silicate in superdeep diamonds from Juina (Mato Grosso State, Brazil). Earth Planet. Sci. Lett. 2007, 259, 384–399. [Google Scholar] [CrossRef]
- Palot, M.; Jacobsen, S.D.; Townsend, J.P.; Nestola, F.; Marquardt, K.; Miyajima, N.; Harris, J.W.; Stachel, T.; McCammon, C.A.; Pearson, D.G. Evidence for H2O-bearing fluids in the lower mantle from diamond inclusion. Lithos 2016, 265, 237–3243. [Google Scholar] [CrossRef] [Green Version]





| P (GPa) | External Modes | O−C−O ν1 | O−C−O ν3 | |||||
|---|---|---|---|---|---|---|---|---|
| 0.6 | 354.56 | 433.56 | 1069.50 | 1099.28 | 1496.66 | |||
| 1.7 | 355.09 | 437.95 | 1071.38 | 1100.94 | 1500.27 | |||
| 2.9 | 355.91 | 441.62 | 1073.93 | 1105.19 | 1504.72 | |||
| 4.5 | 358.75 | 446.55 | 1079.33 | 1109.41 | 1513.64 | |||
| 7.5 | 347.80 | 358.99 | 453.90 | 1086.83 | 1115.00 | 1521.14 | ||
| 9.5 | 350.52 | 370.21 | 457.65 | 1090.27 | 1118.80 | 1527.12 | ||
| 10.9 | 353.64 | 373.91 | 462.45 | 1094.03 | 1122.41 | 1532.52 | ||
| 15.0 | 368.41 | 449.79 | 471.24 | 1100.35 | 1129.64 | 1541.75 | 1501.10 | |
| 16.9 | 370.14 | 452.75 | 474.92 | 1105.21 | 1133.88 | 1546.34 | 1506.03 | |
| 18.9 | 374.39 | 457.62 | 480.92 | 1110.69 | 1139.89 | 1552.74 | 1511.19 | |
| 22.5 | 377.48 | 464.41 | 486.29 | 1114.10 | 1145.50 | 1559.07 | 1518.74 | |
| 23.6 | 379.38 | 468.64 | 490.66 | 1117.88 | 1149.70 | 1561.93 | 1521.73 | |
| 26.2 | 381.76 | 473.72 | 495.23 | 1120.47 | 1154.93 | 1566.39 | 1527.64 | |
| 28.9 | 383.71 | 480.19 | 500.75 | 1124.82 | 1158.14 | 1569.50 | 1534.45 | |
| 26.8 | 386.69 | 485.05 | 505.92 | 1129.56 | 1162.06 | 1570.15 | 1533.91 | |
| 24.9 | 384.44 | 478.48 | 498.02 | 1121.13 | 1152.00 | 1566.88 | 1529.47 | |
| 16.0 | 372.40 | 458.21 | 479.98 | 1104.70 | 1134.86 | 1548.08 | 1505.95 | |
| 6.0 | 356.88 | 448.97 | 1082.56 | 1113.29 | 1519.00 | |||
| 0.9 | 355.65 | 435.62 | 1068.85 | 1100.42 | 1498.64 | |||
| P (GPa) | O−C−O ν4 | O−C−O ν2 | Cu−OH Bending | O−C−O ν1 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.8 | 710.17 | 749.42 | 818.26 | 829.03 | 891.52 | 1039.82 | 1056.97 | 1100.39 | 1111.28 | |
| 2.9 | 712.92 | 748.41 | 817.36 | 829.29 | 882.33 | 1046.21 | 1065.26 | 1107.36 | 1124.71 | |
| 5.1 | 713.42 | 749.39 | 816.44 | 829.77 | 875.99 | 1049.82 | 1070.01 | 1111.64 | 1131.22 | |
| 7.0 | 723.92 | 748.22 | 813.76 | 830.70 | 865.66 | 1054.37 | 1076.79 | 1124.79 | 1149.76 | |
| 8.2 | 722.26 | 746.22 | 812.63 | 830.74 | 863.87 | 1057.79 | 1079.18 | 1131.01 | 1160.99 | |
| 10.5 | 716.99 | 746.54 | 810.98 | 829.79 | 863.95 | 1062.25 | 1083.73 | 1142.73 | 1173.99 | |
| 12.6 | 717.95 | 746.61 | 809.86 | 829.82 | 867.35 | 1070.32 | 1090.08 | 1150.55 | 1182.68 | |
| 14.7 | 712.98 | 746.47 | 808.56 | 828.74 | 869.47 | 1076.35 | 1092.58 | 1153.96 | 1184.35 | |
| 15.8 | 711.33 | 746.60 | 806.78 | 827.57 | 872.52 | 1083.36 | 1098.38 | 1159.50 | 1183.22 | 1206.96 |
| 18.3 | 707.77 | 746.44 | 805.66 | 827.46 | 873.88 | 1086.35 | 1102.45 | 1159.57 | 1183.53 | 1210.16 |
| 20.6 | 704.43 | 748.79 | 803.84 | 825.34 | 875.02 | 1096.77 | 1111.82 | 1164.97 | 1183.63 | 1213.12 |
| 23.0 | 707.69 | 756.48 | 800.00 | 823.64 | 876.26 | 1105.94 | 1121.93 | 1167.55 | 1186.86 | 1215.88 |
| 25.1 | 711.64 | 762.12 | 799.51 | 821.99 | 878.55 | 1109.41 | 1125.73 | 1170.96 | 1188.74 | 1217.97 |
| 27.2 | 716.95 | 766.21 | 799.43 | 824.12 | 878.15 | 1118.28 | 1131.59 | 1173.50 | 1190.95 | 1219.78 |
| 29.2 | 722.55 | 770.75 | 799.87 | 825.47 | 879.81 | 1123.93 | 1135.84 | 1176.57 | 1193.68 | 1221.79 |
| 26.9 | 714.22 | 759.66 | 800.69 | 825.85 | 879.56 | 1118.24 | 1131.29 | 1173.96 | 1192.74 | 1221.59 |
| 20.3 | 710.25 | 755.23 | 804.23 | 825.06 | 875.56 | 1098.10 | 1115.32 | 1168.50 | 1185.95 | 1212.56 |
| 14.3 | 713.56 | 751.03 | 809.32 | 827.55 | 870.62 | 1079.43 | 1094.95 | 1156.57 | 1179.80 | |
| 5.8 | 716.23 | 750.66 | 816.22 | 829.56 | 868.65 | 1056.38 | 1075.70 | 1124.97 | 1146.75 | |
| 0.0001 | 709.66 | 749.32 | 818.92 | 827.20 | 891.36 | 1044.12 | 1058.73 | 1100.55 | 1110.08 | |
| P(GPa) | O–C–O ν3 | O–H Stretches | ||||||||
| 0.8 | 1384.52 | 1423.33 | 1484.29 | 1510.79 | 1544.45 | 3251.55 | 3410.79 | 3485.88 | ||
| 2.9 | 1391.70 | 1427.24 | 1492.21 | 1514.55 | 1550.64 | 3231.31 | 3399.94 | 3477.70 | ||
| 5.1 | 1407.78 | 1440.58 | 1509.61 | 1535.22 | 1564.79 | 3205.45 | 3387.13 | 3453.68 | ||
| 7.0 | 1413.73 | 1447.89 | 1512.54 | 1538.38 | 1570.04 | 3173.99 | 3339.44 | 3429.39 | ||
| 8.2 | 1415.83 | 1448.94 | 1512.94 | 1541.26 | 1571.82 | 3148.03 | 3325.98 | 3427.53 | ||
| 10.5 | 1425.18 | 1456.82 | 1516.06 | 1543.60 | 1574.57 | 3128.78 | 3309.45 | 3421.65 | ||
| 12.6 | 1428.83 | 1460.45 | 1519.62 | 1547.71 | 1579.84 | 3106.58 | 3292.62 | 3411.01 | ||
| 14.7 | 1426.93 | 1460.86 | 1520.14 | 1550.95 | 1581.95 | 3069.74 | 3274.80 | 3402.42 | ||
| 15.8 | 1442.91 | 1468.96 | 1526.70 | 1554.42 | 1587.96 | 3062.83 | 3260.33 | 3391.76 | ||
| 18.3 | 1449.75 | 1473.62 | 1543.34 | 1579.11 | 1602.90 | 3042.73 | 3245.98 | 3386.89 | ||
| 20.6 | 1454.14 | 1480.85 | 1547.90 | 1582.65 | 1605.90 | 3047.87 | 3237.83 | 3383.73 | ||
| 23.0 | 1461.73 | 1499.70 | 1552.97 | 1587.07 | 1611.39 | 3051.26 | 3171.74 | 3361.45 | ||
| 25.1 | 1483.70 | 1510.38 | 1572.76 | 1609.05 | 1636.17 | 3054.36 | 3159.19 | 3358.61 | ||
| 27.2 | 1492.88 | 1511.71 | 1579.78 | 1609.45 | 1639.02 | 3157.10 | 3364.77 | |||
| 29.2 | 1504.24 | 1536.99 | 1582.26 | 1610.19 | 1643.81 | 3154.02 | 3370.67 | |||
| 26.9 | 1495.70 | 1515.07 | 1582.39 | 1610.05 | 1638.81 | 3159.92 | 3366.19 | |||
| 20.3 | 1458.88 | 1484.03 | 1552.76 | 1585.45 | 1620.50 | 3048.75 | 3187.10 | 3364.96 | ||
| 14.3 | 1444.24 | 1468.23 | 1526.67 | 1555.19 | 1590.31 | 3057.87 | 3264.41 | 3389.67 | ||
| 5.8 | 1416.65 | 1445.09 | 1511.61 | 1539.60 | 1569.15 | 3334.45 | 3428.01 | |||
| 0.0001 | 1382.23 | 1421.92 | 1484.97 | 1512.94 | 1542.54 | 3393.86 | 3473.88 | |||
| Assignments | Modes | 0–7 GPa | 7–15 GPa | 15–23 GPa | >~23 GPa |
|---|---|---|---|---|---|
| Cu–O vibrations | νCu-O1 | 2.34(3) | 2.34(3) | 2.34(3) | 2.34(3) |
| νCu-O2 | - | - | 2.24(5) | 2.24(5) | |
| νCu-O3 | 1.06(3) | 1.38(5)/1.69(4) | 1.15(6) | 1.15(6) | |
| O–C–O vibrations | ν3-1 | ~3.5 | ~2.9 | ~2.2 | ~1.6 |
| ν3-2 | - | - | 2.39(3) | 2.39(3) | |
| ν1-1 | 2.12(3) | 2.12(3) | 2.12(3) | 1.69(6) | |
| ν1-2 | 2.12(4) | 2.12(4) | 2.12(4) | 1.38(8) | |
| ν2-1 | −3.60(4) | 0.92(8) | 0.50(2) | 0.50(2) | |
| ν2-2 | ~0.2 | ~−0.2 | −0.60(1) | 0.85(4) | |
| ν2-3 | −0.79(2) | −0.79(2) | −0.79(2) | 0.08(1) | |
| ν4-1 | −0.12(7) | 0.13(2) | 0.13(2) | 2.25(7) | |
| ν4-2 | 0.56(8) | −1.48(4) | −1.48(4) | 2.40(5) | |
| O–H stretches | νOH-1 | −7.52(9) | −3.51(3) | −1.58(1) | 1.61(4) |
| νOH-2 | −5.54(1) | −8.13(2) | −4.71(4) | −2.78(6) | |
| νOH-3 | −10.72(6) | −12.58(4) | −1.03(2) | 1.54(1) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Yuan, X. Vibrational Investigation of Pressure-Induced Phase Transitions of Hydroxycarbonate Malachite Cu2(CO3)(OH)2. Minerals 2020, 10, 277. https://doi.org/10.3390/min10030277
Gao J, Yuan X. Vibrational Investigation of Pressure-Induced Phase Transitions of Hydroxycarbonate Malachite Cu2(CO3)(OH)2. Minerals. 2020; 10(3):277. https://doi.org/10.3390/min10030277
Chicago/Turabian StyleGao, Jing, and Xueyin Yuan. 2020. "Vibrational Investigation of Pressure-Induced Phase Transitions of Hydroxycarbonate Malachite Cu2(CO3)(OH)2" Minerals 10, no. 3: 277. https://doi.org/10.3390/min10030277
APA StyleGao, J., & Yuan, X. (2020). Vibrational Investigation of Pressure-Induced Phase Transitions of Hydroxycarbonate Malachite Cu2(CO3)(OH)2. Minerals, 10(3), 277. https://doi.org/10.3390/min10030277