Nano-Phase KNa(Si6Al2)O16 in Adularia: A New Member in the Alkali Feldspar Series with Ordered K–Na Distribution
Abstract
:1. Introduction
2. Sample and Experimental Methods
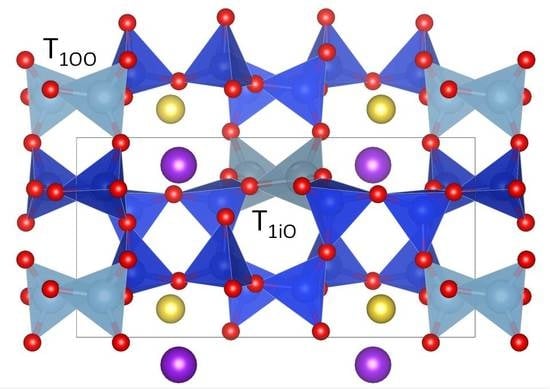
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smith, J.V.; MacKenzie, W. The alkali feldspars V. The nature of orthoclase and microcline perthites and observations concerning the polymorphism of potassium feldspar. Am. Mineral. 1959, 44, 1169–1186. [Google Scholar]
- Goldsmith, J.R.; Laves, F. The sodium content of microclines and the microcline-albite series. Inst. Lucas Mallada C.S.I.C. 1961, 8, 81–96. [Google Scholar]
- Colville, A.A.; Ribbe, P.H. The crystal structure of an adularia and a refinement of the structure of orthoclase. Am. Mineral. 1968, 53, 25–37. [Google Scholar]
- Phillips, M.W.; Ribbe, P.H. The structures of monoclinic potassium-rich feldspars. Am. Mineral. 1973, 58, 263–270. [Google Scholar]
- Blasi, A.; Blasi, C.D.P. Aspects of alkali feldspar characterization: Prospects and relevance to problems outstanding. In Feldspars and Their Reactions; Parsons, I., Ed.; Springer: Boston, MA, USA, 1994; pp. 51–101. [Google Scholar]
- Laves, F.; Goldsmith, J. Comments on the anorthite papers by Megaw and co-workers presented at this symposium. Estudos Geologicos C.S.I.C. 1961, 8, 155–157. [Google Scholar]
- McConnell, J. Electron optical study of effects associated with partial inversion in a silicate phase. Philos. Mag. 1965, 11, 1289–1301. [Google Scholar] [CrossRef]
- McConnell, J. Hallimond Lecture 1970 Electron-Optical study of phase transformations. Mineral. Mag. 1971, 38, 1–20. [Google Scholar] [CrossRef]
- Smith, J.V. Chemical and textural properties. In Feldspar Minerals; Springer: Berlin, Germany, 1974; Volume 2, pp. 152–195. [Google Scholar]
- Smith, J.V.; Brown, W.L. Diffusion, growth, twins and intergrowths. In Feldspar Minerals; Springer: Berlin, Germany, 1988; Volume 1, pp. 398–647. [Google Scholar]
- Hill, T.R. High-Resolution Transmission Electron Microscopy Investigation of Nano-Crystals of Pyroxene and Copper in Oregon Sunstones. Ph.D. Thesis, University of Wisconsin-Madison, Madison, WI, USA, 2009. [Google Scholar]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Shen, Z.; Konishi, H.; Luo, G. Crystal structure of Guinier-Preston zones in orthopyroxene: Z-contrast imaging and ab inito study. Am. Mineral. 2014, 99, 2043–2048. [Google Scholar] [CrossRef]
- Champness, P.; Lorimer, G. Precipitation (exsolution) in an orthopyroxene. J. Mater. Sci. 1973, 8, 467–474. [Google Scholar] [CrossRef]
- Medaris, L.G., Jr.; Driese, S.G.; Stinchcomb, G.E.; Fournelle, J.H.; Lee, S.; Xu, H.; DiPietro, L.; Gopon, P.; Stewart, E.K. Anatomy of a Sub-Cambrian Paleosol in Wisconsin: Mass fluxes of chemical weathering and climatic conditions in North America during formation of the Cambrian Great Unconformity. J. Geol. 2018, 126, 261–283. [Google Scholar]
- Kimata, M.; Saito, S.; Shimizu, M.; Iida, I.; Matsui, T. Low-temperature crystal structures of orthoclase and sanidine. Neues Jahrb. Mineral. Abh. 1996, 171, 199–213. [Google Scholar]
- Zilczer, J.; Loiacono, G. Non-centrosymmetry tests for potassium feldspars: An application of the second-harmonic generator technique. J. Appl. Cryst. 1982, 15, 540–541. [Google Scholar] [CrossRef]
- Hovis, G.L. A refined view of the thermodynamic mixing quantities for alkali feldspars and the quandary of excess configurational entropy. Am. J. Sci. 2017, 317, 597–640. [Google Scholar] [CrossRef]
- Yund, R.; Hofmann, A.; Giletti, B.; Yoder, H. Coherent exsolution in the alkali feldspars. In Geochemical Transport and Kinetics; Hofmann, A.W., Giletti, B.J., Yoder, H.S., Jr., Yund, R.A., Eds.; Carnegie Institution of Washington Publication: Washington, DC, USA, 1974; pp. 173–183. [Google Scholar]







| Label | Atom | x | y | z |
|---|---|---|---|---|
| Mo | Na | 0.76710 | 0.73584 | 0.14141 |
| M2 | K | 0.72382 | 0.24384 | 0.86156 |
| T1oo | Al | 0.99905 | 0.43201 | 0.20296 |
| T1o2 | Si | 0.98176 | 0.42819 | 0.75421 |
| T1io | Al | 0.50176 | 0.93246 | 0.79588 |
| T1i2 | Si | 0.51655 | 0.92662 | 0.24160 |
| T2oo | Si | 0.29038 | 0.36899 | 0.63497 |
| T2o2 | Si | 0.68963 | 0.36265 | 0.32198 |
| T2io | Si | 0.20559 | 0.86970 | 0.35674 |
| T2i2 | Si | 0.80675 | 0.86349 | 0.67794 |
| Oa1_1 | O | 0.99984 | 0.38887 | 0.97310 |
| Oa1_2 | O | 0.51152 | 0.88700 | 0.02930 |
| Oa2o | O | 0.11072 | 0.75651 | 0.28148 |
| Oa22 | O | 0.37894 | 0.25641 | 0.71607 |
| Oboo | O | 0.16227 | 0.39553 | 0.74253 |
| Obo2 | O | 0.80957 | 0.38101 | 0.20447 |
| Obio | O | 0.33133 | 0.89222 | 0.24267 |
| Obi2 | O | 0.69325 | 0.88182 | 0.80208 |
| Ocoo | O | 0.45947 | 0.44707 | 0.72688 |
| Oco2 | O | 0.51662 | 0.43469 | 0.24071 |
| Ocio | O | 0.03766 | 0.94857 | 0.26821 |
| Oci2 | O | 0.98068 | 0.93439 | 0.75671 |
| Odoo | O | 0.18882 | 0.37127 | 0.38839 |
| Odo2 | O | 0.80901 | 0.37269 | 0.57356 |
| Odio | O | 0.31467 | 0.87414 | 0.60170 |
| Odi2 | O | 0.68582 | 0.87183 | 0.42717 |
| Oa | Ob | Oc | Od | Average | |
|---|---|---|---|---|---|
| T1oo | 1.7662 | 1.7456 | 1.7533 | 1.7637 | 1.7572 |
| T1o2 | 1.6131 | 1.6293 | 1.6372 | 1.6396 | 1.6298 |
| T1io | 1.7645 | 1.7370 | 1.7534 | 1.7612 | 1.7540 |
| T1i2 | 1.6103 | 1.6391 | 1.6371 | 1.6349 | 1.6304 |
| T2oo | 1.6336 | 1.6345 | 1.6403 | 1.6080 | 1.6291 |
| T2o2 | 1.6638 | 1.6121 | 1.6178 | 1.6564 | 1.6375 |
| T2io | 1.6522 | 1.6438 | 1.6394 | 1.6026 | 1.6345 |
| T2i2 | 1.6550 | 1.6046 | 1.6158 | 1.6517 | 1.6318 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Jin, S.; Lee, S.; Hobbs, F.W.C. Nano-Phase KNa(Si6Al2)O16 in Adularia: A New Member in the Alkali Feldspar Series with Ordered K–Na Distribution. Minerals 2019, 9, 649. https://doi.org/10.3390/min9110649
Xu H, Jin S, Lee S, Hobbs FWC. Nano-Phase KNa(Si6Al2)O16 in Adularia: A New Member in the Alkali Feldspar Series with Ordered K–Na Distribution. Minerals. 2019; 9(11):649. https://doi.org/10.3390/min9110649
Chicago/Turabian StyleXu, Huifang, Shiyun Jin, Seungyeol Lee, and Franklin W. C. Hobbs. 2019. "Nano-Phase KNa(Si6Al2)O16 in Adularia: A New Member in the Alkali Feldspar Series with Ordered K–Na Distribution" Minerals 9, no. 11: 649. https://doi.org/10.3390/min9110649
APA StyleXu, H., Jin, S., Lee, S., & Hobbs, F. W. C. (2019). Nano-Phase KNa(Si6Al2)O16 in Adularia: A New Member in the Alkali Feldspar Series with Ordered K–Na Distribution. Minerals, 9(11), 649. https://doi.org/10.3390/min9110649