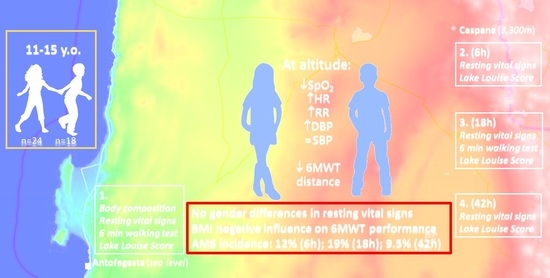
Physiological Responses at Rest and Exercise to High Altitude in Lowland Children and Adolescents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Study Procedure
2.3.1. General Characteristics and Body Composition
2.3.2. Resting Vital Signs
2.3.3. Six-Minute Walking Test
2.3.4. Acute Mountain Sickness Assessment
2.4. Statistical Analysis
3. Results
3.1. General Characteristics and Body Composition
3.2. Resting Vital Signs
3.3. Six-Minute Walking Test
3.4. Acute Mountain Sickness
4. Discussion
4.1. Resting Vital Signs
4.2. Six-Minute Walking Test
4.3. Acute Mountain Sickness Assessment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pollard, A.J.; Niermeyer, S.; Barry, P.; Bärtsch, P.; Berghold, F.; Bishop, R.A.; Clarke, C.; Dhillon, S.; Dietz, T.E.; Durmowicz, A.; et al. Children at high altitude: An international consensus statement by an ad hoc committee of the International Society for Mountain Medicine, March 12, 2001. High Alt. Med. Biol. 2001, 2, 389–403. [Google Scholar] [CrossRef]
- Naeije, R. Physiological adaptation of the cardiovascular system to high altitude. Prog. Cardiovasc. Dis. 2010, 52, 456–466. [Google Scholar] [CrossRef]
- Coppel, J.; Hennis, P.; Gilbert-Kawai, E.; Grocott, M.P.W. The physiological effects of hypobaric hypoxia versus normobaric hypoxia: A systematic review of crossover trials. Extrem. Physiol. Med. 2015, 4, 2. [Google Scholar] [CrossRef] [Green Version]
- Yaron, M.; Niermeyer, S.; Lindgren, K.N.; Honigman, B. Evaluation of diagnostic criteria and incidence of acute mountain sickness in preverbal children. Wilderness Environ. Med. 2002, 13, 21–26. [Google Scholar] [CrossRef]
- Yaron, M.; Niermeyer, S. Travel to high altitude with young children: An approach for clinicians. High Alt. Med. Biol. 2008, 9, 265–269. [Google Scholar] [CrossRef]
- Garlick, V.; O’Connor, A.; Shubkin, C.D. High-altitude illness in the pediatric population: A review of the literature on prevention and treatment. Curr. Opin. Pediatr. 2017, 29, 503–509. [Google Scholar] [CrossRef]
- Hackett, P.H.; Rennie, D.; Levine, H.D. The Incidence, Importance, and Prophylaxis of Acute Mountain Sickness. Lancet 1976, 308, 1149–1155. [Google Scholar] [CrossRef]
- Dallimore, J.; Rowbotham, E.C. Incidence of acute mountain sickness in adolescents. Wilderness Environ. Med. 2009, 20, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Imray, C.H.E.; Kennedy, C.H.; Pattinson, K.; Brearey, S.; Wright, A. Self-assessment of acute mountain sickness in adolescents: A pilot study. Wilderness Environ. Med. 2004, 15, 202–206. [Google Scholar] [CrossRef] [Green Version]
- Pradhan, S.; Yadav, S.; Neupane, P.; Subedi, P. Acute mountain sickness in children at 4380 m in the himalayas. Wilderness Environ. Med. 2009, 20, 359–363. [Google Scholar] [CrossRef] [Green Version]
- Yaron, M.; Waldman, N.; Niermeyer, S.; Nicholas, R.; Honigman, B. The diagnosis of acute mountain sickness in preverbal children. Arch. Pediatr. Adolesc. Med. 1998, 152, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, C.; Chen, Y.; Luo, Y.J. Association between acute mountain sickness (AMS) and age: A meta-analysis. Mil. Med. Res. 2018, 5, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murdoch, D.R. Altitude Illness Among Tourists Flying to 3740 Meters Elevation in the Nepal Himalayas. J. Travel Med. 1995, 2, 255–256. [Google Scholar] [CrossRef]
- Kriemler, S.; Bürgi, F.; Wick, C.; Wick, B.; Keller, M.; Wiget, U.; Schindler, C.; Kaufmann, B.A.; Kohler, M.; Bloch, K.; et al. Prevalence of acute mountain sickness at 3500m within and between families: A prospective cohort study. High Alt. Med. Biol. 2014, 15, 28–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosyreva, A.M.; Dzhalilova, D.S.; Tsvetkov, I.S.; Diatroptov, M.E.; Makarova, O.V. Age-Specific Features of Hypoxia Tolerance and Intensity of Lipopolysaccharide-Induced Systemic Inflammatory Response in Wistar Rats. Bull. Exp. Biol. Med. 2019, 166, 699–703. [Google Scholar] [CrossRef]
- Dzhalilova, D.; Makarova, O. Differences in Tolerance to Hypoxia: Physiological, Biochemical, and Molecular-Biological Characteristics. Biomedicines 2020, 8, 428. [Google Scholar] [CrossRef]
- Horiuchi, M.; Endo, J.; Akatsuka, S.; Uno, T.; Jones, T.E. Prevalence of acute mountain sickness on Mount Fuji: A pilot study. J. Travel Med. 2016, 23, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Canouï-Poitrine, F.; Veerabudun, K.; Larmignat, P.; Letournel, M.; Bastuji-Garin, S.; Richalet, J.P. Risk prediction score for severe high altitude illness: A cohort study. PLoS ONE 2014, 9, e100642. [Google Scholar] [CrossRef] [Green Version]
- Cheng, F.Y.; Jeng, M.J.; Lin, Y.C.; Wang, S.H.; Wu, S.H.; Li, W.C.; Huang, K.F.; Chiu, T.F. Incidence and severity of acute mountain sickness and associated symptoms in children trekking on Xue Mountain, Taiwan. PLoS ONE 2017, 12, e0183207. [Google Scholar] [CrossRef] [Green Version]
- Takken, T.; Evertse, A.; de Waard, F.; Spoorenburg, M.; Kuijpers, M.; Schroer, C.; Hulzebos, E.H. Exercise responses in children and adults with a Fontan circulation at simulated altitude. Congenit. Heart Dis. 2019, 14, 1005–1012. [Google Scholar] [CrossRef] [Green Version]
- Ucrós, S.; Granados, C.M.; Castro-Rodríguez, J.A.; Hill, C.M. Oxygen Saturation in Childhood at High Altitude: A Systematic Review. High Alt. Med. Biol. 2020, 21, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Fulco, C.S.; Rock, P.B.; Cymerman, A. Maximal and submaximal exercise performance at altitude. Aviat. Sp. Environ. Med. 1998, 69, 793–801. [Google Scholar]
- Robert, S. Mazzeo Physiological Responses to Exercise at Altitude. Sport. Med. 2008, 38, 1–8. [Google Scholar]
- López, V.; Moraga, D.; Calderón-Jofre, R.; Moraga, F.A. Heart rate and oxygen saturation in children at high altitude. A different response of Aymaras and non-Aymaras with chronic exposure at 3500 m. J. Health Med. Sci. 2020, 6, 123–129. [Google Scholar]
- Ilarraza-Lomelí, H.; Castañeda-López, J.; Myers, J.; Miranda, I.; Quiroga, P.; Rius, M.D.; Lopez-de-la-Vega, C.; Vallejo, E.; Calderón, J.; Figueroa, J.; et al. Cardiopulmonary exercise testing in healthy children and adolescents at moderately high altitude. Arch. Cardiol. Mex. 2013, 83, 176–182. [Google Scholar] [CrossRef]
- De Groot, J.F.; Takken, T. The six-minute walk test in paediatric populations. J. Physiother. 2011, 57, 128. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, D.M.; Haennel, R.G. Validity and reliability of the 6-min walk test in a cardiac rehabilitation population. J. Cardiopulm. Rehabil. 2000, 20, 156–164. [Google Scholar] [CrossRef]
- Slaughter, M.H.; Lohman, T.G.; Boileau, R.A.; Horswill, C.A.; Stillman, R.J.; Van Loan, M.D.; Bemben, D.A. Skinfold equations for estimations of body fatness in children and youth. Hum. Biol. 1988, 60, 709–723. [Google Scholar]
- Rodríguez, G.; Moreno, L.A.; Blay, M.G.; Blay, V.A.; Fleta, J.; Sarría, A.; Bueno, M. Body fat measurement in adolescents: Comparison of skinfold thickness equations with dual-energy X-ray absorptiometry. Eur. J. Clin. Nutr. 2005, 59, 1158–1166. [Google Scholar] [CrossRef]
- World Health Organization. BMI-for-Age (5–19 Years); World Health Organization: Geneva, Switzerland, 2021; Available online: https://www.who.int/tools/growth-reference-data-for-5to19-years/indicators/bmi-for-age (accessed on 30 July 2021).
- Burrows Argote, R.; Díaz, B.E.; Sciaraffia, M.V.; Gattas, Z.V.; Montoya, C.A.; Lera, M.L. Eating habits and physical activity in schoolchildren, according to type of establishment they attend. Med. J. Chile 2008, 136, 53–63. [Google Scholar] [CrossRef] [Green Version]
- Sutton, J.; Coates, G.; Houston, C. The Lake Louise consensus on the definition and quantification of altitude illness. In Hypoxia and Mountain Medicine; Queen City Printers: Burlington, VT, USA, 1992. [Google Scholar]
- Scrase, E.; Laverty, A.; Gavlak, J.C.D.; Sonnappa, S.; Levett, D.Z.H.; Martin, D.; Grocott, M.P.W.; Stocks, J. The Young Everest Study: Effects of hypoxia at high altitude on cardiorespiratory function and general well-being in healthy children. Arch. Dis. Child. 2009, 94, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Moraga, F.A.; Osorio, J.D.; Vargas, M.E. Acute mountain sickness in tourists with children at Lake Chungará (4400 m) in northern Chile. Wilderness Environ. Med. 2002, 13, 31–35. [Google Scholar] [CrossRef] [Green Version]
- Moraga, F.A.; Pedreros, C.P.; Rodríguez, C.E. Acute mountain sickness in children and their parents after rapid ascent to 3500 m (Putre, Chile). Wilderness Environ. Med. 2008, 19, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Weitz, C.A.; Garruto, R.M. A comparative analysis of arterial oxygen saturation among Tibetans and Han born and raised at high altitude. High Alt. Med. Biol. 2007, 8, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Ricart, A.; Pages, T.; Viscor, G.; Leal, C.; Ventura, J.L. Sex-linked differences in pulse oxymetry. Br. J. Sports Med. 2008, 42, 620–621. [Google Scholar] [CrossRef] [PubMed]
- Huicho, L.; Pawson, I.G.; León-Velarde, F.; Rivera-Ch, M.; Pacheco, A.; Muro, M.; Silva, J. Oxygen saturation and heart rate in healthy school children and adolescents living at high altitude. Am. J. Hum. Biol. 2001, 13, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C.; Lin, W.L.; Wu, J.Y.; Wang, S.H.; Chiu, T.F.; Weng, Y.M.; Hsu, T.Y.; Wu, M.H. Change in oxygen saturation does not predict acute mountain sickness on Jade Mountain. Wilderness Environ. Med. 2012, 23, 122–127. [Google Scholar] [CrossRef] [Green Version]
- Karinen, H.M.; Peltonen, J.E.; Kähönen, M.; Tikkanen, H.O. Prediction of acute mountain sickness by monitoring arterial oxygen saturation during ascent. High Alt. Med. Biol. 2010, 11, 325–332. [Google Scholar] [CrossRef] [Green Version]
- Burtscher, M.; Flatz, M.; Faulhaber, M. Prediction of susceptibility to acute mountain sickness by SaO2 values during short-term exposure to hypoxia. High Alt. Med. Biol. 2004, 5, 335–340. [Google Scholar] [CrossRef]
- Major, S.A.; Hogan, R.J.K.; Yeates, E.; Imray, C.H.E. Peripheral arterial desaturation is further exacerbated by exercise in adolescents with acute mountain sickness. Wilderness Environ. Med. 2012, 23, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Arslan, S.; Arslan, N.; Soylu, A.; Akgün, C.; Tepebasili, I.; Türkmen, M.; Kavukçu, S. High altitude and blood pressure in children. Yale J. Biol. Med. 2003, 76, 145–148. [Google Scholar] [PubMed]
- Lang, M.; Faini, A.; Caravita, S.; Bilo, G.; Anza-Ramìrez, C.; Villafuerte, F.C.; Macarlupu, J.L.; Salvioni, E.; Agostoni, P.; Parati, G. Blood pressure response to six-minute walk test in hypertensive subjects exposed to high altitude: Effects antihypertensive combination treatment. Int. J. Cardiol. 2016, 219, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Gatica, D.; Puppo, H.; Villarroel, G.; Martín, I.S.; Lagos, R.; Montecino, J.J.; Lara, C.; Zenteno, D. Valores de referencia del test de marcha de seis minutos en niños sanos. Rev. Med. Chil. 2012, 140, 1014–1021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Núñez, I.; Mondaca, F.; Casas, B.; Ferreira, C.; Zenteno, D. Normal values of 6-min walk test in healthy children and adolescents: A systematic review and meta-analysis. Rev. Chil. Pediatr. 2018, 89, 128–136. [Google Scholar] [CrossRef] [Green Version]
- Pathare, N.; Haskvitz, E.M.; Selleck, M. 6-Minute Walk Test Performance in Young Children who are Normal Weight and Overweight. Cardiopulm. Phys. Ther. J. 2012, 23, 12–18. [Google Scholar] [CrossRef]
- Raistenskis, J.; Sidlauskiene, A.; Strukcinskiene, B.; Uğur Baysal, S.; Buckus, R. Physical activity and physical fitness in obese, overweight, and normal-weight children. Turkish J. Med. Sci. 2016, 46, 443–450. [Google Scholar] [CrossRef]
- Brand, C.; Reuter, C.P.; Gaya, A.R.; Mota, J.; Duncan, M.; Borfe, L.; Pollo Renner, J.D. Association between cardiorespiratory fitness and cardiometabolic risk factors in Brazilianchildren and adolescents: The mediating role of obesity parameters. Paediatr. Int. Child Health 2020, 1–10. [Google Scholar] [CrossRef]
- Maury-Sintjago, E.; Rodríguez-Fernández, A.; Parra-Flores, J.; Garcia, D.E. Association between body mass index and functional fitness of 9- to 10-year-old Chilean children. Am. J. Hum. Biol. 2019, 31, e23305. [Google Scholar] [CrossRef]
- San Martin, R.; Brito, J.; Siques, P.; León-Velarde, F. Obesity as a Conditioning Factor for High-Altitude Diseases. Obes. Facts 2017, 10, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Kriemler, S.; Jansen, C.; Linka, A.; Kessel-Schaefer, A.; Zehnder, M.; Schürmann, T.; Kohler, M.; Bloch, K.; Brunner-La Rocca, H.P. Higher pulmonary artery pressure in children than in adults upon fast ascent to high altitude. Eur. Respir. J. 2008, 32, 664–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, C.W.; Lin, Y.C.; Chiu, Y.H.; Weng, Y.M.; Li, W.C.; Lin, Y.J.; Wang, S.H.; Hsu, T.Y.; Huang, K.F.; Chiu, T.F. Incidence and risk factors associated with acute mountain sickness in children trekking on Jade Mountain, Taiwan. J. Travel Med. 2016, 23, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Dallimore, J.; Foley, J.A.; Valentine, P. Background rates of acute mountain sickness-like symptoms at low altitude in adolescents using Lake Louise score. Wilderness Environ. Med. 2012, 23, 11–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Anthropometrical Characteristics | n | ||
|---|---|---|---|
| Age (years) | Boys | 18 | 12.5 ± 1.04 |
| Girls | 24 | 12.5 ± 1.14 | |
| All | 42 | 12.5 ± 1.09 | |
| Body mass (Kg) | Boys | 18 | 56.64 ± 10.53 |
| Girls | 24 | 53.56 ± 13.76 | |
| All | 42 | 54.93 ± 12.43 | |
| Height (m) | Boys | 18 | 1.60 ± 0.11 |
| Girls | 24 | 1.55 ± 0.07 | |
| All | 42 | 1.57 ± 0.09 | |
| Body mass index a (kg/m2) | Boys | 18 | 22.09 ± 2.49 |
| Girls | 24 | 22.04 ± 4.67 | |
| All | 42 | 22.06 ± 4.16 | |
| Waist (cm) | Boys | 17 | 72.9 ± 10.9 |
| Girls | 22 | 69.8 ± 9.7 | |
| All | 39 | 71.1 ± 10.2 | |
| Triceps skin fold (mm) | Boys | 17 | 17.9 ± 7.3 |
| Girls | 22 | 21.1 ± 6.6 | |
| All | 39 | 19.7 ± 7.0 | |
| Subscapular skin fold (mm) | Boys | 17 | 15.9 ± 10.1 |
| Girls | 22 | 17.2 ± 9.1 | |
| All | 39 | 16.6 ± 9.4 | |
| % Fat mass b | Boys | 17 | 25.61 ± 10.39 |
| Girls | 22 | 29.79 ± 11.24 | |
| All | 39 | 27.97 ± 10.94 | |
| Physical activity score (1–7) | Boys | 13 | 4.76 ± 1.69 |
| Girls | 21 | 4.04 ± 1.32 | |
| All | 34 | 4.32 ± 1.49 |
| Physiological Parameters | n | SL | HA | |||
|---|---|---|---|---|---|---|
| 6 h | 18 h | 42 h | ||||
| spO2 (%) | Boys | 18 | 98.0 ± 0.3 | 89.2 ± 3.9 | 90.3 ± 2.5 | 89.8 ± 2.8 |
| Girls | 24 | 98.1 ± 0.7 | 88.6 ± 3.9 | 90.8 ± 3.4 | 91.2 ± 2.3 | |
| All | 42 | 98.1 ± 0.5 | 89.5 ± 3.9 *** | 90.5 ± 3.0 *** | 90.6 ± 2.6 *** | |
| HR (bpm) | Boys | 18 | 82.9 ± 12.4 | 105.8 ± 15.2 | 101.8 ± 13.3 | 99.8 ± 12.8 |
| Girls | 24 | 83.6 ± 11.2 | 107.8 ± 13.5 | 103.9 ± 10.5 | 102.2 ± 12.8 | |
| All | 42 | 83.3 ± 11.6 | 107.0 ± 14.1 *** | 103.0 ± 11.7 *** | 101.2 ± 12.7 *** | |
| RR (bpm) | Boys | 18 | 17.9 ± 3.3 | 20.3 ± 3.9 | 20.0 ± 6.8 β | 19.3 ± 3.6 |
| Girls | 24 | 20.1 ± 3.9 | 24.1 ± 10.0 | 25.9 ± 9.8 | 21.9 ± 3.7 | |
| All | 42 | 19.1 ± 3.8 | 22.5 ± 8.1 * | 23.4 ± 9.0 * | 20.8 ± 3.8 * | |
| SBP (mmHg) | Boys | 18 | 116.2 ± 12.2 | 115.5 ± 13.2 | 116.3 ± 11.2 | 117.1 ± 14.8 |
| Girls | 24 | 109.9 ± 10.5 | 111.2 ± 10.7 | 112.8 ± 11.1 | 114.5 ± 10.1 | |
| All | 42 | 112.6 ± 11.5 | 113.1 ± 11.9 | 114.3 ± 11.2 | 115.6 ± 12.3 | |
| DBP (mmHg) | Boys | 18 | 63.8 ± 8.2 | 63.6 ± 9.6 | 69.9 ± 8.5 | 72.8 ± 13.1 |
| Girls | 24 | 61.8 ± 8.2 | 66.3 ± 8.4 | 69.1 ± 7.7 | 69.4 ± 10.2 | |
| All | 42 | 62.7 ± 8.1 | 66.4 ± 8.8 * | 69.4 ± 8.0 * | 70.8 ± 11.5 * | |
| Physiological Parameters | n | SL | HA | ||||
|---|---|---|---|---|---|---|---|
| 6 h | 18 h | 42 h | |||||
| spO2 (%) | Boys | NO | 8 | 97.9 ± 0.4 | 90.0 ± 4.0 *** | 90.6 ± 2 *** | 90.6 ± 3.4 *** |
| OW | 7 | 98.0 ± 0 | 89.1 ± 3.2 *** | 89.7 ± 3.5 *** | 89.3 ± 2.1 *** | ||
| OB | 3 | 98.3 ± 0.6 | 87.3 ± 5.9 *** | 90.0 ± 1.2 *** | 89.0 ± 2.6 *** | ||
| Girls | NO | 12 | 98.3 ± 0.4 | 90.3 ± 3.3 *** | 90.7 ± 3.2 *** | 91.8 ± 2.7 *** | |
| OW | 9 | 98.0 ± 0.5 | 89.8 ± 4.9 *** | 90.8 ± 3 *** | 90.7 ± 2.2 *** | ||
| OB | 3 | 97.3 ± 0.6 | 86.7 ± 1.5 *** | 86.7 ± 4.2 *** | 90.0 ± 0.1 *** | ||
| HR (bpm) | Boys | NO | 8 | 79.1 ± 12.7 | 104.8 ± 15.1 *** | 102.9 ± 11.5 *** | 100.5 ± 12.2 *** |
| OW | 7 | 81.7 ± 11.2 | 100.9 ± 10.4 *** | 94.9 ± 9.4 *** | 94.4 ± 11.6 *** | ||
| OB | 3 | 96 ± 6.9 | 120.3 ± 20.5 *** | 115 ± 18.3 *** | 100.3 ± 14.4 *** | ||
| Girls | NO | 12 | 83.8 ± 11.9 | 105.7 ± 14.1 *** | 106.3 ± 9.9 *** | 100.2 ± 15.8 *** | |
| OW | 9 | 85.9 ± 11.4 | 109.1 ± 12.2 *** | 98 ± 8,7 *** | 103.9 ± 10.1 *** | ||
| OB | 3 | 77 ± 7.5 β | 112.7 ± 18.7 *** β | 112 ± 11.3 *** | 105.3 ± 7.0 *** | ||
| RR (bpm) | Boys | NO | 8 | 17.3 ± 3.9 | 20 ± 3.9 | 18.3 ± 3.5 | 18.9 ± 3.4 |
| OW | 7 | 18 ± 3.3 | 19.3 ± 3.2 | 18.4 ± 3.2 | 19.3 ± 4.6 | ||
| OB | 3 | 19.3 ± 1.2 | 23.3 ± 5.9 * | 28.3 ± 14 *** | 20.7 ± 2.3 | ||
| Girls | NO | 12 | 20 ± 4.1 | 22.3 ± 9.2 | 23.7 ± 8 β | 22.1 ± 2.7 β | |
| OW | 9 | 19.9 ± 2.3 | 25.7 ± 11 * # β | 26.4 ± 11.1 * # β | 20.1 ± 2.9 | ||
| OB | 3 | 21 ± 7.8 | 26.7 ± 12.4 * # β | 33 ± 12.1 *** # β | 26.3 ± 6.0 * # β | ||
| SBP (mmHg) | Boys | NO | 8 | 119.8 ± 14.7 | 112.8 ± 16.5 | 114.9 ± 15 | 113.3 ± 11.6 |
| OW | 7 | 110 ± 8.5 | 114.4 ± 9.9 | 116.1 ± 8.2 | 115.9 ± 18.9 | ||
| OB | 3 | 121 ± 7.9 | 125.3 ± 7.1 | 120.3 ± 7.2 | 130 ± 2.0 * | ||
| Girls | NO | 12 | 105.8 ± 10.7 | 106.7 ± 9.3 | 112.6 ± 12.4 * | 111.8 ± 10.5 * | |
| OW | 9 | 112.4 ± 9.8 | 115.7 ± 10.5 | 113.3 ± 11 | 115.6 ± 10.3 | ||
| OB | 3 | 118.7 ± 2.5 | 117.3 ± 10.7 | 111.7 ± 8.3 | 122.3 ± 4.2 * | ||
| DBP (mmHg) | Boys | NO | 8 | 63.4 ± 8.6 | 65 ± 7.9 | 72 ± 11.3 * | 70.5 ± 7.5 * |
| OW | 7 | 61.1 ± 7.5 | 66.3 ± 12.1 | 66.3 ± 5.4 | 67.4 ± 7.5 * | ||
| OB | 3 | 71.3 ± 5.9 | 71.7 ± 8.7 | 72.7 ± 2.1 | 91.3 ± 21.5 * | ||
| Girls | NO | 8 | 59.3 ± 6.4 | 64.2 ± 8.8 | 66.7 ± 9 | 68.4 ± 12.2 * | |
| OW | 7 | 64.2 ± 10.5 | 68.6 ± 7.7 | 70.8 ± 6.0 | 67.4 ± 7.1 | ||
| OB | 3 | 64.7 ± 4.5 β | 67.7 ± 9.5 | 73.7 ± 4.0 * | 79 ± 1.0 *** # | ||
| LLS | Boys | NO | 8 | - | 0.8 ± 0.9 | 0.9 ± 1 | 1.4 ± 1.6 |
| OW | 7 | - | 1.0 ± 0.8 | 1.0 ± 1.3 | 0.4 ± 0.5 | ||
| OB | 3 | - | 1.7 ± 1.5 | 2.3 ± 1.2 | 1.3 ± 1.2 | ||
| Girls | NO | 12 | - | 1 ± 1.2 | 0.9 ± 0.8 | 0.9 ± 0.8 | |
| OW | 9 | - | 1.6 ± 1.6 | 1.6 ± 1.8 | 2.1 ± 1.8 | ||
| OB | 3 | - | 2.7 ± 1.2 | 2 ± 1.7 | 1.3 ± 0.6 | ||
| Antofagasta (SL) | Caspana (HA 3300 m) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 h | 18 h | 42 h | ||||||||||||||
| Lake Louise Score | S1 | S2 | S3 | % | S1 | S2 | S3 | % | S1 | S2 | S3 | % | S1 | S2 | S3 | % |
| Headache | 1 | 0 | 0 | 2.4 | 15 | 2 | 0 | 40.4 | 10 | 1 | 0 | 26.2 | 4 | 1 | 0 | 11.9 |
| Gastrointestinal | 1 | 0 | 0 | 2.4 | 9 | 2 | 0 | 26.2 | 8 | 1 | 0 | 21.4 | 3 | 1 | 0 | 9.5 |
| Fatigue | 4 | 0 | 0 | 9.5 | 14 | 1 | 0 | 35.7 | 7 | 2 | 0 | 21.5 | 6 | 1 | 0 | 16.7 |
| Dizziness | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 9.5 | 3 | 1 | 0 | 9.5 | 2 | 0 | 0 | 4.8 |
| Sleep disturbances | 3 | 0 | 0 | 7.1 | 3 | 0 | 0 | 9.5 | 16 | 11 | 1 | 61.9 | 14 | 9 | 0 | 54.7 |
| AMS incidence | N = 0 | 0 | N = 5 | 11.9 | N = 8 | 19 | N = 4 | 9.5 | ||||||||
| Lake Louise Score | n | SL | HA | |||
|---|---|---|---|---|---|---|
| 6 h | 18 h | 42 h | ||||
| Headache | Boys | 18 | 0.00 ± 0.0 | 0.22 ± 0.4 | 0.11 ± 0.3 | 0.0 ± 0.0 |
| Girls | 24 | 0.40 ± 0.2 | 0.54 ± 0.7 | 0.25 ± 0.5 | 0.17 ± 0.5 | |
| All | 42 | 0.20 ± 0.2 | 0.58 ± 0.1 | 0.19 ± 0.5 | 0.10 ± 0.4 | |
| Gastrointestinal | Boys | 18 | 0.00 ± 0.0 | 0.17 ± 0.4 | 0.06 ± 0.2 | 0.13 ± 0.5 |
| Girls | 24 | 0.40 ± 0.2 | 0.38 ± 0.6 | 0.38 ± 0.6 * | 0.11 ± 0.3 | |
| All | 42 | 0.21 ± 0.2 | 0.29 ± 0.5 | 0.24 ± 0.5 | 0.12 ± 0.4 | |
| Fatigue | Boys | 18 | 0.17 ± 0.4 | 0.39 ± 0.5 | 0.44 ± 0.7 | 0.11 ± 0.3 |
| Girls | 24 | 0.39 ± 0.2 | 0.33 ± 0.6 | 0.13 ± 0.3 | 0.29 ± 0.6 | |
| All | 42 | 0.10 ± 0.3 | 0.36 ± 0.6 | 0.26 ± 0.5 | 0.21 ± 0.5 | |
| Dizziness | Boys | 18 | 0.00 ± 0.0 | 0.17 ± 0.4 | 0.22 ± 0.6 | 0.06 ± 0.2 |
| Girls | 24 | 0.00 ± 0.0 | 0.40 ± 0.2 | 0.04 ± 0.2 | 0.08 ± 0.3 | |
| All | 42 | 0.00 ± 0.0 | 0.10 ± 0.3 | 0.12 ± 0.4 | 0.07 ± 0.3 | |
| Sleep disturbances | Boys | 18 | 0.11 ± 0.3 | 0.17 ± 0.4 | 0.72 ± 0.82 | 0.72 ± 0.8 |
| Girls | 24 | 0.00 ± 0.0 | 0.40 ± 0.2 | 1.04 ± 0.9 | 0.75 ± 0.8 | |
| All | 42 | 0.05 ± 0.2 | 0.10 ± 0.3 | 0.90 ± 0.9 | 0.74 ± 0.8 | |
| AMS (LLS) | Boys | 18 | 2.01 ± 0.0 | 1.94 ± 0.2 | 1.94 ± 0.2 | 1.94 ± 0.2 |
| Girls | 24 | 1.80 ± 0.6 | 1.96 ± 0.2 | 1.88 ± 0.3 | 1.92 ± 0.3 | |
| All | 42 | 1.90 ± 0.4 | 1.95 ± 0.2 | 1.90 ± 0.3 | 1.93 ± 0.3 | |
| BMI | n | SL | HA | |||
|---|---|---|---|---|---|---|
| 6 h | 18 h | 42 h | ||||
| Headache | N | 20 | 0.00 ± 0.0 * | 0.25 ± 0.4 * | 0.10 ± 0.3 | 0.05 ± 0.2 |
| OW | 16 | 0.00 ± 0.0 * | 0.38 ± 0.6 * | 0.19 ± 0.5 | 0.29 ± 0.5 | |
| O | 6 | 0.17 ± 0.4 * | 1.00 ± 0.6 * | 0.50 ± 0.6 | 0.04 ± 0.3 * | |
| Gastrointestinal | N | 20 | 0.05 ± 0.2 | 0.30 ± 0.6 | 0.15 ± 0.4 * | 0.10 ± 0.0 * |
| OW | 16 | 0.00 ± 0.0 | 0.31 ± 0.6 | 0.31 ± 0.6 * | 0.01 ± 0.8 * | |
| O | 6 | 0.00 ± 0.0 | 0.17 ± 0.4 | 0.33 ± 0.5 | 0.50 ± 0.0 | |
| Fatigue | N | 20 | 0.10 ± 0.3 | 0.30 ± 0.6 | 0.10 ± 0.3 | 0.15 ± 0.5 |
| OW | 16 | 0.06 ± 0.3 | 0.44 ± 0.6 | 0.31 ± 0.6 | 0.31 ± 0.6 | |
| O | 6 | 0.17 ± 0.4 | 0.33 ± 0.5 | 0.67 ± 0.8 | 0.17 ± 0.4 | |
| Dizziness | N | 20 | 0.00 ± 0.0 | 0.17 ± 0.2 | 0.10 ± 0.3 * | 0.00 ± 0.0 |
| OW | 16 | 0.00 ± 0.0 | 0.50 ± 0.3 | 0.00 ± 0.0 * | 0.06 ± 0.3 * | |
| O | 6 | 0.00 ± 0.0 | 0.13 ± 0.4 | 0.51 ± 0.8 * | 0.33 ± 0.5 * | |
| Sleep disturbances | N | 20 | 0.10 ± 0.3 | 0.15 ± 0.4 | 0.91 ± 0.9 | 0.65 ± 0.8 * |
| OW | 16 | 0.00 ± 0.0 | 0.06 ± 0.3 | 0.88 ± 1.1 | 0.69 ± 0.8 | |
| O | 6 | 0.00 ± 0.0 | 0.00 ± 0.0 | 1.00 ± 0.0 | 1.17 ± 0.1 | |
| AMS | N | 20 | 1.90 ± 0.5 | 2.00 ± 0.0 | 1.95 ± 0.2 | 2.00 ± 0.3 |
| OW | 16 | 1.88 ± 0.5 | 1.94 ± 0.3 | 1.94 ± 0.3 | 1.88 ± 0.4 | |
| O | 6 | 2.01 ± 0.1 | 1.83 ± 0.4 | 1.67 ± 0.5 | 1.83 ± 0.3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lang, M.; Vizcaíno-Muñoz, G.; Jopia, P.; Silva-Urra, J.; Viscor, G. Physiological Responses at Rest and Exercise to High Altitude in Lowland Children and Adolescents. Life 2021, 11, 1009. https://doi.org/10.3390/life11101009
Lang M, Vizcaíno-Muñoz G, Jopia P, Silva-Urra J, Viscor G. Physiological Responses at Rest and Exercise to High Altitude in Lowland Children and Adolescents. Life. 2021; 11(10):1009. https://doi.org/10.3390/life11101009
Chicago/Turabian StyleLang, Morin, Guillem Vizcaíno-Muñoz, Paulina Jopia, Juan Silva-Urra, and Ginés Viscor. 2021. "Physiological Responses at Rest and Exercise to High Altitude in Lowland Children and Adolescents" Life 11, no. 10: 1009. https://doi.org/10.3390/life11101009
APA StyleLang, M., Vizcaíno-Muñoz, G., Jopia, P., Silva-Urra, J., & Viscor, G. (2021). Physiological Responses at Rest and Exercise to High Altitude in Lowland Children and Adolescents. Life, 11(10), 1009. https://doi.org/10.3390/life11101009