A Flavonoid on the Brain: Quercetin as a Potential Therapeutic Agent in Central Nervous System Disorders
Abstract
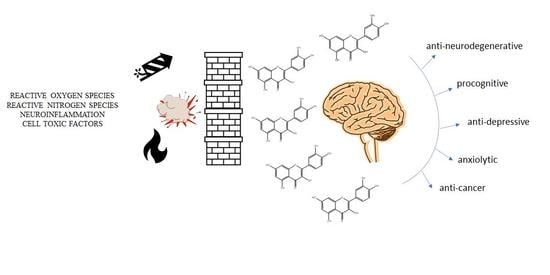
:1. Introduction
Methods
2. Metabolism and Bioavailability of Quercetin
3. Quercetin-Loaded Nanocarriers—New Delivery to Better Availability
4. Quercetin in Neurodegenerative Diseases—Dementia and Alzheimer’s Disease, Parkinson’s, Huntington’s Diseases
4.1. Dementia and Alzheimer’s Disease
4.1.1. Cholinergic Effect
4.1.2. Pro-Neurotrophic Effect
4.1.3. Anti-Inflammatory Effect
4.1.4. Antioxidant Effect
4.1.5. Anti-Neurotoxic Protein Aggregates
4.1.6. Improving Metabolic Disruption Effect
4.2. Parkinson’s Disease
4.3. Huntington’s Disease
4.4. Other Neurodegenerative Diseases
5. Effects of Quercetin in Mental Disorders—Depression, Anxiety
5.1. Depressive Disorders
5.2. Anxiety Disorders
6. Therapeutic Potential of Quercetin in Brain Cancer
6.1. Cytotoxicity and Mechanisms
6.2. Synergism
6.3. Novel Drug Delivery Systems
7. Clinical Studies on Quercetin in CNS Diseases
7.1. Case Study—Glioblastoma
7.2. Clinical Study—Alzheimer’s Disease
8. Discussion and Conclusions
9. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3-NP | 3-nitropropionic acid |
| 5-HT | serotonin/serotonin receptor |
| A | adenosine |
| A1 | adenosine receptor 1 |
| Aβ | β-amyloid |
| Ach | acetylcholine |
| AchE | acetylcholinesterase |
| AD | Alzheimer’s disease |
| ADA | adenosine deaminase |
| α-syn | α-synuclein |
| AMP | adenosine monophosphate |
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor |
| AMPK | AMP activated protein kinase |
| APP | amyloid precursor protein |
| BACE1 | β-secretase 1 |
| Bcl | B-cell lymphoma |
| BDNF | brain-derived neurotrophic factors |
| CDK | cyclin-dependent kinase |
| CNS | central nervous system |
| COX | cyclooxygenase |
| CREB | cyclic-AMP-response element-binding protein |
| DA | dopamine |
| D | dopaminergic receptor |
| EGFR | epidermal growth factor receptor |
| EphA2 | ephrin type-A receptor 2 |
| GABA | γ-aminobutyric acid |
| GLU | glutamate |
| GlyR | glycine receptor |
| GSK3β | glycogen synthase kinase 3 β |
| GSH | glutathione |
| GST | glutathione transferase |
| HD | Huntington’s disease |
| HPA | hypothalamus-pituitary-adrenal |
| HSP70 | heat shock protein 70 |
| IL | interleukin |
| iNOS | inducible nitric oxide synthase |
| ISR | integrated stress response |
| JNK | c-Jun N-terminal kinase |
| LC3 | microtubule-associated proteins 1A/1B light chain 3B |
| LOX | lipoxygenase |
| M | muscarinic acetylcholine receptor |
| MAO-B | monoamine oxidase-B |
| MAPK | mitogen-activated protein kinases |
| MMP | metalloproteinase |
| mTOR | mammalian target of rapamycin kinase |
| N | nicotinic acetylcholine receptor |
| NGF | nerve growth factor |
| NMDA | N-methyl-d-aspartate receptor |
| NMRPTase | nicotinamide phosphoribosyltransferase |
| NOS | nitric oxide synthase |
| NfκB | nuclear-kappa B factor |
| NFTs | neurofibrillary tangles |
| Nrf | nuclear factor-like 2 |
| NRLP-3 | NLR Family Pyrin Domain Containing 3 |
| PAI-1 | plasminogen activator inhibitor-1 |
| PD | Parkinson’s disease |
| PGC-1α | peroxisome proliferator-activated receptor-gamma coactivator |
| PI3K | phosphoinositide 3-kinases |
| PON2 | paraoxonase-2 |
| Que | quercetin |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| SIRT | sirtuin |
| SOD | superoxide dismutase |
| SPs | senile plaques |
| TLR | toll-like receptors |
| TNF-α | tumor necrosis factor-α |
| VEGF | vascular endothelial growth factor |
References
- Kim, J.K.; Park, S.U. Quercetin and its role in biological functions: An updated review. EXCLI J. 2018, 17, 856. [Google Scholar] [PubMed]
- Wang, W.; Sun, C.; Mao, L.; Ma, P.; Liu, F.; Yang, J.; Gao, Y. The biological activities, chemical stability, metabolism and delivery systems of quercetin: A review. Trends Food Sci. Technol. 2016, 56, 21–38. [Google Scholar] [CrossRef]
- Erlund, I. Review of the flavonoids quercetin, hesperetin, and naringenin. Dietary sources, bioactivities, bioavailability, and epidemiology. Nutr. Res. 2004, 24, 851–874. [Google Scholar] [CrossRef]
- Ozgen, S.; Kilinc, O.K.; Selamoğlu, Z. Antioxidant activity of quercetin: A mechanistic review. Turk. J. Agric. Food Sci. Technol. 2016, 4, 1134–1138. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, inflammation and immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Samini, M. The neuro-protective effects of quercetin. Res. J. Pharm. Technol. 2019, 12, 561–568. [Google Scholar] [CrossRef]
- Silvestro, S.; Bramanti, P.; Mazzon, E. Role of Quercetin in Depressive-Like Behaviors: Findings from Animal Models. Appl. Sci. 2021, 11, 7116. [Google Scholar] [CrossRef]
- Khan, H.; Ullah, H.; Aschner, M.; Cheang, W.S.; Akkol, E.K. Neuroprotective effects of quercetin in Alzheimer’s disease. Biomolecules 2020, 10, 59. [Google Scholar] [CrossRef] [Green Version]
- Amanzadeh, E.; Esmaeili, A.; Rahgozar, S.; Nourbakhshnia, M. Application of quercetin in neurological disorders: From nutrition to nanomedicine. Rev. Neurosci. 2019, 30, 555–572. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Bolwell, P.G.; Bramley, P.M.; Pridham, J.B. The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radic. Res. 1995, 22, 375–383. [Google Scholar] [CrossRef]
- Floyd, R.A.; Hensley, K. Oxidative stress in brain aging: Implications for therapeutics of neurodegenerative diseases. Neurobiol. Aging 2002, 23, 795–807. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Halliwell, B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef] [Green Version]
- Troubat, R.; Barone, P.; Leman, S.; Desmidt, T.; Cressant, A.; Atanasova, B.; Camus, V. Neuroinflammation and depression: A review. Eur. J. Neurosci. 2021, 53, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Dajas, F.; Abin-Carriquiry, J.A.; Arredondo, F.; Blasina, F.; Echeverry, C.; Martínez, M.; Vaamonde, L. Quercetin in brain diseases: Potential and limits. Neurochem. Int. 2015, 89, 140–148. [Google Scholar] [CrossRef]
- David, A.V.A.; Arulmoli, R.; Parasuraman, S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn. Rev. 2016, 10, 84. [Google Scholar]
- Yang, D.; Wang, T.; Long, M.; Li, P. Quercetin: Its main pharmacological activity and potential application in clinical medicine. Oxidative Med. Cell. Longev. 2020, 2020, 8825327. [Google Scholar] [CrossRef]
- Dajas, F.; Rivera, F.; Blasina, F.; Arredondo, F.; Echeverry, C.; Lafon, L.; Morquio, A.; Heinzen, H. Cell culture protection and in vivo neuroprotective capacity of flavonoids. Neurotox. Res. 2003, 5, 425–432. [Google Scholar] [CrossRef]
- Costa, L.G.; Garrick, J.M.; Roquè, P.J.; Pellacani, C. Mechanisms of neuroprotection by quercetin: Counteracting oxidative stress and more. Oxidative Med. Cell. Longev. 2016, 2016, 2986796. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.M.; Zheng, G.H.; Cheng, C.; Sun, J.M. Quercetin protects mouse brain against lead-induced neurotoxicity. J. Agric. Food Chem. 2013, 61, 7630–7635. [Google Scholar] [CrossRef]
- Zaplatic, E.; Bule, M.; Shah, S.Z.A.; Uddin, M.S.; Niaz, K. Molecular mechanisms underlying protective role of quercetin in attenuating Alzheimer’s disease. Life Sci. 2019, 224, 109–119. [Google Scholar] [CrossRef]
- Shi, G.J.; Li, Y.; Cao, Q.H.; Wu, H.X.; Tang, X.Y.; Gao, X.H.; Yang, Y. In vitro and in vivo evidence that quercetin protects against diabetes and its complications: A systematic review of the literature. Biomed. Pharmacother. 2019, 109, 1085–1099. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Arellano, L.; Salazar-García, M.; Corona, J.C. Neuroprotective effects of quercetin in pediatric neurological diseases. Molecules 2020, 25, 5597. [Google Scholar] [CrossRef] [PubMed]
- Benameur, T.; Soleti, R.; Porro, C. The potential neuroprotective role of free and encapsulated quercetin mediated by miRNA against neurological diseases. Nutrients 2021, 13, 1318. [Google Scholar] [CrossRef] [PubMed]
- Grewal, A.K.; Singh, T.G.; Sharma, D.; Sharma, V.; Singh, M.; Rahman, M.H.; Abdel-Daim, M.M. Mechanistic insights and perspectives involved in neuroprotective action of quercetin. Biomed. Pharmacother. 2021, 140, 111729. [Google Scholar] [CrossRef]
- Islam, M.S.; Quispe, C.; Hossain, R.; Islam, M.T.; Al-Harrasi, A.; Al-Rawahi, A.; Martorell, M.; Mamurova, A.; Seilkhan, A.; Altybaeva, N.; et al. Neuropharmacological effects of quercetin: A literature-based review. Front. Pharmacol. 2021, 12, 665031. [Google Scholar] [CrossRef]
- Jakaria, M.; Azam, S.; Jo, S.H.; Kim, I.S.; Dash, R.; Choi, D.K. Potential therapeutic targets of quercetin and its derivatives: Its role in the therapy of cognitive impairment. J. Clin. Med. 2019, 8, 1789. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.W.; Chen, J.Y.; Ouyang, D.; Lu, J.H. Quercetin in animal models of Alzheimer’s disease: A systematic review of preclinical studies. Int. J. Mol. Sci. 2020, 21, 493. [Google Scholar] [CrossRef] [Green Version]
- Tamtaji, O.R.; Hadinezhad, T.; Fallah, M.; Shahmirzadi, A.R.; Taghizadeh, M.; Behnam, M.; Asemi, Z. The therapeutic potential of quercetin in Parkinson’s disease: Insights into its molecular and cellular regulation. Curr. Drug Targets 2020, 21, 509–518. [Google Scholar] [CrossRef]
- Graefe, E.U.; Wittig, J.; Mueller, S.; Riethling, A.K.; Uehleke, B.; Drewelow, B.; Veit, M. Pharmacokinetics and bioavailability of quercetin glycosides in humans. J. Clin. Pharmacol. 2001, 41, 492–499. [Google Scholar] [CrossRef]
- Kaşıkcı, M.B.; Bağdatlıoğlu, N. Bioavailability of quercetin. Curr. Res. Nutr. Food Sci. J. 2016, 4, 146–151. [Google Scholar] [CrossRef]
- Ishisaka, A.; Ichikawa, S.; Sakakibara, H.; Piskula, M.K.; Nakamura, T.; Kato, Y.; Terao, J. Accumulation of orally administered quercetin in brain tissue and its antioxidative effects in rats. Free Rad. Biol. Med. 2011, 51, 1329–1336. [Google Scholar] [CrossRef] [PubMed]
- Najafabadi, R.E.; Kazemipour, N.; Esmaeili, A.; Beheshti, S.; Nazifi, S. Using superparamagnetic iron oxide nanoparticles to enhance bioavailabiliyty of quercetin in the intact rat brain. BMC Pharmacol. Toxicol. 2018, 19, 59. [Google Scholar]
- Liu, L.; Tang, Y.; Gao, C.; Li, Y.; Chen, S.; Xiong, T.; Li, J.; Du, M.; Gong, Z.; Chen, H.; et al. Characterization and biodistribution in vivo of quercetin-loaded cationic nanostructured lipid carriers. Colloids Surf. B Biointerfaces 2014, 115, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Bagad, M.; Khan, Z.A. Poly(n-butylcyanoacrylate) nanoparticles for oral delivery of quercetin: Preparation, characterization, and pharmacokinetics and biodistribution studies in Wistar rats. Int. J. Nanomed. 2015, 10, 3921–3935. [Google Scholar]
- Kumar, P.; Sharma, G.; Kumar, R.; Singh, B.; Malik, R.; Katare, O.P.; Raza, K. Promises of a biocompatible nanocarrier in improved brain delivery of quercetin: Biochemical, pharmacokinetic and biodistribution evidences. Int. J. Pharm. 2016, 515, 307–314. [Google Scholar] [CrossRef]
- Qi, Y.; Guo, L.; Jiang, Y.; Shi, Y.; Sui, H.; Zhao, L. Brain delivery of quercetin-loaded exosomes improved cognitive function in AD mice by inhibiting phosphorylated tau-mediated neurofibrillary tangles. Drug Deliv. 2020, 27, 74. [Google Scholar] [CrossRef]
- Liu, Y.; Gong, Y.; Xie, W.; Huang, A.; Yuan, X.; Zhou, H.; Zhu, X.; Chen, X.; Liu, J.; Liu, J.; et al. Microbubbles in combination with focused ultrasound for the delivery of quercetin-modified sulfur nanoparticles through the blood brain barier into the brain parenchyma and relief of endoplasmic reticulum stress to treat Alzheimer’s disease. Nanoscale 2020, 12, 6498. [Google Scholar] [CrossRef]
- Thipkaew, C.; Wattanathorn, J.; Muchimapura, S. Electrospun nanofibers loaded with quercetin promote the recovery of focal entrapment neuropathy in a rat model of streptozotocin-induced diabetes. Biomed. Res. Int. 2017, 2017, 2017493. [Google Scholar] [CrossRef]
- Ebrahimpour, S.; Shahidi, S.B.; Abbasi, M.; Tavakali, Z.; Esmaeili, A. Quercetin-conjugated superparamagnetic iron oxide nanopa. Sci. Rep. 2020, 10, 1597. [Google Scholar] [CrossRef]
- Savale, S.K. Formulation and evaluation of quercetin nanoemulsions for treatment of brain tumor via intranasal pathway. Asian J. Biomater. Res. 2017, 3, 28–32. [Google Scholar]
- Li, C.; Yu, D.G.; Williams, G.R.; Wang, Z.H. Fast-dissolving core-shell composite microparticles of quercetin fabricated using a coaxial electrospray process. PLoS ONE 2014, 9, e92106. [Google Scholar] [CrossRef] [PubMed]
- Testa, G.; Gamba, P.; Badilli, U.; Gargiulo, S.; Maina, M.; Guina, T.; Calfapietra, S.; Biasi, F.; Cavalli, R.; Poli, G.; et al. Loading into nanoparticles improves quercetin’s efficacy in preventing neuroinflammation induced by oxysterols. PLoS ONE 2014, 9, e96795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Zhou, H.; Yin, T.; Gong, Y.; Yuan, G.; Chen, L.; Liu, J. Quercetin-modified gold-palladium nanoparticles as a potential autophagy inducer for the treatment of Alzheimer’s disease. J. Colloid Interface Sci. 2019, 552, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Wang, X.; Cai, S.; Liu, X.; Zhang, Y.; Yang, L.; Wang, C.; Yang, R. Quercetin nanoparticles with enhanced bioavailability as multifunctional agents toward amyloid induced neurotoxicity. J. Mater. Chem. B 2018, 6, 1387. [Google Scholar] [CrossRef]
- Wang, G.; Wang, J.J.; Luo, J.; Wang, L.; Chen, X.; Zhang, L.; Jiang, S. PEG2000-DPSE-coated quercetin nanoparticles remarkably enhanced anticancer effects through induced programed cell death on C6 glioma cells. J. Biomed. Mater. Res. Part A 2013, 101, 3076–3085. [Google Scholar] [CrossRef]
- Bardestani, A.; Ebrahimpour, S.; Esmaeili, A.; Esmaeili, A. Quercetin attenuates neurotoxicity induced by iron oxide nanoparticles. J. Nanobiotechnol. 2021, 19, 327. [Google Scholar] [CrossRef]
- Qi, Y.; Yi, P.; He, T.; Song, X.; Liu, Y.; Li, Q.; Zheng, J.; Song, R.; Liu, C.; Zhang, Z.; et al. Quercetin-loaded selenium nanoparticles inhibit amyloid-β aggregation and exhibit antioxidant activity. Colloid Surf. A 2020, 602, 125058. [Google Scholar] [CrossRef]
- World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 10 February 2022).
- Iliyasu, M.O.; Musa, S.A.; Oladele, S.B.; Iliya, I.A. The study of attenuating effects of quercetin on spatial memory impairment and degenerative changes in hippocampus of lead exposed rats. Afr. J. Cell. Pathol. 2021, 13, 1–10. [Google Scholar]
- Alshammari, G.M.; Al-Qahtani, W.H.; Alshuniaber, M.A.; Yagoub, A.E.A.; Al-Khalifah, A.S.; Al-Harbi, L.N.; Yahya, M.A. Quercetin improves the impairment in memory function and attenuates hippocampal damage in cadmium chloride-intoxicated male rats by suppressing acetylcholinesterase and concomitant activation of SIRT1 signaling. J. Funct. Foods 2021, 86, 104675. [Google Scholar] [CrossRef]
- Bohnen, N.I.; Grothe, M.J.; Ray, N.J.; Müller, M.L.; Teipel, S.J. Recent advances in cholinergic imaging and cognitive decline—revisiting the cholinergic hypothesis of dementia. Curr. Geriatr. Rep. 2018, 7, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braun, J.B.; Ruchel, J.B.; Adefegha, S.A.; Coelho, A.P.V.; Trelles, K.B.; Signor, C.; Rubin, M.A.; Oliveira, J.S.; Dornelles, G.L.; de Andrade, C.M.; et al. Neuroprotective effects of pretreatment with quercetin as assessed by acetylcholinesterase assay and behavioral testing in poloxamer-407 induced hyperlipidemic rats. Biomed. Pharmacother. 2017, 88, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Othman, A.; Amen, Y.; Shimizu, K. A novel acylated flavonol tetraglycoside and rare oleanane saponins with a unique acetal-linked dicarboxylic acid substituent from the xero-halophyte Bassia Indica. Fitoterapia 2021, 152, 104907. [Google Scholar] [CrossRef]
- Gupta, R.; Shukla, R.K.; Chandravanshi, L.P.; Srivastava, P.; Dhuriya, Y.K.; Shanker, J.; Singh, M.P.; Pant, A.B.; Khanna, V.K. Protective role of quercetin in cadmium-induced cholinergic dysfunctions in rat brain by modulating mitochondrial integrity and MAP kinase signaling. Mol. Neurobiol. 2017, 54, 4560–4583. [Google Scholar] [CrossRef]
- Maciel, R.M.; Carvalho, F.B.; Olabiyi, A.A.; Schmatz, R.; Gutierres, J.M.; Stefanello, N.; Zanini, D.; Rosa, M.M.; Andrade, C.M.; Rubin, M.A.; et al. Neuroprotective effects of quercetin on memory and anxiogenic-like behavior in diabetic rats: Role of ectonucleotidases and acetylcholinesterase activities. Biomed. Pharmacother. 2016, 84, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Marucci, G.; Buccioni, M.; Dal Ben, D.; Lambertucci, C.; Volpini, R.; Amenta, F. Efficacy of acetylcholinesterase inhibitors in Alzheimer’s disease. Neuropharmacology 2021, 190, 108352. [Google Scholar] [CrossRef]
- Moreta, M.P.G.; Burgos-Alonso, N.; Torrecilla, M.; Marco-Contelles, J.; Bruzos-Cidón, C. Efficacy of Acetylcholinesterase Inhibitors on Cognitive Function in Alzheimer’s Disease. Review of Reviews. Biomedicines 2021, 9, 1689. [Google Scholar] [CrossRef]
- Arancio, O.; Chao, M.V. Neurotrophins, synaptic plasticity and dementia. Curr. Opin. Neurobiol. 2007, 17, 325–330. [Google Scholar] [CrossRef]
- Yao, R.Q.; Qi, D.S.; Yu, H.L.; Liu, J.; Yang, L.H.; Wu, X.X. Quercetin attenuates cell apoptosis in focal cerebral ischemia rat brain via activation of BDNF–TrkB–PI3K/Akt signaling pathway. Neurochem. Res. 2012, 37, 2777–2786. [Google Scholar] [CrossRef]
- Rahvar, M.; Owji, A.A.; Mashayekhi, F.J. Effect of quercetin on the brain-derived neurotrophic factor gene expression in the rat brain. Bratisl. Lek. Listy 2018, 119, 28–31. [Google Scholar] [CrossRef] [Green Version]
- Selvakumar, K.; Bavithra, S.; Krishnamoorthy, G.; Arunakaran, J. Impact of quercetin on tight junctional proteins and BDNF signaling molecules in hippocampus of PCBs-exposed rats. Interdiscip. Toxicol. 2018, 11, 294–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walton, M.R.; Dragunow, M. Is CREB a key to neuronal survival? Trends Neurosci. 2000, 23, 48–53. [Google Scholar] [CrossRef]
- Batabyal, A.; Rivi, V.; Benatti, C.; Blom, J.M.C.; Lukowiak, K. Long-term memory of configural learning is enhanced via CREB upregulation by the flavonoid quercetin in Lymnaea stagnalis. J. Exp. Biol. 2021, 224, jeb242761. [Google Scholar] [CrossRef] [PubMed]
- Esplugues, J.V. NO as a signalling molecule in the nervous system. Br. J. Pharmacol. 2002, 135, 1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdalla, F.H.; Cardoso, A.M.; Pereira, L.B.; Schmatz, R.; Gonçalves, J.F.; Stefanello, N.; Fiorenza, A.M.; Gutierres, J.M.; Serres, J.D.; Zanini, D.; et al. Neuroprotective effect of quercetin in ectoenzymes and acetylcholinesterase activities in cerebral cortex synaptosomes of cadmium-exposed rats. Mol. Cell. Biochem. 2013, 381, 1–8. [Google Scholar] [CrossRef]
- Abdalla, F.H.; Schmatz, R.; Cardoso, A.M.; Carvalho, F.B.; Baldissarelli, J.; de Oliveira, J.S.; Rosa, M.M.; Gonçalves Nunes, M.A.; Rubin, M.A.; da Cruz, I.B.; et al. Quercetin protects the impairment of memory and anxiogenic-like behavior in rats exposed to cadmium: Possible involvement of the acetylcholinesterase and Na+, K+-ATPase activities. Physiol. Behav. 2014, 135, 152–167. [Google Scholar] [CrossRef]
- Halder, S.; Kar, R.; Galav, V.; Mehta, A.K.; Bhattacharya, S.K.; Mediratta, P.K.; Banerjee, B.D. Cadmium exposure during lactation causes learning and memory-impairment in F1 generation mice: Amelioration by quercetin. Drug Chem. Toxicol. 2016, 39, 272–278. [Google Scholar] [CrossRef]
- Li, H.; Chen, F.J.; Yang, W.L.; Qiao, H.Z.; Zhang, S.J. Quercetin improves cognitive disorder in aging mice by inhibiting NLRP3 inflammasome activation. Food Funct. 2021, 12, 717–725. [Google Scholar] [CrossRef]
- Dodla, M.C.; Mumaw, J.; Stice, S.L. Role of astrocytes, soluble factors, cells adhesion molecules and neurotrophins in functional synapse formation: Implications for human embryonic stem cell derived neurons. Curr. Stem Cell Res. Ther. 2010, 5, 251–260. [Google Scholar] [CrossRef] [Green Version]
- Diniz, L.P.; Matias, I.C.P.; Garcia, M.N.; Gomes, F.C.A. Astrocytic control of neural circuit formation: Highlights on TGF-beta signaling. Neurochem. Int. 2014, 78, 18–27. [Google Scholar] [CrossRef]
- Sharma, V.; Mishra, M.; Ghosh, S.; Tewari, R.; Basu, A.; Seth, P.; Sen, E. Modulation of interleukin-1β mediated inflammatory response in human astrocytes by flavonoids: Implications in neuroprotection. Brain Res. Bull. 2007, 73, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Yarza, R.; Vela, S.; Solas, M.; Ramirez, M.J. c-Jun N-terminal kinase (JNK) signaling as a therapeutic target for Alzheimer’s disease. Front. Pharmacol. 2016, 6, 321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakagawa, T.; Ohta, K. Quercetin regulates the integrated stress response to improve memory. Int. J. Mol. Sci. 2019, 20, 2761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakagawa, K.; Ueda, M.; Itoh, M.; Islam, S.; Tana Nakagawa, T. Dietary quercetin ameliorates memory impairment in a murine model of Alzheimer’s disease with obesity and diabetes, suppressing ATF4 expression. J. Neurol. Neurosci. 2017, 8, 234. [Google Scholar] [CrossRef]
- Zafir, A.; Banu, N. Induction of oxidative stress by restraint stress and corticosterone treatments in rats. Indian J. Biochem. Biophys. 2009, 46, 53–58. [Google Scholar]
- Schroeter, H.; Spencer, J.P.; Rice-Evans, C.; Williams, R.J. Flavonoids protect neurons from oxidized lowdensity- lipoprotein-induced apoptosis involving c-Jun N-terminal kinase (JNK), c-Jun and caspase-3. Biochem. J. 2001, 358, 547–557. [Google Scholar] [CrossRef]
- Das, T.K.; Jana, P.; Chakrabarti, S.K.; Abdul Hamid, M.R. Curcumin downregulates GSK3 and Cdk5 in scopolamine-induced Alzheimer’s disease rats abrogating Aβ 40/42 and tau hyperphosphorylation. J. Alzheimers Dis. Rep. 2019, 3, 257–267. [Google Scholar] [CrossRef] [Green Version]
- Kuo, Y.C.; Chen, C.L.; Rajesh, R. Optimized liposomes with transactivator of transcription peptide and anti-apoptotic drugs to target hippocampal neurons and prevent tau-hyperphosphorylated neurodegeneration. Acta Biomater. 2019, 87, 207–222. [Google Scholar] [CrossRef]
- Xie, L.; Helmerhorst, E.; Taddei, K.; Plewright, B.; Van Bronswijk, W.; Martins, R. Alzheimer’s β-amyloid peptides compete for insulin binding to the insulin receptor. J. Neurosci. 2002, 22, RC221. [Google Scholar] [CrossRef] [Green Version]
- Van Himbergen, T.M.; Beiser, A.S.; Ai, M.; Seshadri, S.; Otokozawa, S.; Au, R.; Schaefer, E.J. Biomarkers for insulin resistance and inflammation and the risk for all-cause dementia and Alzheimer disease: Results from the Framingham Heart Study. Arch. Neurol. 2012, 69, 594–600. [Google Scholar] [CrossRef] [Green Version]
- Hu, T.; Shi, J.J.; Fang, J.; Wang, Q.; Chen, Y.B.; Zhang, S.J. Quercetin ameliorates diabetic encephalopathy through SIRT1/ER stress pathway in db/db mice. Aging 2020, 12, 7015. [Google Scholar] [CrossRef] [PubMed]
- Molaei, A.; Hatami, H.; Dehghan, G.; Sadeghian, R.; Khajehnasiri, N. Synergistic effects of quercetin and regular exercise on the recovery of spatial memory and reduction of parameters of oxidative stress in animal model of Alzheimer’s disease. EXCLI J. 2020, 19, 596. [Google Scholar] [PubMed]
- Mzhelskaya, K.V.; Shipelin, V.A.; Shumakova, A.A.; Musaeva, A.D.; Soto, J.S.; Riger, N.A.; Trusov, N.V.; Kirbaeva, N.V.; Apryatin, S.A.; Gmoshinski, I.V. Effects of quercetin on the neuromotor function and behavioral responses of Wistar and Zucker rats fed a high-fat and high-carbohydrate diet. Behav. Brain Res. 2020, 378, 112270. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Hong, T.; Yang, Z.; Zhang, Y.; Wang, L.; Dong, M.; Zhao, J.; Mu, J.; Meng, Y. Effect of quercetin in the 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-induced mouse model of Parkinson’s disease. Evid. Based Complement. Alternat. Med. 2012, 2012, 928643. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Yuan, X.; Du, L.D.; He, G.R.; Du, G.H. Antagonism of quercetin against tremor induced by unilateral striatal lesion of 6-OHDA in rats. J. Asian Nat. Prod. Res. 2016, 18, 65–71. [Google Scholar] [CrossRef]
- El-Horany, H.E.; El-latif, R.N.A.; ElBatsh, M.M.; Emam, M.N. Ameliorative effect of quercetin on neurochemical and behavioral deficits in rotenone rat model of Parkinson’s disease: Modulating autophagy (quercetin on experimental Parkinson’s disease). J. Biochem. Mol. Toxicol. 2016, 30, 360–369. [Google Scholar] [CrossRef]
- Sandhir, R.; Mehrotra, A. Quercetin supplementation is effective in improving mitochondrial dysfunctions induced by 3-nitropropionic acid: Implications in Huntington’s disease. BBA Mol. Basis Dis. 2013, 1832, 421–430. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, J.; Singh, R.; Dutta, D.; Naskar, A.; Rajamma, U.; Mohanakumar, K.P. Quercetin improves behavioral deficiencies, restores astrocytes and microglia, and reduces serotonin metabolism in 3-nitropropionic acid-induced rat model of Huntington’s disease. CNS Neurosci. Ther. 2014, 20, 10–19. [Google Scholar] [CrossRef]
- Jain, D.; Gangshettiwar, A. Combination of lycopene, quercetin and poloxamer 188 alleviates anxiety and depression in 3-nitropropionic acid-induced Huntington’s disease in rats. J. Intercul. Ethnopharmacol. 2014, 3, 186. [Google Scholar] [CrossRef]
- Mirzazadeh, E.; Khezri, S.; Abtahi Froushani, S.M. Effects of Quercetin on Improving the Damage Caused by Free Radicals in the Rat Models of Multiple Sclerosis. ISMJ 2019, 22, 1–15. [Google Scholar] [CrossRef]
- Bhatia, N.K.; Modi, P.; Sharma, S.; Deep, S. Quercetin and baicalein act as potent antiamyloidogenic and fibril destabilizing agents for SOD1 fibrils. ACS Chem. Neurosci. 2020, 11, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.X.; Zhang, R.Y.; Rui, W.J.; Wang, Z.Q.; Feng, X. Quercetin alleviates chronic unpredictable mild stress-induced depressive-like behaviors by promoting adult hippocampal neurogenesis via FoxG1/CREB/BDNF signaling pathway. Behav. Brain Res. 2021, 406, 113245. [Google Scholar] [CrossRef] [PubMed]
- Martinowich, K.; Manji, H.; Lu, B. New insights into BDNF function in depression and anxiety. Nat. Neurosci. 2007, 10, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Anacker, C.; Zunszain, P.A.; Cattaneo, A.; Carvalho, L.A.; Garabedian, M.J.; Thuret, S.; Price, J.; Pariante, C.M. Antidepressants increase human hippocampal neurogenesis by activating the glucocorticoid receptor. Mol. Psychiatry 2011, 16, 738–750. [Google Scholar] [CrossRef] [Green Version]
- Kohler, O.; Krogh, J.; Mors, O.; Eriksen Benros, M. Inflammation in depression and the potential for anti-inflammatory treatment. Curr. Neuropharmacol. 2016, 14, 732–742. [Google Scholar] [CrossRef] [Green Version]
- Roumestan, C.; Michel, A.; Bichon, F.; Portet, K.; Detoc, M.; Henriquet, C.; Jaffuel, D.; Mathieu, M. Anti-inflammatory properties of desipramine and fluoxetine. Respir. Res. 2007, 8, 35. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Xu, X.; Jiang, T.; Jin, L.; Zhao, X.D.; Cheng, J.H.; Jin, X.J.; Ma, J.; Piao, H.N.; Piao, L.X. Sertraline ameliorates inflammation in CUMS mice and inhibits TNF-α-induced inflammation in microglia cells. Int. Immunopharmacol. 2019, 67, 119–128. [Google Scholar] [CrossRef]
- Monje, M.L.; Toda, H.; Palmer, T.D. Inflammatory blockade restores adult hippocampal neurogenesis. Science 2003, 302, 1760–1765. [Google Scholar] [CrossRef]
- Black, C.N.; Bot, M.; Scheffer, P.G.; Cuijpers, P.; Penninx, B.W. Is depression associated with increased oxidative stress? A systematic review and meta-analysis. Psychoneuroendocrinology 2015, 51, 164–175. [Google Scholar] [CrossRef] [Green Version]
- Bhatt, S.; Nagappa, A.N.; Patil, C.R. Role of oxidative stress in depression. Drug Discov. Today 2020, 25, 1270–1276. [Google Scholar] [CrossRef]
- Merzoug, S.; Toumi, M.L.; Tahraoui, A. Quercetin mitigates Adriamycin-induced anxiety-and depression-like behaviors, immune dysfunction, and brain oxidative stress in rats. Naunyn Schmiedeberg’s Arch. Pharmacol. 2014, 387, 921–933. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Yeom, M.; Shim, I.; Lee, H.; Hahm, D.H. Protective effects of quercetin on anxiety-like symptoms and neuroinflammation induced by lipopolysaccharide in rats. Evid. Based Complement. Altern. Med. 2020, 2020, 4892415. [Google Scholar] [CrossRef] [PubMed]
- Kosari-Nasab, M.; Shokouhi, G.; Ghorbanihaghjo, A.; Mesgari-Abbasi, M.; Salari, A.A. Quercetin mitigates anxiety-like behavior and normalizes hypothalamus–pituitary–adrenal axis function in a mouse model of mild traumatic brain injury. Behav. Pharmacol. 2019, 30, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Holzmann, I.; da Silva, L.M.; da Silva, J.A.C.; Steimbach, V.M.B.; de Souza, M.M. Antidepressant-like effect of quercetin in bulbectomized mice and involvement of the antioxidant defenses, and the glutamatergic and oxidonitrergic pathways. Pharmacol. Biochem. Behav. 2015, 136, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Anjaneyulu, M.; Chopra, K.; Kaur, I. Antidepressant activity of quercetin, a bioflavonoid, in streptozotocin-induced diabetic mice. J. Med. Food 2003, 6, 391–395. [Google Scholar] [CrossRef]
- Toumi, M.L.; Merzoug, S.; Baudin, B.; Tahraoui, A. Quercetin alleviates predator stress-induced anxiety-like and brain oxidative signs in pregnant rats and immune count disturbance in their offspring. Pharmacol. Biochem. Behav. 2013, 107, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.W.; Lee, S. Anxiolytic effects of quercetin: Involvement of GABAergic system. J. Life Sci 2014, 24, 290–296. [Google Scholar] [CrossRef]
- Zhang, J.L.; Liu, M.; Cui, W.; Yang, L.; Zhang, C.N. Quercetin affects shoaling and anxiety behaviors in zebrafish: Involvement of neuroinflammation and neuron apoptosis. Fish Shellfish. Immunol. 2020, 105, 359–368. [Google Scholar] [CrossRef]
- Shrivastava, N.; Dey, A.; Chatterjee, S.S.; Kumar, V. Adaptogenic potential of triethylene glycol and quercetin in stressed mice. Pharm. Pharmacol. Int. J. 2015, 2, 197–206. [Google Scholar]
- Al-Hasawi, N.A.; Amine, S.A.; Novotny, L. The In Vitro Anti-Proliferative Interaction of Flavonoid Quercetin and Toxic Metal Cadmium in the 1321N1 Human Astrocytoma Cell Line. Sci. Pharm. 2018, 86, 36. [Google Scholar] [CrossRef] [Green Version]
- Jakubowicz-Gil, J.; Langner, E.; Wertel, I.; Piersiak, T.; Rzeski, W. Temozolomide, quercetin and cell death in the MOGGCCM astrocytoma cell line. Chem. Biol. Interact. 2010, 188, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Jakubowicz-Gil, J.; Langner, E.; Bądziul, D.; Wertel, I.; Rzeski, W. Quercetin and sorafenib as a novel and effective couple in programmed cell death induction in human gliomas. Neurotox. Res. 2014, 26, 64–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugantha Priya, E.; Selvakumar, K.; Bavithra, S.; Elumalai, P.; Arunkumar, R.; Raja Singh, P.; Brindha Mercy, A.; Arunakaran, J. Anti-cancer activity of quercetin in neuroblastoma: An in vitro approach. Neurol. Sci. 2014, 35, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Lagerweij, T.; Hiddingh, L.; Biesmans, D.; Crommentuijn, M.H.; Cloos, J.; Li, X.N.; Kogiso, M.; Tannous, B.A.; Vandertop, W.P.; Noske, D.P.; et al. A chemical screen for medulloblastoma identifies quercetin as a putative radiosensitizer. Oncotarget 2015, 7, 35776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Souza, P.O.; Bianchi, S.E.; Figueiró, F.; Heimfarth, L.; Moresco, K.S.; Gonçalves, R.M.; Hoppe, J.B.; Klein, C.P.; Salbego, C.G.; Gelain, D.P.; et al. Anticancer activity of flavonoids isolated from Achyrocline satureioides in gliomas cell lines. Toxicol. In Vitro 2018, 51, 23–33. [Google Scholar] [CrossRef]
- Jang, E.; Kim, I.Y.; Kim, H.; Lee, D.M.; Seo, D.Y.; Lee, J.A.; Choi, K.S.; Kim, E. Quercetin and chloroquine synergistically kill glioma cells by inducing organelle stress and disrupting Ca2+ homeostasis. Biochem. Pharmacol. 2020, 178, 114098. [Google Scholar] [CrossRef]
- Rizvi, S.M.D.; Hussain, T.; Mehmood, K.; Moin, A.; Alanazi, A.S.; Subaiea, G.M. Molecular docking and dynamic simulation study to explore quercetin as a multi-potent candidate against gliomas. Trop. J. Pharm. Res. 2021, 20, 815–823. [Google Scholar] [CrossRef]
- da Silva, A.B.; Coelho, P.L.C.; das Neves Oliveira, M.; Oliveira, J.L.; Amparo, J.A.O.; da Silva, K.C.; Soares, J.R.P.; Pitanga, B.P.S.; Dos Santos Souza, C.; de Faria Lopes, G.P.; et al. The flavonoid rutin and its aglycone quercetin modulate the microglia inflammatory profile improving antiglioma activity. Brain Behav. Immun. 2020, 85, 170–185. [Google Scholar] [CrossRef]
- Kim, H.; Moon, J.Y.; Ahn, K.S.; Cho, S.K. Quercetin induces mitochondrial mediated apoptosis and protective autophagy in human glioblastoma U373MG cells. Oxid. Med. Cell. Longev. 2013, 2013, 596496. [Google Scholar] [CrossRef] [Green Version]
- Kusaczuk, M.; Krętowski, R.; Naumowicz, M.; Stypułkowska, A.; Cechowska-Pasko, M. A Preliminary Study of the Effect of Quercetin on Cytotoxicity, Apoptosis, and Stress Responses in Glioblastoma Cell Lines. Int. J. Mol. Sci. 2022, 23, 1345. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, Z.G.; Yang, J.Q.; Zhou, Y.; Meng, L.H.; Wang, H.; Li, C.L. Low concentration of quercetin antagonizes the invasion and angiogenesis of human glioblastoma U251 cells. Oncotargets Ther. 2017, 10, 4023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, H.C.; Jiang, Q.; Yu, Y.; Mei, J.P.; Cui, Y.K.; Zhao, W.J. Quercetin promotes cell apoptosis and inhibits the expression of MMP-9 and fibronectin via the AKT and ERK signalling pathways in human glioma cells. Neurochem. Int. 2015, 80, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Jung, N.; Lee, S.; Sohng, J.K.; Jung, H.J. Apigenin inhibits cancer stem cell-like phenotypes in human glioblastoma cells via suppression of c-Met signaling. Phytother. Res. 2016, 30, 1833–1840. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Shen, C.; Li, C.; Liu, Y.; Gao, D.; Shi, C.; Peng, F.; Liu, Z.; Zhao, B.; Zheng, Z.; et al. Inhibition of autophagy induced by quercetin at a late stage enhances cytotoxic effects on glioma cells. Tumour. Biol. 2016, 37, 3549–3560. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tang, C.; Li, L.; Li, R.; Fan, Y. Quercetin blocks t-AUCB-induced autophagy by Hsp27 and Atg7 inhibition in glioblastoma cells in vitro. J. Neurooncol. 2016, 129, 39–45. [Google Scholar] [CrossRef]
- Li, J.; Tang, C.; Li, L.; Li, R.; Fan, Y. Quercetin sensitizes glioblastoma to t-AUCB by dual inhibition of Hsp27 and COX-2 in vitro and in vivo. J. Exp. Clin. Cancer Res. 2016, 35, 61. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Tang, Z.G.; Lin, Y.; Qu, X.G.; Lv, W.; Wang, G.B.; Li, C.L. Effects of quercetin on proliferation and migration of human glioblastoma U251 cells. Biomed. Pharmacother. 2017, 92, 33–38. [Google Scholar] [CrossRef]
- Michaud-Levesque, J.; Bousquet-Gagnon, N.; Béliveau, R. Quercetin abrogates IL-6/STAT3 signaling and inhibits glioblastoma cell line growth and migration. Exp. Cell Res. 2012, 318, 925–935. [Google Scholar] [CrossRef]
- Sang, D.P.; Li, R.J.; Lan, Q. Quercetin sensitizes human glioblastoma cells to temozolomide in vitro via inhibition of Hsp27. Acta Pharmacol. Sin. 2014, 35, 832–838. [Google Scholar] [CrossRef] [Green Version]
- Pozsgai, E.; Bellyei, S.; Cseh, A.; Boronkai, A.; Racz, B.; Szabo, A.; Sumegi, B.; Hocsak, E. Quercetin increases the efficacy of glioblastoma treatment compared to standard chemoradiotherapy by the suppression of PI-3-kinase-Akt pathway. Nutr. Cancer 2013, 65, 1059–1066. [Google Scholar] [CrossRef] [Green Version]
- Taylor, M.A.; Khathayer, F.; Ray, S.K. Quercetin and sodium butyrate synergistically increase apoptosis in rat C6 and human T98G glioblastoma cells through inhibition of autophagy. Neurochem. Res. 2019, 44, 1715–1725. [Google Scholar] [CrossRef] [PubMed]
- Tsiailanis, A.D.; Renziehausen, A.; Kiriakidi, S.; Vrettos, E.I.; Markopoulos, G.S.; Sayyad, N.; Hirmiz, B.; Aguilar, M.I.; Del Borgo, M.P.; Kolettas, E.; et al. Enhancement of glioblastoma multiforme therapy through a novel Quercetin-Losartan hybrid. Free Radic. Biol. Med. 2020, 160, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Bhandarkar, S.; Prabhakar, B.; Shende, P. Quercetin-loaded platelets as a potential targeted therapy for glioblastoma multiforme cell line U373-MG. Biotechnol. J. 2021, 16, 2100271. [Google Scholar] [CrossRef] [PubMed]
- Ersoz, M.; Erdemir, A.; Derman, S.; Arasoglu, T.; Mansuroglu, B. Quercetin-loaded nanoparticles enhance cytotoxicity and antioxidant activity on C6 glioma cells. Pharm. Dev. Technol. 2020, 25, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, J.J.; Yang, G.Y.; Du, S.M.; Zeng, N.; Li, D.S.; Li, R.M.; Chen, J.Y.; Feng, J.B.; Yuan, S.H.; et al. Effects of quercetin nanoliposomes on C6 glioma cells through induction of type III programmed cell death. Int. J. Nanomed. 2012, 7, 271–280. [Google Scholar]
- Lou, M.; Zhang, L.N.; Ji, P.G.; Feng, F.Q.; Liu, J.H.; Yang, C.; Li, B.F.; Wang, L. Quercetin nanoparticles induced autophagy and apoptosis through AKT/ERK/Caspase-3 signaling pathway in human neuroglioma cells: In vitro and in vivo. Biomed. Pharmacother. 2016, 84, 1–9. [Google Scholar] [CrossRef]
- Wang, G.; Wang, J.J.; Chen, X.L.; Du, L.; Li, F. Quercetin-loaded freeze-dried nanomicelles: Improving absorption and anti-glioma efficiency in vitro and in vivo. J. Control. Release 2016, 235, 276–290. [Google Scholar] [CrossRef]
- Barbarisi, M.; Iaffaioli, R.V.; Armenia, E.; Schiavo, L.; De Sena, G.; Tafuto, S.; Barbarisi, A.; Quagliariello, V. Novel nanohydrogel of hyaluronic acid loaded with quercetin alone and in combination with temozolomide as new therapeutic tool, CD44 targeted based, of glioblastoma multiforme. J. Cell Physiol. 2018, 233, 6550–6564. [Google Scholar] [CrossRef]
- Hu, J.; Wang, J.; Wang, G.; Yao, Z.; Dang, X. Pharmacokinetics and antitumor efficacy of DSPE-PEG2000 polymeric liposomes loaded with quercetin and temozolomide: Analysis of their effectiveness in enhancing the chemosensitization of drug-resistant glioma cells. Int. J. Mol. Med. 2016, 37, 690–702. [Google Scholar] [CrossRef] [Green Version]
- Nezami, M.A.; Duma, C. Proof of concept in a case study of glioblastoma multiforme successfully treated with IV Quercetin in combination with leading edge gamma knife and standard treatments. J. Cancer Ther. 2018, 9, 522–528. [Google Scholar] [CrossRef] [Green Version]
- Olson, C.; Thornton, J.; Adam, G.; Lieberman, H. Effects of 2 adenosine antagonists, quercetin and caffeine, on vigilance and mood. J. Clin. Psychopharmacol. 2010, 30, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Broman-Fulks, J.J.; Canu, W.H.; Trout, K.L.; Nieman, D.C. The effects of quercetin supplementation on cognitive functioning in a community sample: A randomized, placebo-controlled trial. Ther. Adv. Psychopharmacol. 2012, 2, 131–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.I.; Tchkonia, T.; Pirtskhalava, T.; Gower, A.C.; Ding, H.; Giorgadze, N.; Palmer, A.K.; Ikeno, Y.; Hubbard, G.B.; Lenburg, M.; et al. The Achilles’ heel of senescent cells: From transcriptome to senolytic drugs. Aging Cell 2015, 14, 644–658. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, M.M.; Garbarino, V.R.; Zilli, E.M.; Petersen, R.C.; Kirkland, J.L.; Tchkonia, T.; Musi, N.; Seshadri, S.; Craft, S.; Orr, M.E. Senolytic therapy to modulate the progression of Alzheimer’s disease (SToMP-AD): A pilot clinical trial. J. Prev. Alzheimer’s Dis. 2022, 9, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Rifaai, R.A.; Mokhemer, S.A.; Saber, E.A.; Abd El-Aleem, S.A.; El-Tahawy, N.F.G. Neuroprotective effect of quercetin nanoparticles: A possible prophylactic and therapeutic role in alzheimer’s disease. J. Chem. Neuroanat. 2020, 107, 101795. [Google Scholar] [CrossRef]




| Formulations | Animal Model/Doses/Route of Administration | Conclusions | Ref. |
|---|---|---|---|
| Quercetin-loaded cationic nanostructured lipid carriers (QR-CNLC) | In vivo study Healthy male C57BL/6J mice; oral administration of: (a) quercetin suspended in 0.5%, w/v CMC-Na aqueous solution (b) QR-CNLC at a dose of 25 mg/kg bwt | QR-CNLC failed to accumulate higher quercetin in brain tissue than quercetin suspension | [34] |
| Quercetin conjugated with superparamagnetic iron oxide nanoparticles (QT-SPION) | In vivo study: Healthy Wistar male rats orally fed by gavage with: (a) quercetin solution (b) QT-SPION at a dose of 50 and 100 mg/kg/d for 7 days | QT-SPION ↑ the bioavailability of quercetin in the brain | [33] |
| Quercetin-loaded poly(n-butylcyano acrylate) nanoparticles (QT-PBCA NPs); QT-PBCA NPs coated with polysorbate-80 (P-80) on their surfaces (QT-PBCA + P-80) | In vivo study Wistar male albino rats; administration of: (a) quercetin (b) QT-PBCA NPs (c) QT-PBCA + P-80 at a dose of 50 mg/kg bwt via oral feeding cannula | QT-PBCA NPs ↑ the quercetin bioavailability by 2.38-fold QT-PBCA + P-80 ↑ the quercetin bioavailability by 4.93-fold | [35] |
| Quercetin-loaded nano lipidic carriers employing phospholipids and tocopherol acetate (NLCs); Quercetin-loaded solid lipid nanoparticles (SLNs) | In vivo study Wistar male rats; oral gavage of: (a) quercetin (b) NLCs (c) SLNs in equivalent quercetin doses of 50 mg/kg | SLNs ↑ the brain delivery of quercetin by 3.2 times ↑ the bioavailability of quercetin by 3.5-fold NLCs ↑ the brain delivery of quercetin by 5.6 times ↑ the bioavailability of quercetin by 5.4-fold ↑ neuroprotective activity | [36] |
| Plasma exosomes loaded with quercetin (Exo-Que) | In vivo study Okadaic acid-induced model of tauopathy and cognitive deficiency; AD mice; peritoneal injection of (a) quercetin (b) Exo-Que | Exo-Que ↑ quercetin concentration by 2.5-fold in cerebrum and 1.5-fold in cerebellum ↑ effect in MWM test | [37] |
| Quercetin-modified sulfur nanoparticles (Qc@SNPs) embedded in microbubbles (MB) (Qc@SNPs-MB) | In vivo study Fluorescence real-time imaging concentration of ruthenium-labelled NPs: 1 mg/kg MWM test in AD mice, NPs given i.v., 2 days/week for 5 weeks, concentration of both NPs: 5 mg/kg Concentration of NPs = 10 μg/mL | Qc@SNPs ↑ the brain targeting and bioavailability of quercetin ↓ ER stress in nerve cells | [38] |
| Quercetin-loaded zein-based nanofibers developed using electrospinning technique | In vivo study Streptozotocin induced diabetic rat model; adult Wistar male rats; animals exposed to crush injury and subjected to zein-based nanofibers loaded with quercetin at concentrations of: (a) 5% (b) 10% (c) 15% for 21 days | Different concentrations of quercetin can be loaded into nanofibers without differences in their diameters. The release of quercetin from tested nanofibers was >60% within first 6 h, obtained a maximum within 24 h, and lasted at least 72 h. Quercetin-loaded, zein-based nanofibers ↑ the effect of quercetin in neuropathic injury in rats | [39] |
| Quercetin conjugated superparamagnetic iron oxide nanoparticles (QCSPIONs) | In vivo study Sreptozotocin induced diabetic rat model; adult Wistar male rats orally fed by gavage with: (a) SPIONs (b) quercetin (c) QCSPIONs at a dose of 25 mg/kg for a period of 35 consecutive days | QCSPIONs ↑ SOD1 and CAT expression levels ↑ learning and memory in diabetic rats ↑ Nrf2 and antioxidant genes expression level by miR-27a regulation | [40] |
| Formulations | Assay | Conclusions | Ref. |
| Quercetin-loaded nanoemulsion prepared using spontaneous emulsification technique (QUR-loaded NE) | In vitro study Dialysis membrane method; isolated sheep nasal mucosa; QUR-loaded NE | QUR-loaded NE for intranasal administration seems to be a promising delivery system for anticancer agents to achieve CNS targets | [41] |
| Fast-dissolving core-shell composite microparticles of quercetin fabricated using coaxial electrospraying | In vitro study Permeation study performer using a RYJ-6A Diffusion Test Apparatus Quercetin contents: (a) M2 (262 mg) (b) M3 (187 mg) (c) M5 (120 mg) (d) quercetin (20 mg) | Fast-dissolving core-shell composite microparticles of quercetin ↑ the dissolution and permeation rates of quercetin | [42] |
| Quercetin-loaded β-CD dodecylcarbonate nanoparticles | In vitro study Dialysis bag method; SH-SY5Y cells; quercetin-loaded β-CD dodecylcarbonate nanoparticles | Quercetin-loaded nanoparticles ↑ BBB permeation, bioavailability, and access to target cells ↑ neuroprotective efficacy | [43] |
| Quercetin-modified polysorbate 80 (P-80)-coated AuPd nanoparticles (Qu@P-80@AuPd) | In vitro study Transwell co-culture system; bEnd.3 cells; SH-SY5Y cells to co-culture; flow cytometric method to measure transport efficiency through BBB of: (a) Concave cubic Qu@AuPd at a dose of 10 μg/mL (b) Concave cubic Qu@P-80@AuPd at a dose of 10 μg/mL | Concave cubic Qu@P-80@AuPd ↑ BBB permeability and good biocompatibility in Transwell assay, MTT, and apoptosis Concave cubic Qu@P-80@AuPd could have a potential in AD treatment as an autophagy inducer | [44] |
| Quercetin-loaded poly(n-butylcyanoacrylate) (PBCA) nanoparticles (QT-PBCA NPs) Quercetin-loaded poly(n-butylcyanoacrylate) (PBCA) nanoparticles coated with polysorbate-80 (P-80) (QT-PBCA + P-80) | In vitro study Dialysis bag method; (a) quercetin (b) QT-PBCA NPs (c) QT-PBCA + P-80 | QT-PBCA + P-80 ↑ oral bioavailability of quercetin ↑ the BBB penetration and CNS efficacy | [35] |
| Quercetin nanoparticles developed by pulsed laser ablation in water (Que NPs) | In vitro study Dialysis-based in vitro drug release assay; (a) Que NPs (b) quercetin powder in PBS | Que NPs ↑ bioavailability of quercetin ↑ efficacy of quercetin as a result of prolonged residence time in systemic circulation | [45] |
| PEG2000-DPSE-coated quercetin nanoparticles (PEG2000-DPSE-QUE-NPS) | In vitro study Annexin V-FITC K; Mitochondrial Membrane Potential Assay Kit; glioma C6 cells; PEG2000-DPSE-QUE-NPS | PEG2000-DPSE-QUE-NPS ↑ solubility of quercetin ↑ efficacy of quercetin in inhibiting glioma C6 cells through induced apoptosis and necrosis. | [46] |
| Quercetin-loaded nanolipidic carriers employing phospholipids and tocopherol acetate (NLCs); Quercetin-loaded solid lipid nanoparticles (SLNs) | In vitro study DPPH antioxidant assay; Caco-2 cellular permeability study; (a) quercetin (b) NLCs (c) SLNs (d) ascorbic acid | NLCs and SLNs ↑ quercetin intestinal permeability ↑ antioxidant effect | [36] |
| In Vivo Study | Treatments | Results | Ref. |
|---|---|---|---|
| EPM in pregnant female Wistar rats acutely stressed by a predator (a cat) | quercetin: 50 mg/kg p.o., for 6 days (from 14th to the 19th day of gestation) | Anxiolytic effect of quercetin. A significant decrease in the elevated by the stressor corticosterone level, alleviation of oxidative stress (reduced GSH, increased GST). | [93] |
| EPM in 2.5 mg/kg CD-intoxicated male Wistar rats | quercetin: 5, 25, 50 mg/kg p.o., administered 5 days a week for 45 days | Inhibiting anxiogenic effect of Cd at all the doses. | [46] |
| EPM in male Wistar rats with 3-nitropropionic acid (3-NP)-induced Huntington’s disease | quercetin: 50 mg/kg p.o., lycopene: 25 mg/kg p.o.; given along with 3-NP for 14 days | Anxiolytic effect of quercetin given along with lycopene. The effect was not observable in case of a single substance, but it seemed the effect of lycopene was stronger than quercetin. | [94] |
| EPM in ICR mice | quercetin: 1.25, 2.5, 5, 10 mg/kg p.o., 1 h before the test; buspirone: 2 mg/kg i.p.; 30 min before the test | Anxiolytic effect of 5 mg/kg quercetin (bell-shaped dose–response curve), comparable with 2 mg/kg buspirone, with no muscle relaxant effect or influence on locomotor activity. The effect was mediated by GABA-ergic system. | [95] |
| EPM in adriamycin (ADR)-injected male Wistar rats | quercetin: 60 mg/kg i.p.; 24, 5, and 1 h before the test session | Anxiolytic effect of quercetin. Oxidative stress level was alleviated (GSH maintained at high level; products of lipid peroxidation eliminated). | [97] |
| MBT in male albino mice | quercetin: 5 mg/kg, p.o., triethylene glycol (TEG): 5 mg/kg p.o.; once daily for 11 (standard, one-zone MBT) or 12 days (two-zones MBT) | Anti-obsessive-compulsive effect of quercetin but only in 12th day of experiment in 2-zone version of MBT. | [100] |
| EPM in STZ-induced diabetic male Wistar rats | quercetin: 5, 25, 50 mg/kg p.o.; for 40 days | Anxiolytic effect of quercetin, significant at all tested doses. | [27] |
| EPM, light-dark box, zero maze in mild traumatic brain injury (mTBI)-induced NMRI mice | quercetin: 50 mg/kg p.o., diazepam 3 mg/kg p.o.; once daily for 14 consecutive days (days 10–24 postinjury) | In all the tests, quercetin exerted significant anxiolytic effect, which was comparable to diazepam (although the dose was much higher). HPA axis was normalized by the drugs (ACTH and corticosterone level were decreased vs. mTBI group). | [96] |
| EPM in LPS-lateral ventricle-injected in male SD rats | quercetin: 50 and 100 mg/kg i.p., ibuprofen (the dose not showed) i.p.; once daily for 21 days after LPS injection | Anxiolytic effect of quercetin was dose-dependent but only at the dose of 100 mg/kg was a significant effect noted. Anxiety index was comparable low with ibuprofen. A reduction in inflammatory response was observed: a decrease in inflammatory markers: enzyme, COX-2, and cytokines, e.g., IL-1β, IL-6, NF-κB, in expression of inducible NOS and an increase in expression of BDNF. | [98] |
| tank tests in zebrafish (Danio rerio) | quercetin: 0.01, 0.1, 1, 10, 100, 1000 μg/L | At the lower doses anxiolytic effect, but the highest dose was angiogenic. The molecular mechanism involves alteration in inflammatory (an increase in antioxidant enzymes, e.g., SOD, a decrease in pro-inflammatory enzyme, COX-2, and cytokines, e.g., IL-1β, IL-6, IL-10, TNF-α), a suppression in an apoptotic response. | [99] |
| Biological Effect | Cellular Mechanism | References |
|---|---|---|
| apoptosis (intrinsic pathway) | cytochrome c release mitochondria membrane depolimerization caspase-3 and -9 PARP-1 cleavage MAPK cascade Bcl-2, survivin p53 NF-κB TNFα | [114,116,120,121,122,123] |
| cell cycle arrest | cyclin D1, D2 p21 CDK2 | [124] |
| autophagy | Beclin-1 protein LC3 protein | [120,125,126,127] |
| inhibition of angiogenesis | capillary formation endothelial cells proliferation mTOR | [128] |
| inhibition of metastasis and migration | VEGF, MMP-2, MMP-9, fibronectin migration ability in wound healing and/or transwell assay | [123,124,126,127,128,129] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wróbel-Biedrawa, D.; Grabowska, K.; Galanty, A.; Sobolewska, D.; Podolak, I. A Flavonoid on the Brain: Quercetin as a Potential Therapeutic Agent in Central Nervous System Disorders. Life 2022, 12, 591. https://doi.org/10.3390/life12040591
Wróbel-Biedrawa D, Grabowska K, Galanty A, Sobolewska D, Podolak I. A Flavonoid on the Brain: Quercetin as a Potential Therapeutic Agent in Central Nervous System Disorders. Life. 2022; 12(4):591. https://doi.org/10.3390/life12040591
Chicago/Turabian StyleWróbel-Biedrawa, Dagmara, Karolina Grabowska, Agnieszka Galanty, Danuta Sobolewska, and Irma Podolak. 2022. "A Flavonoid on the Brain: Quercetin as a Potential Therapeutic Agent in Central Nervous System Disorders" Life 12, no. 4: 591. https://doi.org/10.3390/life12040591
APA StyleWróbel-Biedrawa, D., Grabowska, K., Galanty, A., Sobolewska, D., & Podolak, I. (2022). A Flavonoid on the Brain: Quercetin as a Potential Therapeutic Agent in Central Nervous System Disorders. Life, 12(4), 591. https://doi.org/10.3390/life12040591