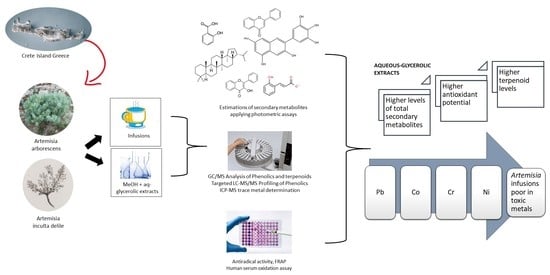
Artemisia arborescens and Artemisia inculta from Crete; Secondary Metabolites, Trace Metals and In Vitro Antioxidant Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Standards and Solvents
2.2. Sampling and Preparation
2.3. Preparation of Samples
2.3.1. Decoctions
2.3.2. Herbal Extracts
2.4. Determination of Total Phenolic Content
2.5. Determination of Total Flavonoid Content (TFC)
2.6. Determination of Phenolic Classes
2.7. Determination of Total Terpenes
2.8. Assessment of Antioxidant Activity
2.9. GC/MS Analysis of Phenolic Compounds and Terpenoids
2.10. Targeted LC-MS/MS Profiling of Phenolic Compounds in Glycerolic Extracts
2.11. Trace Metals Determination
3. Results and Discussion
3.1. Secondary Metabolites
3.2. Antioxidant Properties
3.3. GC-MS Profiling
3.4. HPLC-MS Profiling in Hydro-Glycolic Extracts
3.5. Trace Metals
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leonti, M.; Casu, L. Traditional medicines and globalization: Current and future perspectives in ethnopharmacology. Front. Pharmacol. 2013, 4, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alami Merrouni, I.; Elachouri, M. Anticancer medicinal plants used by Moroccan people: Ethnobotanical, preclinical, phytochemical and clinical evidence. J. Ethnopharmacol. 2021, 266, 113435. [Google Scholar] [CrossRef] [PubMed]
- Bussmann, R.W.; Glenn, A. Medicinal plants used in Northern Peru for reproductive problems and female health. J. Ethnobiol. Ethnomed. 2010, 6, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali-Shtayeh, M.S.; Jamous, R.M.; Al-Shafie, J.H.; Elgharabah, W.A.; Kherfan, F.A.; Qarariah, K.H.; Khdair, I.S.; Soos, I.M.; Musleh, A.A.; Isa, B.A.; et al. Traditional knowledge of wild edible plants used in Palestine (Northern West Bank): A comparative study. J. Ethnobiol. Ethnomed. 2008, 4, 13. [Google Scholar] [CrossRef] [Green Version]
- Hassan, W.; Noreen, H.; Rehman, S.; Gul, S.; Amjad Kamal, M.; Paul Kamdem, J.; Zaman, B.; BT da Rocha, J. Oxidative Stress and Antioxidant Potential of One Hundred Medicinal Plants. Curr. Top. Med. Chem. 2017, 17, 1336–1370. [Google Scholar] [CrossRef]
- Watson, B.; Kennel, E. Artemisia spp. Available online: https://www.herbsociety.org (accessed on 19 March 2022).
- Feng, X.; Cao, S.; Qiu, F.; Zhang, B. Traditional application and modern pharmacological research of Artemisia annua L. Pharmacol. Ther. 2020, 216, 107650. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Herrera-Bravo, J.; Semwal, P.; Painuli, S.; Badoni, H.; Ezzat, S.M.; Farid, M.M.; Merghany, R.M.; Aborehab, N.M.; Salem, M.A.; et al. Artemisia spp.: An Update on Its Chemical Composition, Pharmacological and Toxicological Profiles. Oxidative Med. Cell. Longev. 2022, 2022, 5628601. [Google Scholar] [CrossRef]
- Watson, L.E.; Bates, P.L.; Evans, T.M.; Unwin, M.M.; Estes, J.R. Molecular phylogeny of Subtribe Artemisiinae (Asteraceae), including Artemisia and its allied and segregate genera. BMC Evol. Biol. 2002, 2, 17. [Google Scholar] [CrossRef]
- Algieri, F.; Rodriguez-Nogales, A.; Rodriguez-Cabezas, M.E.; Risco, S.; Ocete, M.A.; Galvez, J. Botanical Drugs as an Emerging Strategy in Inflammatory Bowel Disease: A Review. Mediat. Inflamm. 2015, 2015, 179616. [Google Scholar] [CrossRef] [Green Version]
- Ekiert, H.; Klimek-Szczykutowicz, M.; Rzepiela, A.; Klin, P.; Szopa, A. Artemisia Species with High Biological Values as a Potential Source of Medicinal and Cosmetic Raw Materials. Molecules 2022, 27, 6427. [Google Scholar] [CrossRef]
- Jaradat, N.; Qneibi, M.; Hawash, M.; Al-Maharik, N.; Qadi, M.; Abualhasan, M.N.; Ayesh, O.; Bsharat, J.; Khadir, M.; Morshed, R.; et al. Assessing Artemisia arborescens essential oil compositions, antimicrobial, cytotoxic, anti-inflammatory, and neuroprotective effects gathered from two geographic locations in Palestine. Ind. Crop. Prod. 2022, 176, 114360. [Google Scholar] [CrossRef]
- Russo, A.; Bruno, M.; Avola, R.; Cardile, V.; Rigano, D. Chamazulene-Rich Artemisia arborescens Essential Oils Affect the Cell Growth of Human Melanoma Cells. Plants 2020, 9, 1000. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.-W.; Chen, D.; Chen, L.; He, B.; Li, Y. A comprehensive overview of Artemisinin and its derivatives as anticancer agents. Eur. J. Med. Chem. 2022, 247, 115000. [Google Scholar] [CrossRef]
- Dhibi, S.; Bouzenna, H.; Samout, N.; Tlili, Z.; Elfeki, A.; Hfaiedh, N. Nephroprotective and antioxidant properties of Artemisia arborescens hydroalcoholic extract against oestroprogestative-induced kidney damages in rats. Biomed. Pharmacother. 2016, 82, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Dhibi, S.; Ettaya, A.; Elfeki, A.; Hfaiedh, N. Protective effects of Artemisia arborescens essential oil on oestroprogestative treatment induced hepatotoxicity. Nutr. Res. Pract. 2015, 9, 466. [Google Scholar] [CrossRef] [Green Version]
- Ijomone, O.M.; Ifenatuoha, C.W.; Aluko, O.M.; Ijomone, O.K.; Aschner, M. The aging brain: Impact of heavy metal neurotoxicity. Crit. Rev. Toxicol. 2020, 50, 801–814. [Google Scholar] [CrossRef]
- Fu, Z.; Xi, S. The effects of heavy metals on human metabolism. Toxicol. Mech. Methods 2020, 30, 167–176. [Google Scholar] [CrossRef]
- Shehata, E.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Extraction optimisation using water/glycerol for the efficient recovery of polyphenolic antioxidants from two Artemisia species. Sep. Purif. Technol. 2015, 149, 462–469. [Google Scholar] [CrossRef]
- Karakashov, B.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Optimisation of polyphenol extraction from Hypericum perforatum (St. John’s Wort) using aqueous glycerol and response surface methodology. J. Appl. Res. Med. Aromat. Plants 2015, 2, 1–8. [Google Scholar] [CrossRef]
- Mitić, S.S.; Paunović, D.Đ.; Pavlović, A.N.; Tošić, S.B.; Stojković, M.B.; Mitić, M.N. Phenolic profiles and total antioxidant capacity of marketed beers in Serbia. Int. J. Food Prop. 2013, 17, 908–922. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Tornberg, E.; Gekas, V. Recovery and preservation of phenols from olive waste in ethanolic extracts. J. Chem. Technol. Biotechnol. 2010, 85, 1148–1155. [Google Scholar] [CrossRef]
- Fan, J.-P.; He, C.-H. Simultaneous quantification of three major bioactive triterpene acids in the leaves of diospyros kaki by high-performance liquid chromatography method. J. Pharm. Biomed. Anal. 2006, 41, 950–956. [Google Scholar] [CrossRef]
- Lantzouraki, D.Z.; Sinanoglou, V.J.; Zoumpoulakis, P.G.; Glamočlija, J.; Ćirić, A.; Soković, M.; Heropoulos, G.; Proestos, C. Antiradical–antimicrobial activity and phenolic profile of pomegranate (Punica granatum L.) juices from different cultivars: A comparative study. RSC Adv. 2015, 5, 2602–2614. [Google Scholar] [CrossRef]
- Lantzouraki, D.Z.; Tsiaka, T.; Soteriou, N.; Asimomiti, G.; Spanidi, E.; Natskoulis, P.; Gardikis, K.; Sinanoglou, V.J.; Zoumpoulakis, P. Antioxidant profiles of Vitis vinifera L. and Salvia triloba L. leaves using high-energy extraction methodologies. J. AOAC Int. 2020, 103, 413–421. [Google Scholar] [CrossRef]
- Kaliora, A.C.; Batzaki, C.; Christea, M.G.; Kalogeropoulos, N. Nutritional evaluation and functional properties of traditional composite salad dishes. LWT—Food Sci. Technol. 2015, 62, 775–782. [Google Scholar] [CrossRef]
- Lantzouraki, D.Z.; Sinanoglou, V.J.; Tsiaka, T.; Proestos, C.; Zoumpoulakis, P. Total phenolic content, antioxidant capacity and phytochemical profiling of grape and pomegranate wines. RSC Adv. 2015, 5, 101683–101692. [Google Scholar] [CrossRef]
- van der Laan, T.; Boom, I.; Maliepaard, J.; Dubbelman, A.-C.; Harms, A.C.; Hankemeier, T. Data-independent acquisition for the quantification and identification of metabolites in plasma. Metabolites 2020, 10, 514. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Huan, T. Comparison of full-scan, data-dependent, and data-independent acquisition modes in liquid chromatography–mass spectrometry based untargeted metabolomics. Anal. Chem. 2020, 92, 8072–8080. [Google Scholar] [CrossRef]
- Davies, V.; Wandy, J.; Weidt, S.; van der Hooft, J.J.; Miller, A.; Daly, R.; Rogers, S. Rapid development of improved data-dependent acquisition strategies. Anal. Chem. 2021, 93, 5676–5683. [Google Scholar] [CrossRef] [PubMed]
- Grigoriou, C.; Costopoulou, D.; Vassiliadou, I.; Karavoltsos, S.; Sakellari, A.; Bakeas, E.; Leondiadis, L. Polycyclic aromatic hydrocarbons and trace elements dietary intake in inhabitants of Athens, Greece, based on a duplicate portion study. Food Chem. Toxicol. 2022, 165, 113087. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency (USEPA). Guidelines establishing test procedures for the analysis of pollutants (App. B, Part 136, definition and procedures for the determination of the method detection limit). In U.S. Code of Federal Regulations; U.S. Government Publishing Office: Washington, DC, USA, 1997; pp. 265–267. [Google Scholar]
- Anibogwu, R.; Jesus, K.D.; Pradhan, S.; Pashikanti, S.; Mateen, S.; Sharma, K. Extraction, Isolation and Characterization of Bioactive Compounds from Artemisia and Their Biological Significance: A Review. Molecules 2021, 26, 6995. [Google Scholar] [CrossRef] [PubMed]
- Ghafoori, H.; Sariri, R.; Naghavi, M.R. Study of effect of extraction conditions on the biochemical composition and antioxidant activity of Artemisia Absinthium by HPLC and TLC. J. Liq. Chromatogr. Relat. Technol. 2014, 37, 1558–1567. [Google Scholar] [CrossRef]
- Singh, R.; Verma, P.; Singh, G. Total phenolic, flavonoids and tannin contents in different extracts of Artemisia absinthium. J. Intercult. Ethnopharmacol. 2012, 1, 101. [Google Scholar] [CrossRef]
- Bourgou, S.; BettaiebRebey, I.; Mkadmini, K.; Isoda, H.; Ksouri, R.; Ksouri, W.M. LC-ESI-TOF-MS and GC-MS profiling of Artemisia herba-alba and evaluation of its bioactive properties. Food Res. Int. 2017, 99, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Singh, P. The Genus Artemisia: A 2012–2017 Literature Review on Chemical Composition, Antimicrobial, Insecticidal and Antioxidant Activities of Essential Oils. Medicines 2017, 4, 68. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, I.S.; Cavaco, T.; Brodelius, M. Phenolic composition and antioxidant capacity of six Artemisia species. Ind. Crop. Prod. 2011, 33, 382–388. [Google Scholar] [CrossRef]
- Ornano, L.; Venditti, A.; Ballero, M.; Sanna, C.; Quassinti, L.; Bramucci, M.; Lupidi, G.; Papa, F.; Vittori, S.; Maggi, F.; et al. Chemopreventive and Antioxidant Activity of the Chamazulene-Rich Essential Oil Obtained from Artemisia arborescens L. Growing on the Isle of La Maddalena, Sardinia, Italy. Chem. Biodivers. 2013, 10, 1464–1474. [Google Scholar] [CrossRef]
- Gouveia, S.; Castilho, P.C. Antioxidant potential of Artemisia argentea L’Hér alcoholic extract and its relation with the phenolic composition. Food Res. Int. 2011, 44, 1620–1631. [Google Scholar] [CrossRef]
- Khan, I.; Karim, N.; Ahmad, W.; Abdelhalim, A.; Chebib, M. GABA-A Receptor Modulation and Anticonvulsant, Anxiolytic, and Antidepressant Activities of Constituents from Artemisia indica Linn. Evid. Based Complement. Altern. Med. 2016, 2016, 1215393. [Google Scholar] [CrossRef] [Green Version]
- Jyoti, M.A.; Nam, K.-W.; Jang, W.S.; Kim, Y.-H.; Kim, S.-K.; Lee, B.-E.; Song, H.-Y. Antimycobacterial activity of methanolic plant extract of Artemisia capillaris containing ursolic acid and hydroquinone against Mycobacterium tuberculosis. J. Infect. Chemother. 2016, 22, 200–208. [Google Scholar] [CrossRef]
- Peñas-Fuentes, J.L.; Siles, E.; Rufino-Palomares, E.E.; Pérez-Jiménez, A.; Reyes-Zurita, F.J.; Lupiáñez, J.A.; Fuentes-Almagro, C.; Peragón-Sánchez, J. Effects of Erythrodiol on the Antioxidant Response and Proteome of HepG2 Cells. Antioxidants 2021, 11, 73. [Google Scholar] [CrossRef] [PubMed]
- Carmo, J.; Cavalcante-Araújo, P.; Silva, J.; Ferro, J.; Correia, A.C.; Lagente, V.; Barreto, E. Uvaol Improves the Functioning of Fibroblasts and Endothelial Cells and Accelerates the Healing of Cutaneous Wounds in Mice. Molecules 2020, 25, 4982. [Google Scholar] [CrossRef]
- López-Ruiz, R.; Romero-González, R.; Garrido Frenich, A. Metabolomics approaches for the determination of multiple contaminants in food. Curr. Opin. Food Sci. 2019, 28, 49–57. [Google Scholar] [CrossRef]
- Slimestad, R.; Johny, A.; Thomsen, M.G.; Karlsen, C.R.; Rosnes, J.T. Chemical profiling and biological activity of extracts from nine Norwegian medicinal and aromatic plants. Molecules 2022, 27, 7335. [Google Scholar] [CrossRef]
- Mamatova, A.S.; Korona-Glowniak, I.; Skalicka-Woźniak, K.; Józefczyk, A.; Wojtanowski, K.K.; Baj, T.; Sakipova, Z.B.; Malm, A. Phytochemical composition of wormwood (Artemisia gmelinii) extracts in respect of their antimicrobial activity. BMC Complement. Altern. Med. 2019, 19, 288. [Google Scholar] [CrossRef] [Green Version]
- Minda, D.; Ghiulai, R.; Banciu, C.D.; Pavel, I.Z.; Danciu, C.; Racoviceanu, R.; Soica, C.; Budu, O.D.; Muntean, D.; Diaconeasa, Z.; et al. Phytochemical profile, antioxidant and wound healing potential of three Artemisia species: In vitro and in OVO evaluation. Appl. Sci. 2022, 12, 1359. [Google Scholar] [CrossRef]
- Gong, G.; Guan, Y.-Y.; Zhang, Z.-L.; Rahman, K.; Wang, S.-J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A review of Pharmacological Effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.F.S.; Luthria, D.L.; Sasaki, T.; Heyerick, A. Flavonoids from Artemisia annua L. as antioxidants and their potential synergism with artemisinin against malaria and cancer. Molecules 2010, 15, 3135–3170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Y.; Sun, G.; Dong, X.; Wang, M.; Qin, M.; Yu, Y.; Sun, X. Isorhamnetin attenuates atherosclerosis by inhibiting macrophage apoptosis via PI3K/AKT activation and HO-1 induction. PLoS ONE 2015, 10, e0120259. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Su, H.; Bi, Y.; Li, J.; Feng, L.; Sheng, W. Anti-proliferative effect of isorhamnetin on Hela cells through inducing G2/M cell cycle arrest. Exp. Ther. Med. 2018, 15, 3917–3923. [Google Scholar] [CrossRef] [Green Version]
- Larrazábal-Fuentes, M.J.; Fernández-Galleguillos, C.; Palma-Ramírez, J.; Romero-Parra, J.; Sepúlveda, K.; Galetovic, A.; González, J.; Paredes, A.; Bórquez, J.; Simirgiotis, M.J.; et al. Chemical profiling, antioxidant, anticholinesterase, and antiprotozoal potentials of Artemisia Copa phil. (Asteraceae). Front. Pharmacol. 2020, 11, 1911. [Google Scholar] [CrossRef]
- Alam, W.; Khan, H.; Shah, M.A.; Cauli, O.; Saso, L. Kaempferol as a dietary anti-inflammatory agent: Current therapeutic standing. Molecules 2020, 25, 4073. [Google Scholar] [CrossRef]
- Araniti, F.; Gullì, T.; Marrelli, M.; Statti, G.; Gelsomino, A.; Abenavoli, M.R. Artemisia arborescens L. Leaf Litter: Phytotoxic activity and phytochemical characterization. Acta Physiol. Plant. 2016, 38, 128. [Google Scholar] [CrossRef]
- Younsi, F.; Trimech, R.; Boulila, A.; Ezzine, O.; Dhahri, S.; Boussaid, M.; Messaoud, C. Essential oil and phenolic compounds of Artemisia herba-alba (asso.): Composition, Antioxidant, antiacetylcholinesterase, and antibacterial activities. Int. J. Food Prop. 2015, 19, 1425–1438. [Google Scholar] [CrossRef] [Green Version]
- Dhifallah, A.; Selmi, H.; Ouerghui, A.; Sammeri, H.; Aouini, D.; Rouissi, H. Comparative study of phenolic compounds and antiradical activities of four extracts of Tunisian Artemisia herba Alba. Pharm. Chem. J. 2022, 56, 226–232. [Google Scholar] [CrossRef]
- Mohammed, M.J.; Anand, U.; Altemimi, A.B.; Tripathi, V.; Guo, Y.; Pratap-Singh, A. Phenolic composition, antioxidant capacity and antibacterial activity of white wormwood (Artemisia herba-alba). Plants 2021, 10, 164. [Google Scholar] [CrossRef]
- Mehdizadeh, A.; Karimi, E.; Oskoueian, E. Nano-liposomal encapsulation of Artemisia aucheri phenolics as a potential phytobiotic against campylobacter jejuni infection in mice. Food Sci. Nutr. 2022, 10, 3314–3322. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Marengo, B.; Zuccari, G. Oxidative stress, antioxidant capabilities, and bioavailability: Ellagic acid or urolithins? Antioxidants 2020, 9, 707. [Google Scholar] [CrossRef]
- Gil, T.-Y.; Hong, C.-H.; An, H.-J. Anti-inflammatory effects of ellagic acid on keratinocytes via MAPK and stat pathways. Int. J. Mol. Sci. 2021, 22, 1277. [Google Scholar] [CrossRef] [PubMed]
- Jordão, J.; Porto, H.; Lopes, F.; Batista, A.; Rocha, M. Protective effects of ellagic acid on cardiovascular injuries caused by hypertension in rats. Planta Med. 2017, 83, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Arzani, A.; Zeinali, H.; Razmjo, K. Iron and magnesium concentrations of mint accessions (Mentha spp.). Plant Physiol. Biochem. 2007, 45, 323–329. [Google Scholar] [CrossRef]
- Michalke, B.; Fernsebner, K. New insights into manganese toxicity and speciation. J. Trace Elem. Med. Biol. 2014, 28, 106–116. [Google Scholar] [CrossRef]
- Gimou, M.-M.; Pouillot, R.; Charrondiere, U.R.; Noël, L.; Guérin, T.; Leblanc, J.-C. Dietary exposure and health risk assessment for 14 toxic and essential trace elements in Yaoundé: The Cameroonian total diet study. Food Addit. Contam. 2014, 31, 1064–1080. [Google Scholar] [CrossRef]
- Rose, M.; Baxter, M.; Brereton, N.; Baskaran, C. Dietary exposure to metals and other elements in the 2006 UK Total Diet Study and some trends over the last 30 years. Food Addit. Contam. 2010, 27, 1380–1404. [Google Scholar] [CrossRef] [Green Version]
- International Agency for Research on Cancer. Chromium, nickel and welding. IARC Monogr. Eval. Carcinog. Risks Hum. 1990, 49, 647–648. [Google Scholar]
- International Agency for Research on Cancer. Beryllium, cadmium, mercury, and exposures in the glass manufacturing industry. IARC Monogr. Eval. Carcinog. Risks Hum. 1993, 19, 360–363. [Google Scholar]
- Pennington, J. Commission Directive 2004/ 73/EC, 29th Time Council Directive 67/548EEC. OJEC 2004, 152, 1–311. Available online: https://op.europa.eu/en/publication-detail/-/publication/7bf52e98-781e-4c0a-aec9-3795a32f5984 (accessed on 5 May 2023).
- WHO. Quality Control Methods for Medicinal Plant Materials; World Health Organization: Geneva, Switzerland, 1998; Available online: https://www.who.int/docs/default-source/medicines/norms-and-standards/guidelines/quality-control/quality-control-methods-for-medicinal-plant-materials.pdf?sfvrsn=b451e7c6_0 (accessed on 15 March 2023).
- Chizzola, R.; Michitsch, H.; Franz, C. Monitoring of metallic micronutrients and heavy metals in herbs, spices and medicinal plants from Austria. Eur. Food Res. Technol. 2003, 216, 407–411. [Google Scholar] [CrossRef]
- Begaa, S.; Messaoudi, M.; Benarfa, A. Statistical Approach and Neutron Activation Analysis for Determining Essential and Toxic Elements in Two Kinds of Algerian Artemisia Plant. Biol. Trace Elem. Res. 2020, 199, 2399–2405. [Google Scholar] [CrossRef] [PubMed]
- Ait Bouzid, H.; Oubannin, S.; Ibourki, M.; Bijla, L.; Hamdouch, A.; Sakar, E.H.; Harhar, H.; Majourhat, K.; Koubachi, J.; Gharby, S. Comparative evaluation of chemical composition, antioxidant capacity, and some contaminants in six Moroccan medicinal and Aromatic. Biocatal. Agric. Biotechnol. 2022, 47, 102569. [Google Scholar] [CrossRef]
- Zhong, W.-S.; Ren, T.; Zhao, L.-J. Determination of Pb (Lead), Cd (Cadmium), Cr (Chromium), Cu (Copper), and Ni (Nickel) in Chinese tea with high-resolution continuum source graphite furnace atomic absorption spectrometry. J. Food Drug Anal. 2016, 24, 46–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalny, P.; Fijałek, Z.; Daszczuk, A.; Ostapczuk, P. Determination of selected microelements in polish herbs and their infusions. Sci. Total Environ. 2007, 381, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Pohl, P.; Bielawska-Pohl, A.; Dzimitrowicz, A.; Greda, K.; Jamroz, P.; Lesniewicz, A.; Szymczycha-Madeja, A.; Welna, M. Understanding element composition of medicinal plants used in herbalism—A case study by analytical atomic spectrometry. J. Pharm. Biomed. Anal. 2018, 159, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, H.; Hokura, A.; Κatsuki, F.; Itoh, A.; Haraguchi, H. Multielement Determination and Speciation of Major-to-Trace Elements in Black Tea Leaves by ICP-AES and ICP-MS with the Aid of Size Exclusion Chromatography. Anal. Sci. 2001, 17, 391–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NDA. Scientific Opinion on Dietary Reference Values for chromium. EFSA J. 2014, 12, 3845. [Google Scholar] [CrossRef] [Green Version]
- National Health and Medical Research Council. Nutrient Reference Values for Australia and New Zealand Including Recommended Dietary Intakes; National Health and Medical Research Council: Canberra, Australia, 2006.
- Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.; Nebbia, C.S.; et al. Update of the risk assessment of nickel in food and drinking water. EFSA J. 2020, 18, e06268. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; Cobalt and Cobalt Compounds 52: Lyon, France, 1991. [Google Scholar]
- AFSSA (Agence Française de Sécurité Sanitaire des Aliments/French Agency for Food Safety). Opinion of the French Food Safety Agency on a Request for Scientific and Technical Support Regarding the Migration of Cobalt from Porcelain Oven-Dishes Intended to Come in Contact with Food. Available online: https://www.anses.fr/Documents/MCDA2010sa0095EN.pdf (accessed on 11 May 2010).
- EFSA. Opinion of the Scientific Panel on contaminants in the food chain [CONTAM] related to cadmium as undesirable substance in animal feed. EFSA J. 2004, 2, 72. [Google Scholar] [CrossRef]
- Joint FAO/WHO Expert Committee on Food Additives; Meeting and World Health Organization. Safety Evaluation of Certain Contaminants in Food; Food and Agriculture Organization of the United Nations: Rome, Italy, 2011. [Google Scholar]
| A. arborescens | A. inculta | |||||
|---|---|---|---|---|---|---|
| Decoction | Methanolic | Aqueous-Glycerolic | Decoction | Methanolic | Aqueous-Glycerolic | |
| TPC (mg CAE·g−1 dm) | 8.29 ± 0.27 d | 7.3 ± 1.1 d | 32.82 ± 0.50 b | 9.74 ± 0.15 c | 10.80 ± 0.90 c | 36.0 ± 2.5 a |
| TFC (mg CE·g−1 dm) | 6.05 ± 0.17 c | 4.48 ± 0.37 d | 19.57 ± 0.76 a | 6.04 ± 0.72 c | 7.0 ± 1.3 c | 16.77 ± 0.36 b |
| THCC (mg CAE·g−1 dm) | 0.0985 ± 0.0035 e | 0.095 ± 0.017 d,e | 0.310 ± 0.010 b | 0.1117 ± 0.0012 d | 0.1257 ± 0.0050 c | 0.338 ± 0.013 a |
| TFnolC (mg QE·g−1 dm) | 0.0859 ± 0.0025 e | 0.100 ± 0.016 d,e | 0.293 ± 0.015 b | 0.0988 ± 0.0015 d | 0.1383 ± 0.0048 c | 0.370 ± 0.013 a |
| TAC (μg CNE·g−1 dm) | 0.651 ± 0.032 d | 1.424 ± 0.028 b | 2.680 ± 0.082 a | 0.462 ± 0.011 e | 0.764 ± 0.058 c | 2.61 ± 0.14 a |
| TTC (mg UAE·g−1 dm) | 0.229 ± 0.029 d | 1.859 ± 0.160 a | 0.374 ± 0.021 c | 0.215 ± 0.021 d | 1.857 ± 0.346 a | 0.438 ± 0.024 b |
| A. arborescens | A. inculta | |||||
|---|---|---|---|---|---|---|
| Decoction | Methanolic | Aqueous-Glycerolic | Decoction | Methanolic | Aqueous-Glycerolic | |
| Antiradical activity (mg TE·g−1 dm) | 5.18 ± 0.50 e | 7.524 ± 0.039 d | 30.9 ± 1.2 a | 5.68 ± 0.21 e | 8.23 ± 0.40 c | 27.8 ± 1.1 b |
| FRAP (mg AAE·g−1 dm) | 3.45 ± 0.14 e | 5.36 ± 0.44 c | 21.77 ± 0.70 a | 2.92 ± 0.25 e | 4.31 ± 0.47 d | 18.19 ± 0.27 b |
| TSO (sec) | 1107.7 ± 8.1 f | 627.8 ± 366.3 a | 2007.25 ± 171.25 f | 2120 ± 493.8 b | 1400.3 ± 377.2 f | 3850.25 ± 320.25 c |
| Phenolic Compounds | Molecular Formula | A. arborescens | A. inculta | ||||
|---|---|---|---|---|---|---|---|
| Decoction | Methanolic | Aqueous-Glycerolic | Decoction | Methanolic | Aqueous-Glycerolic | ||
| Caffeic acid | C9H8O4 | 94.6 ± 7.5 d | 121.9 ± 9.0 c | 39.1 ± 4.5 e | 382 ± 12 a | 289.6 ± 6.7 b | 19.1 ± 3.0 f |
| Chlorogenic acid | C16H18O9 | 537.1 ± 8.9 e | 5754 ± 70 a | 1669.2 ± 1.3 d | 2332 ± 179 c | 5052 ± 56 b | 285 ± 19 f |
| Chrysin | C15H10O4 | nd | 3.89 ± 0.05 b | nd | nd | 6.51 ± 0.13 a | nd |
| p-Coumaric acid | C9H8O3 | 1.52 ± 0.13 d | 2.97 ± 0.32 c | nd | 37.78 ± 0.26 b | 43.2 ± 3.5 a | nd |
| Ferulic acid | C10H10O4 | 16.26 ± 0.59 b | 2.54 ± 0.16 d | 1.64 ± 0.05 e | 25.3 ± 1.4 a | 16.8 ± 1.4 b | 3.94 ± 0.06 c |
| Gallic acid | C7H6O5 | nd | 0.94 ± 0.02 a | nd | nd | 0.67 ± 0.06 a | nd |
| p-Hydroxybenzoic acid | C7H6O3 | 7.89 ± 0.16 c | 1.16 ± 0.10 e | 3.82 ± 0.46 d | 45.4 ± 2.6 a | nd | 23.99 ± 0.42 b |
| p-Hydroxyphenylacetic acid | C8H8O3 | nd | 0.39 ± 0.05 b | nd | nd | 0.44 ± 0.03 b | 5.17 ± 0.68 a |
| Kaempferol | C15H10O6 | nd | 1.15 ± 0.11 b | nd | nd | 3.19 ± 0.18 a | nd |
| Naringenin | C15H12O5 | nd | 3.07 ± 0.28 c | nd | 15.84 ± 0.25 b | 40.1 ± 2.7 a | 36.5 ± 2.4 a |
| Phloretic acid | C9H10O3 | nd | nd | 1.70 ± 0.09 a | nd | 0.49 ± 0.01 b | 1.02 ± 0.02 c |
| Protocatechuic acid | C7H6O4 | 8.73 ± 0.80 b | 6.44 ± 0.33 b,c | nd | 21.2 ± 1.0 a | 5.51 ± 0.61 c | nd |
| Quercetin | C15H10O7 | nd | 7.21 ± 0.31 c | nd | 14.93 ± 0.89 b | 23.04 ± 0.54 a | nd |
| Resveratrol | C14H12O3 | nd | 0.36 ± 0.04 b | 1.62 ± 0.09 a | nd | 0.24 ± 0.03 b | nd |
| Sinapic acid | C11H12O5 | nd | nd | 30.1 ± 3.0 | nd | nd | nd |
| Syringic acid | C9H10O5 | 6.47 ± 0.51 b | 2.96 ± 0.29 c | 6.34 ± 0.47 b | 10.39 ± 0.26 a | 5.84 ± 0.37 b | 7.38 ± 0.13 b |
| Tyrosol | C8H10O2 | nd | 0.05 ± 0.01 b | nd | nd | 0.14 ± 0.01 a | nd |
| Vanillic acid | C8H8O4 | 6.66 ± 0.70 d | 2.20 ± 0.21 f | 3.06 ± 0.17 e | 21.1 ± 1.1 a | 13.96 ± 0.44 b | 9.18 ± 0.58 c |
| Total Phenolic Compounds | 679 ± 19 e | 5911 ± 81 a | 1756 ± 10 d | 2906 ± 199 c | 5502 ± 72 b | 392 ± 27 f | |
| Terpenoids | |||||||
| Erythrodiol | C30H50O2 | nd | nd | 487.8 ± 14 a | nd | nd | 420 ± 30 a |
| Oleanolic acid | C30H48O3 | nd | 8.54 ± 0.86 c | 242.6 ± 18 b | nd | 7.14 ± 0.68 c | 480 ± 45 a |
| Ursolic acid | C30H48O3 | nd | 14.24 ± 0.93 c | 35.2 ± 5.0 b | nd | 15.58 ± 0.63 c | 82.4 ± 7.9 a |
| Uvaol | C30H50O2 | nd | nd | 712.9 ± 18 a | nd | nd | 584 ± 42 b |
| Total Terpenoids | nd | 22.8 ± 1.8 b | 1478 ± 56 a | nd | 22.7 ± 1.3 b | 1568 ± 126 a | |
| Phenolic Compound | Molecular Formula | [M–H]—(m/z) 1,2 | MS 2 Product Ions (m/z) | A. arborescens | A. inculta |
|---|---|---|---|---|---|
| Caffeic acid hexoside | C15H18O9 | 341.11 | 179, 161, 135 | + | |
| Chlorogenic acid | C16H18O9 | 353.15 | 217, 191 | + | + |
| Dihydrokaempferol 3-O-glucoside | C21H22O11 | 449.09 | 287 | + | |
| Dihydrokaempferol-3-O-rhamnoside (Engeletin) | C21H22O10 | 433.00 | 269, 179, 151 | + | |
| Procyanidin B2 | C30H26O12 | 577.24 | 425 | + | |
| Ellagic acid | C14H6O8 | 301.06 | 301, 257, 229, 185 | + | |
| Ellagic acid-O-hexoside | C20H16O13 | 463.11 | 301, 300, 283, 257, 229 | + | |
| Gallic acid derivative | not defined | 243.27 | 169, 225, 151, 139, 125 | + | |
| Hexose ester of protocatechuic acid | C13H15O9 | 314.77 | 153 | + | |
| p-Hydroxybenzoic acid | C7H6O3 | 137.06 | 93 | + | |
| Isorhamnetin | C16H12O7 | 315.20 | 300, 301 | + | + |
| Kaempferol-3-O-glucoside (Astragalin) | C21H20O11 | 447.24 | 285, 255, 327 | + | + |
| Kaempferol-3-O-rutinoside (Nictoflorin) | C27H30O15 | 593.26 | 285 | + | + |
| Phlorizin | C21H24O10 | 435.20 | 297, 273, 167 | + | |
| Pyrogallol | C6H6O3 | 125.06 | 106, 97, 81 | + | |
| Quercetin-3-O-glucuronide (Miquelianin) | C21H18O13 | 477.26 | 301 | + | |
| Quercetin-3-O-glucoside | C21H20O12 | 463.19 | 301 | + | |
| Quercetin-O-xyloside | C20H18O11 | 433.19 | 301 | + | |
| Syringaldehyde | C9H10O4 | 181.12 | 166 | + | |
| Syringetin-3-O-glucoside | C23H24O13 | 507.25 | 345 | + | |
| Syringetin-hexoside | C23H24O13 | 507.25 | 345, 327, 315 | + | |
| Valoneic acid bilactone | C21H10O13 | 469.04 | 425, 407 | + |
| Cd | Co | Cr | Cu | Fe | Mn | Ni | Pb | Zn | |
|---|---|---|---|---|---|---|---|---|---|
| A. arborescens | |||||||||
| Herbal tissue | 0.621 ± 0.056 | 0.295 ± 0.027 | 2.42 ± 0.03 | 9.38 ± 1.02 | 228 ± 25 | 87.9 ± 10.0 | 22.6 ± 1.9 | 0.676 ± 0.056 | 56.1 ± 6.0 |
| Infusion | 0.254 ± 0.021 | 0.554 ± 0.049 | 1.12 ± 0.14 | 16.4 ± 1.88 | 44.8 ± 5.2 | 101 ± 11 | 41.9 ± 5.1 | 0.298 ± 0.031 | 52.6 ± 4.8 |
| % EE | 13.0 | 59.7 | 14.8 | 55.6 | 6.2 | 36.6 | 29.1 | 14.0 | 29.8 |
| A. inculta | |||||||||
| Herbal tissue | 0.064 ± 0.007 | 0.117 ± 0.011 | 0.781 ± 0.063 | 10.1 ± 0.9 | 175 ± 18 | 28.6 ± 3.4 | 1.47 ± 0.12 | 0.174 ± 0.016 | 33.8 ± 2.9 |
| Infusion | 0.059 ± 0.006 | 0.156 ± 0.013 | 1.04 ± 0.12 | 21.1 ± 1.9 | 45.8 ± 5.7 | 27.8 ± 2.2 | 4.77 ± 0.51 | 0.303 ± 0.036 | |
| % EE | 24.6 | 35.5 | 35.5 | 41.3 | 7.0 | 26.0 | 36.5 | 46.6 | 42.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lantzouraki, D.Z.; Amerikanou, C.; Karavoltsos, S.; Kafourou, V.; Sakellari, A.; Tagkouli, D.; Zoumpoulakis, P.; Makris, D.P.; Kalogeropoulos, N.; Kaliora, A.C. Artemisia arborescens and Artemisia inculta from Crete; Secondary Metabolites, Trace Metals and In Vitro Antioxidant Activities. Life 2023, 13, 1416. https://doi.org/10.3390/life13061416
Lantzouraki DZ, Amerikanou C, Karavoltsos S, Kafourou V, Sakellari A, Tagkouli D, Zoumpoulakis P, Makris DP, Kalogeropoulos N, Kaliora AC. Artemisia arborescens and Artemisia inculta from Crete; Secondary Metabolites, Trace Metals and In Vitro Antioxidant Activities. Life. 2023; 13(6):1416. https://doi.org/10.3390/life13061416
Chicago/Turabian StyleLantzouraki, Dimitra Z., Charalampia Amerikanou, Sotirios Karavoltsos, Vasiliki Kafourou, Aikaterini Sakellari, Dimitra Tagkouli, Panagiotis Zoumpoulakis, Dimitris P. Makris, Nick Kalogeropoulos, and Andriana C. Kaliora. 2023. "Artemisia arborescens and Artemisia inculta from Crete; Secondary Metabolites, Trace Metals and In Vitro Antioxidant Activities" Life 13, no. 6: 1416. https://doi.org/10.3390/life13061416
APA StyleLantzouraki, D. Z., Amerikanou, C., Karavoltsos, S., Kafourou, V., Sakellari, A., Tagkouli, D., Zoumpoulakis, P., Makris, D. P., Kalogeropoulos, N., & Kaliora, A. C. (2023). Artemisia arborescens and Artemisia inculta from Crete; Secondary Metabolites, Trace Metals and In Vitro Antioxidant Activities. Life, 13(6), 1416. https://doi.org/10.3390/life13061416