Cultivation of Chroococcidiopsis thermalis Using Available In Situ Resources to Sustain Life on Mars
Abstract
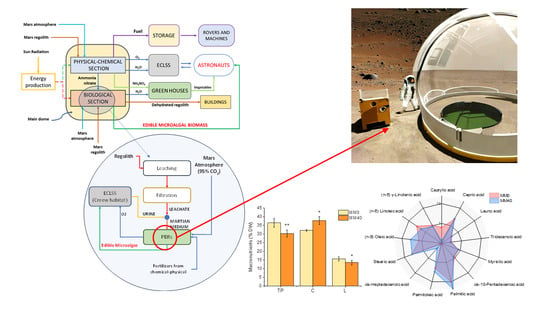
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation and Composition of the Martian Medium (MM)
2.3. Culture Conditions and Growth Experiments to Optimize MM Content for Cultivation Purposes
2.4. Sample Preparation for Chemical Characterization Analysis
2.5. Determination of Total Carbohydrates, Lipids, and Total Soluble Proteins
2.6. Determination of Fatty Acid Methyl Esters (FAMEs) Using GC-FID Analysis
2.7. Lipidomic Analysis
2.8. Chlorophyll-a, Total Carotenoids, Phycocyanin and Allophycocyanin
2.9. Antioxidant Power Determination
2.10. Preparation of Chroococcidiopsis Culture Extracts for MTT Assay
2.11. MTT Assay for In Vitro Cytotoxicity Activity
2.12. Statistical Analysis
3. Results and Discussion
3.1. Optimization of the Martian Culture Medium MM
3.2. Analysis of the Macronutrient’s Composition of Chroococcidiopsis Thermalis Biomass
3.3. Fatty Acid Methyl Esters (FAMEs)
3.4. Results from Lipidomic Analyses
3.5. Chlorophyll-a, Total Carotenoids, Phycocyanin and Allophycocyanin
3.6. Antioxidant Activity
3.7. Cytotoxicity of C. thermalis Extracts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| FA | C:N | MM0 (DW%) | MM40 (DW%) | p Value |
|---|---|---|---|---|
| Caprylic acid | C8:0 | 0.64 ± 0.05 | 0.13 ± 0.02 | **** |
| Capric acid | C10:0 | 2.30 ± 0.04 | 2.50 ± 0.10 | ** |
| Lauric acid | C12:0 | 0.40 ± 0.02 | 0.40 ± 0.04 | ns |
| Tridecanoic acid | C13:0 | 0.31 ± 0.01 | 0.28 ± 0.02 | ns |
| Myristic acid | C14:0 | 0.80 ± 0.02 | 0.80 ± 0.04 | ns |
| cis-10-Pentadecenoic acid | C15:1 | 0.90 ± 0.02 | 0.77 ± 0.03 | ns |
| Palmitic acid | C16:0 | 10.40 ± 0.10 | 24.00 ± 0.1 | **** |
| Palmitoleic acid | C16:1 | 2.02 ± 0.02 | 3.07 ± 0.03 | **** |
| cis-Heptadecenoic acid | C17:1 | 0.11 ± 0.02 | 0.22 ± 0.02 | ns |
| Stearic acid | C18:0 | 3.90 ± 0.05 | 3.90 ± 0.04 | ns |
| (n-9) Oleic acid | C18:1 | 1.43 ± 0.03 | 3.70 ± 0.03 | **** |
| (n-6) Linoleic acid | C18:2 | 3.30 ± 0.05 | 1.80 ± 0.05 | **** |
| (n-6) γ-Linolenic acid | C18:3 | 1.06 ± 0.04 | 0.93 ± 0.03 | ns |
| (n-3) α-Linolenic acid (ALA) | C18:3 | 0.80 ± 0.02 | 1.80 ± 0.01 | **** |
| Total saturates | 18.7 ± 0.1 | 32.0 ± 0.2 | **** | |
| Total monoenes | 4.4 ± 0.1 | 7.7 ± 0.1 | **** | |
| Total PUFA | 5.10 ± 0.05 | 4.5 ± 0.1 | **** | |
| S/U * | 1.95 | 2.60 | not tested |
| Lipid Species (C:N) | Fatty Acyl Chains in MS/MS Mode | Formula | Calculated m/z | Observed m/z of Molecular Ions | Error (ppm) |
|---|---|---|---|---|---|
| MGDG annotated as [M+NH4]+ | |||||
| MGDG 31:2 | 15:1/16:1 | C40H72O10 | 730.5464 | 730.5479 | 2.1 |
| MGDG 32:1 | 16:0/16:1 | C41H76O10 | 746.5777 | 746.5820 | 5.8 |
| MGDG 32:2 | 16:1/16:1 | C41H74O10 | 744.5620 | 744.5578 | −5.6 |
| MGDG 34:1 | 16:0/18:1 | C43H80O10 | 774.6090 | 774.6065 | −3.2 |
| MGDG 34:2 | 16:0/18:2 | C43H78O10 | 772.5933 | 772.5937 | 0.5 |
| MGDG 34:3 | 16:0/18:3 | C43H76O10 | 770.5777 | 770.5757 | −2.6 |
| MGDG 34:4 | 16:1/18:3 | C43H74O10 | 768.5620 | 768.5610 | −1.3 |
| DGDG annotated as [M+NH4]+ | |||||
| DGDG 32:1 | 16:0/16:1 | C47H86O15 | 908.6305 | 908.6266 | −4.3 |
| DGDG 34:2 | 16:1/18:1 | C49H88O15 | 934.6461 | 934.6433 | −3.0 |
| DGDG 34:3 | 16:0/18:3 | C49H86O15 | 932.6305 | 932.6304 | −0.1 |
| DGDG 34:4 | 16:1/18:3 | C49H84O15 | 930.6148 | 930.6147 | −0.1 |
| PC annotated as [M+H]+ | |||||
| PC 32:1 | C40H78NO8P | 732.5538 | 732.5590 | 7.1 | |
| PC 32:2 | C40H76NO8P | 730.5381 | 730.5420 | 5.3 | |
| PC 36:2 | 18:1/18:1 | C44H84NO8P | 786.6007 | 786.6033 | 3.3 |
| PC 36:4 | C44H80NO8P | 782.5694 | 782.5628 | −8.4 | |
| TG annotated as [M+NH4]+ | |||||
| TG 42:0 | 12:0/14:0/16:0 | C45H86O6 | 740.6763 | 740.6810 | −6.35 |
| TG 44:0 | 14:0/14:0/16:0 | C47H90O6 | 768.7084 | 768.7100 | −3.12 |
| TG 45:0 | 14:0/15:0/16:0 | C48H92O6 | 782.7232 | 782.7235 | −0.35 |
| TG 46:0 | 14:0/16:0/16:0 | C49H94O6 | 796.7389 | 796.7418 | −3.64 |
| TG 46:1 | 14:0/16:0/16:1 | C49H92O6 | 794.7232 | 794.7260 | −3.52 |
| TG 47:0 | 15:0/16:0/16:0 | C50H96O6 | 810.7545 | 810.7582 | −4.56 |
| TG 48:0 | 16:0/16:0/16:0 | C51H98O6 | 824.7702 | 824.7750 | −5.82 |
| TG 49:0 | 16:0/16:0/17:0 | C52H100O6 | 838.7858 | 838.7911 | −6.32 |
| TG 49:1 | 15:0/16:0/18:1 | C52H98O6 | 836.7702 | 836.7750 | −5.74 |
| TG 50:1 | 16:0/16:0/18:1 | C53H100O6 | 850.7858 | 850.7886 | −3.29 |
| TG 52:2 | 16:0/18:1/18:1 | C55H102O6 | 876.8015 | 876.8047 | −3.65 |
| TG 54:0 | 18:0/18:0/18:0 | C57H110O6 | 908.8641 | 908.8601 | 4.40 |
| TG 54:3 | 18:1/18:1/18:1 | C57H104O6 | 902.8171 | 902.8202 | −3.43 |
| TG 54:5 | 18:1/18:1/18:3 | C57H100O6 | 898.7858 | 898.7809 | 5.45 |
| TG 55:0 | 16:0/15:0/24:0 | C58H112O6 | 922.8797 | 922.8784 | 1.41 |
| SQDG annotated as [M-H]- | |||||
| SQDG 32:0 | 16:0/16:0 | C41H78O12S | 793.5141 | 793.5155 | 1.76 |
| SQDG 32:1 | 16:0/16:1 | C41H76O12S | 791.4985 | 791.4999 | 1.77 |
| SQDG 34:1 | 16:0/18:1 | C43H80O12S | 819.5298 | 819.5299 | 0.12 |
| SQDG 34:2 | 16:0/18:2 | C43H78O12S | 817.5141 | 817.5140 | −0.12 |
| SQDG 34:3 | 16:0/18:3 | C43H76O12S | 815.4985 | 815.4993 | 0.98 |
| PG annotated as [M-H]− and as [M+NH4]+ | |||||
| PG 34:2 | 16:1/18:1 | C40H75O10P | 745.5025/764.5436 | 745.5024/764.5474 | −0.1/5.0 |
| PG 34:3 | 16:0/18:3 | C40H73O10P | 743.4869/762.5280 | 743.4884/762.5313 | 2.1/4.3 |
| Lipid Species (C:N) | MM-0 | MM-40 | p Value |
|---|---|---|---|
| MGDG 31:2 | 0.09 ± 0.01 | 0.09 ± 0.01 | ns |
| MGDG 32:1 | 0.40 ± 0.06 | 0.07 ± 0.01 | **** |
| MGDG 32:2 | 0.34 ± 0.01 | 0.12 ± 0.02 | *** |
| MGDG 34:1 | 0.38 ± 0.03 | 0.09 ± 0.02 | **** |
| MGDG 34:2 | 0.07 ± 0.01 | 0.68 ± 0.03 | **** |
| MGDG 34:3 | 4.58 ± 0.36 | 0.07 ± 0.01 | **** |
| MGDG 34:4 | 2.68 ± 0.18 | 1.88 ± 0.02 | **** |
| DGDG 32:1 | 0.07 ± 0.01 | 0.30 ± 0.01 | *** |
| DGDG 34:2 | 0.06 ± 0.01 | 0.03 ± 0.01 | ns |
| DGDG 34:3 | 0.03 ± 0.02 | 0.32 ± 0.03 | **** |
| DGDG 34:4 | 0.25 ± 0.03 | 0.08 ± 0.01 | * |
| PC 32:2 | 0.09 ± 0.01 | 0.09 ± 0.01 | ns |
| PC 36:2 | 0.02 ± 0.01 | 0.05 ± 0.01 | ns |
| PC 36:4 | 0.02 ± 0.01 | 0.03 ± 0.01 | ns |
| TG 42:0 | 0.37 ± 0.02 | 0.25 ± 0.03 | ns |
| TG 44:0 | 0.28 ± 0.02 | 0.14 ± 0.04 | ns |
| TG 45:0 | 0.14 ± 0.01 | 0.51 ± 0.07 | **** |
| TG 46:0 | 0.92 ± 0.04 | 1.05 ± 0.09 | ns |
| TG 46:1 | 0.12 ± 0.02 | 0.06 ± 0.01 | ns |
| TG 47:0 | 0.81 ± 0.08 | 0.31 ± 0.04 | **** |
| TG 48:0 | 0.08 ± 0.01 | 0.05 ± 0.01 | ns |
| TG 49:0 | 0.11 ± 0.02 | 0.27 ± 0.04 | * |
| TG 49:1 | 0.06 ± 0.01 | 0.03 ± 0.01 | ns |
| TG 50:1 | 0.53 ± 0.04 | 0.57 ± 0.04 | ns |
| TG 52:2 | 0.04 ± 0.01 | 0.21 ± 0.03 | * |
| TG 54:0 | 1.22 ± 0.12 | 1.05 ± 0.08 | * |
| TG 54:3 | 1.80 ± 0.1 | 1.94 ± 0.04 | ns |
| TG 54:5 | 0.13 ± 0.01 | 0.15 ± 0.05 | ns |
| TG 55:0 | 1.73 ± 0.01 | 2.13 ± 0.1 | **** |
| SQDG 32:0 | 0.10 ± 0.01 | 0.13 ± 0.04 | ns |
| SQDG 32:1 | 0.14 ± 0.02 | 0.43 ± 0.09 | **** |
| SQDG 34:1 | 0.08 ± 0.01 | 0.03 ± 0.01 | ns |
| SQDG 34:2 | 0.70 ± 0.09 | 0.48 ± 0.04 | *** |
| SQDG 34:3 | 0.83 ± 0.04 | 0.56 ± 0.08 | **** |
| PG 34:2 | 0.14 ± 0.03 | 0.14 ± 0.03 | ns |
| PG 34:3 | 0.03 ± 0.01 | 0.04 ± 0.01 | ns |
References
- Kovic, M. Risks of Space Colonization. Futures 2021, 126, 102638. [Google Scholar] [CrossRef]
- Revellame, E.D.; Aguda, R.; Chistoserdov, A.; Fortela, D.L.; Hernandez, R.A.; Zappi, M.E. Microalgae Cultivation for Space Exploration: Assessing the Potential for a New Generation of Waste to Human Life-Support System for Long Duration Space Travel and Planetary Human Habitation. Algal Res. 2021, 55, 102258. [Google Scholar] [CrossRef]
- Alemany, L.; Peiro, E.; Arnau, C.; Garcia, D.; Poughon, L.; Cornet, J.F.; Dussap, C.G.; Gerbi, O.; Lamaze, B.; Lasseur, C.; et al. Continuous Controlled Long-Term Operation and Modeling of a Closed Loop Connecting an Air-Lift Photobioreactor and an Animal Compartment for the Development of a Life Support System. Biochem. Eng. J. 2019, 151, 107323. [Google Scholar] [CrossRef]
- Poughon, L.; Laroche, C.; Creuly, C.; Dussap, C.G.; Paille, C.; Lasseur, C.; Monsieurs, P.; Heylen, W.; Coninx, I.; Mastroleo, F.; et al. Limnospira Indica PCC8005 Growth in Photobioreactor: Model and Simulation of the ISS and Ground Experiments. Life Sci. Space Res. 2020, 25, 53–65. [Google Scholar] [CrossRef]
- Revellame, E.D.; Aguda, R.; Gatdula, K.M.; Holmes, W.; Fortela, D.L.; Sharp, W.; Gang, D.; Chistoserdov, A.; Hernandez, R.; Zappi, M.E. Microalgae in Bioregenerative Life Support Systems for Space Applications. Algal Res. 2024, 77, 103332. [Google Scholar] [CrossRef]
- Cao, G.; Concas, A.; Fais, G.; Gabrielli, G.; Manca, A.; Pantaleo, A. Process and Kit to Investigate Microgravity Effect on Animal/Vegetable Cells under Extraterrestrial Cultivation Conditions and Cultivation Process Thereof to Sustain Manned Space Missions 2021; World Intellectual Property Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Fais, G.; Manca, A.; Concas, A.; Pantaleo, A.; Cao, G. A Novel Process to Grow Edible Microalgae on Mars by Exploiting in Situ-Available Resources: Experimental Investigation. Acta Astronaut. 2022, 201, 454–463. [Google Scholar] [CrossRef]
- Concas, A.; Fais, G.; Enna, M.; Zucchelli, S.; Caboni, P.; Lai, N.; Cincotti, A.; Cao, G. Modeling and Experimental Assessment of Synechococcus Nidulans Cultivation Using Simulated Martian Medium and Astronauts’ Urine. Acta Astronaut. 2023, 205, 185–198. [Google Scholar] [CrossRef]
- Brughitta, E.; Atzori, F.; Gamboni, E.; Foddi, S.; Casula, M.; Fais, G.; Manca, A.; Pantaleo, A.; Cao, G.; Concas, A. Cultivation of Cyanobacteria and Microalgae Using Simulated In-Situ Available Resources for the Production of Useful Bio-Compounds on Mars: Modelling of Experiments. Chem. Eng. Trans. 2023, 98, 111–116. [Google Scholar]
- Peters, G.H.; Abbey, W.; Bearman, G.H.; Mungas, G.S.; Smith, J.A.; Anderson, R.C.; Douglas, S.; Beegle, L.W. Mojave Mars Simulant-Characterization of a New Geologic Mars Analog. Icarus 2008, 197, 470–479. [Google Scholar] [CrossRef]
- Bruno, F.; Ceriani, A.; Zhan, Z.; Caruso, G.; Del Mastro, A. Virtual Reality to Simulate an Inflatable Modular Hydroponics Greenhouse on Mars. In Proceedings of the ASME Design Engineering Technical Conference, Virtual, 17–19 August 2020. [Google Scholar]
- Kozicki, J.; Kozicka, J. Human Friendly Architectural Design for a Small Martian Base. Adv. Space Res. 2011, 48, 1997–2004. [Google Scholar] [CrossRef]
- Bolonkin, A.A. Inflatable Dome for Moon, Mars, Asteroids and Satellites. In Proceedings of the A Collection of Technical Papers—AIAA Space 2007 Conference, Long Beach, CA, USA, 18–20 September 2007. [Google Scholar]
- Hublitz, I.; Henninger, D.L.; Drake, B.G.; Eckart, P. Engineering Concepts for Inflatable Mars Surface Greenhouses. Adv. Space Res. 2004, 34, 1546–1551. [Google Scholar] [CrossRef]
- Fais, G.; Manca, A.; Bolognesi, F.; Borselli, M.; Concas, A.; Busutti, M.; Broggi, G.; Sanna, P.; Castillo-Aleman, Y.M.; Rivero-Jiménez, R.A.; et al. Wide Range Applications of Spirulina: From Earth to Space Missions. Mar. Drugs 2022, 20, 299. [Google Scholar] [CrossRef]
- Jung, F.; Krüger-Genge, A.; Waldeck, P.; Küpper, J.H. Spirulina Platensis, a Super Food? J. Cell. Biotechnol. 2019, 5, 43–54. [Google Scholar] [CrossRef]
- Soni, R.A.; Sudhakar, K.; Rana, R.S. Spirulina—From Growth to Nutritional Product: A Review. Trends Food Sci. Technol. 2017, 69, 157–171. [Google Scholar] [CrossRef]
- Bothe, H. The Cyanobacterium Chroococcidiopsis and Its Potential for Life on Mars. J. Astrobiol. Space Sci. 2019, 2, 398–412. [Google Scholar]
- Verseux, C.; Baqué, M.; Lehto, K.; De Vera, J.P.P.; Rothschild, L.J.; Billi, D. Sustainable Life Support on Mars—The Potential Roles of Cyanobacteria. Int. J. Astrobiol. 2016, 15, 65–92. [Google Scholar] [CrossRef]
- Verseux, C.; Heinicke, C.; Ramalho, T.P.; Determann, J.; Duckhorn, M.; Smagin, M.; Avila, M. A Low-Pressure, N2/CO2 Atmosphere Is Suitable for Cyanobacterium-Based Life-Support Systems on Mars. Front. Microbiol. 2021, 12, 611798. [Google Scholar] [CrossRef]
- Imre Friedmann, E.; Ocampo-Friedmann, R. A Primitive Cyanobacterium as Pioneer Microorganism for Terraforming Mars. Adv. Space Res. 1995, 15, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Aguiló, P.; Jeroni, N.; Giacomo, G.; Sebastià, F.; Bauçà, C.; Cao, G. Singular Adaptations in the Carbon Assimilation Mechanism of the Polyextremophile Cyanobacterium Chroococcidiopsis Thermalis. Photosynth. Res. 2023, 156, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Billi, D.; Wilmotte, A.; Mckay, C.P. Desert Strains of Chroococcidiopsis: A Platform to Investigate Genetic Diversity in Extreme Environments and Explore Survival Potential beyond Earth. EPSC Abstr. 2010, 5, 2–4. [Google Scholar]
- Billi, D.; Gallego Fernandez, B.; Fagliarone, C.; Chiavarini, S.; Rothschild, L.J. Exploiting a Perchlorate-Tolerant Desert Cyanobacterium to Support Bacterial Growth for in Situ Resource Utilization on Mars. Int. J. Astrobiol. 2021, 20, 29–35. [Google Scholar] [CrossRef]
- Caiyan, L.; Xianyuan, Z.; Tong, Y.; Xiaoyan, L.; Gaohong, W. Protection and Damage Repair Mechanisms Contributed To the Survival of Chroococcidiopsis Sp. Exposed To a Mars-Like Near Space Environment. Microbiol. Spectr. 2022, 10, e03440-22. [Google Scholar] [CrossRef]
- Cockell, C.S.; Schuerger, A.C.; Billi, D.; Friedmann, E.I.; Panitz, C. Effects of a Simulated Martian UV Flux on the Cyanobacterium, Chroococcidiopsis Sp. 029. Astrobiology 2005, 5, 127–140. [Google Scholar] [CrossRef]
- Murray, B.; Ertekin, E.; Dailey, M.; Soulier, N.T.; Shen, G.; Bryant, D.A.; Perez-Fernandez, C.; Diruggiero, J. Adaptation of Cyanobacteria to the Endolithic Light Spectrum in Hyper-Arid Deserts. Microorganisms 2022, 10, 1198. [Google Scholar] [CrossRef]
- Antonopoulou, S.; Karantonis, H.C.; Nomikos, T.; Oikonomou, A.; Fragopoulou, E.; Pantazidou, A. Bioactive Polar Lipids from Chroococcidiopsis Sp. (Cyanobacteria). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2005, 142, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Montero-Lobato, Z.; Fuentes, J.L.; Garbayo, I.; Ascaso, C.; Wierzchos, J.; Vega, J.M.; Vílchez, C. Identification, Biochemical Composition and Phycobiliproteins Production of Chroococcidiopsis Sp. from Arid Environment. Process Biochem. 2020, 97, 112–120. [Google Scholar] [CrossRef]
- Řezanka, T.; Víden, I.; Go, J.V.; Dor, I.; Dembitsky, V.M. Polar Lipids and Fatty Acids of Three Wild Cyanobacterial Strains of the Genus Chroococcidiopsis. Folia Microbiol. 2003, 48, 781–786. [Google Scholar] [CrossRef]
- Clark, B.C.; Kounaves, S.P. Evidence for the Distribution of Perchlorates on Mars. Int. J. Astrobiol. 2016, 15, 311–318. [Google Scholar] [CrossRef]
- Misra, G.; Smith, W.; Garner, M.; Loureiro, R. Potential Biological Remediation Strategies for Removing Perchlorate from Martian Regolith. New Space 2021, 9, 217–227. [Google Scholar] [CrossRef]
- Davila, A.F.; Willson, D.; Coates, J.D.; McKay, C.P. Perchlorate on Mars: A Chemical Hazard and a Resource for Humans. Int. J. Astrobiol. 2013, 12, 321–325. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, L.; Li, Y.; Sun, Q.; Sun, M.; Huang, Y.; Li, Z.; Tang, D.; Wang, Y.; Xiao, L. In-Situ Utilization of Regolith Resource and Future Exploration of Additive Manufacturing for Lunar/Martian Habitats: A Review. Appl. Clay Sci. 2022, 229, 106673. [Google Scholar] [CrossRef]
- Olsson-Francis, K.; Cockell, C.S. Use of Cyanobacteria for In-Situ Resource Use in Space Applications. Planet. Space Sci. 2010, 58, 1279–1285. [Google Scholar] [CrossRef]
- Caporale, A.G.; Palladino, M.; De Pascale, S.; Duri, L.G.; Rouphael, Y.; Adamo, P. How to Make the Lunar and Martian Soils Suitable for Food Production—Assessing the Changes after Manure Addition and Implications for Plant Growth. J. Environ. Manag. 2023, 325, 116455. [Google Scholar] [CrossRef]
- Rapp, D. Use of Extraterrestrial Resources for Human Space Missions to Moon or Mars; Springer: Cham, Switzerland, 2013; ISBN 9783642327629. [Google Scholar]
- Santomartino, R.; Zea, L.; Cockell, C.S. The Smallest Space Miners: Principles of Space Biomining. Extremophiles 2022, 26, 7. [Google Scholar] [CrossRef]
- Eichler, A.; Hadland, N.; Pickett, D.; Masaitis, D.; Handy, D.; Perez, A.; Batcheldor, D.; Wheeler, B.; Palmer, A. Challenging the Agricultural Viability of Martian Regolith Simulants. Icarus 2021, 354, 114022. [Google Scholar] [CrossRef]
- Ramalho, T.P.; Chopin, G.; Pérez-Carrascal, O.M.; Tromas, N.; Verseux, C. Selection of Anabaena Sp. PCC 7938 as a Cyanobacterium Model for Biological ISRU on Mars. Appl. Environ. Microbiol. 2022, 88, e00594-22. [Google Scholar] [CrossRef] [PubMed]
- Macário, I.P.E.; Veloso, T.; Frankenbach, S.; Serôdio, J.; Passos, H.; Sousa, C.; Gonçalves, F.J.M.; Ventura, S.P.M.; Pereira, J.L. Cyanobacteria as Candidates to Support Mars Colonization: Growth and Biofertilization Potential Using Mars Regolith as a Resource. Front. Microbiol. 2022, 13, 840098. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, B.G.; Rothschild, L.J.; Fagliarone, C.; Chiavarini, S.; Billi, D. Feasibility as Feedstock of the Cyanobacterium Chroococcidiopsis Sp. 029 Cultivated with Urine-Supplemented Moon and Mars Regolith Simulants. Algal Res. 2023, 71, 103044. [Google Scholar] [CrossRef]
- Allen, C.C.; Morris, R.V.; Karen, M.J.; Golden, D.C.; Lindstrom, M.M.; Lockwood, J.P. Martian Regolith Simulant Jsc Mars-1. Lunar Planet. Sci. Conf. 1998, XXIX, 1690. [Google Scholar]
- Sarigul, N.; Korkmaz, F.; Kurultak, İ. A New Artificial Urine Protocol to Better Imitate Human Urine. Sci. Rep. 2019, 9, 20159. [Google Scholar] [CrossRef] [PubMed]
- Staub, R. Ernährungphysiologish-Autökologische Untersuchung an Den Planktonischen Blaualge Oscillatoria Rubescens DC. Schweizerische Zeitschrift Für Hydrologie. Ph.D. Thesis, ETH Zürich, Zürich, Switzerland, 1961; pp. 82–168. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Chen, Y.; Vaidyanathan, S. Simultaneous Assay of Pigments, Carbohydrates, Proteins and Lipids in Microalgae. Anal. Chim. Acta 2013, 776, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Brenton, A.G.; Godfrey, A.R. Accurate Mass Measurement: Terminology and Treatment of Data. J. Am. Soc. Mass Spectrom. 2010, 21, 1821–1835. [Google Scholar] [CrossRef] [PubMed]
- Zavrel, T.; Sinetova, M.; Červený, J. Measurement of Chlorophyll a and Carotenoids Concentration in Cyanobacteria. Bio-Protocol 2015, 5, e1467. [Google Scholar] [CrossRef]
- Ritchie, R.J. Consistent Sets of Spectrophotometric Chlorophyll Equations for Acetone, Methanol and Ethanol Solvents. Photosynth. Res. 2006, 89, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as Well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Lobban, C.S.; Chapman, D.J.; Kremer, B.P. Experimental Phycology: A Laboratory Manual; CUP Archive: Cambridge, UK, 1988; ISBN 052134834X. [Google Scholar]
- Zavřel, T.; Chmelík, D.; Sinetova, M.A.; Červený, J. Spectrophotometric Determination of Phycobiliprotein Content in Cyanobacterium Synechocystis. J. Vis. Exp. 2018, 139, e58076. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Flores Hernandez, F.Y.; Khandual, S.; Ramírez López, I.G. Cytotoxic Effect of Spirulina Platensis Extracts on Human Acute Leukemia Kasumi-1 and Chronic Myelogenous Leukemia K-562 Cell Lines. Asian Pac. J. Trop. Biomed. 2017, 7, 14–19. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Varshney, P.; Mikulic, P.; Vonshak, A.; Beardall, J.; Wangikar, P.P. Extremophilic Micro-Algae and Their Potential Contribution in Biotechnology. Bioresour. Technol. 2015, 184, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Babu, P.; Chandel, A.K.; Singh, O.V. (Eds.) Survival Mechanisms of Extremophiles BT. In Extremophiles and Their Applications in Medical Processes; Springer International Publishing: Cham, Switzerland, 2015; pp. 9–23. ISBN 978-3-319-12808-5. [Google Scholar]
- Yadav, P.; Singh, R.P.; Rana, S.; Joshi, D.; Kumar, D.; Bhardwaj, N.; Gupta, R.K.; Kumar, A. Mechanisms of Stress Tolerance in Cyanobacteria under Extreme Conditions. Stresses 2022, 2, 531–549. [Google Scholar] [CrossRef]
- Csonka, L.N. Physiological and Genetic Responses of Bacteria to Osmotic Stress. Microbiol. Rev. 1989, 53, 121–147. [Google Scholar] [CrossRef]
- Concas, A.; Lutzu, G.A.; Turgut Dunford, N. Experiments and Modeling of Komvophoron Sp. Growth in Hydraulic Fracturing Wastewater. Chem. Eng. J. 2021, 426, 131299. [Google Scholar] [CrossRef]
- Neubek, D.J. Nutrition Requirements, Standards, and Operating Bands for Exploration Missions; Nutritional Biochemistry Laboratory: Washington, DC, USA, 2005. [Google Scholar]
- Stein, T.P.; Leskiw, M.J. Oxidant Damage during and after Spaceflight. Am. J. Physiol.—Endocrinol. Metab. 2000, 278, 375–382. [Google Scholar] [CrossRef]
- Smith, S.M.; Zwart, S.R.; Heer, M. Evidence Report: Risk Factor of Inadequate Nutrition; National Aeronautics and Space Administration: Houston, TX, USA, 2015. [Google Scholar]
- Carpentier, W.R.; Charles, J.B.; Shelhamer, M.; Hackler, A.S.; Johnson, T.L.; Domingo, C.M.M.; Sutton, J.P.; Scott, G.B.I.; Wotring, V.E. Biomedical Findings from NASA’s Project Mercury: A Case Series. NPJ Microgravity 2018, 4, 6. [Google Scholar] [CrossRef]
- Atwater, W.O.; Woods, C.D. The Chemical Composition of American Food Materials. Publisher: Washington: Government Printing Office. 1896. Available online: https://www.ars.usda.gov/ARSUserFiles/80400530/pdf/hist/oes_1896_bul_28.pdf (accessed on 22 February 2023).
- Sirmons, T.A.; Roma, P.G.; Whitmire, A.M.; Smith, S.M.; Zwart, S.R.; Young, M.; Douglas, G.L. Meal Replacement in Isolated and Confined Mission Environments: Consumption, Acceptability, and Implications for Physical and Behavioral Health. Physiol. Behav. 2020, 219, 112829. [Google Scholar] [CrossRef]
- Strable, M.S.; Ntambi, J.M. Genetic Control of de Novo Lipogenesis: Role in Diet-Induced Obesity. Crit. Rev. Biochem. Mol. Biol. 2010, 45, 199–214. [Google Scholar] [CrossRef]
- Silbernagel, G.; Kovarova, M.; Cegan, A.; Machann, J.; Schick, F.; Lehmann, R.; Häring, H.-U.; Stefan, N.; Schleicher, E.; Fritsche, A.; et al. High Hepatic SCD1 Activity Is Associated with Low Liver Fat Content in Healthy Subjects under a Lipogenic Diet. J. Clin. Endocrinol. Metab. 2012, 97, E2288–E2292. [Google Scholar] [CrossRef]
- Carta, G.; Murru, E.; Banni, S.; Manca, C. Palmitic Acid: Physiological Role, Metabolism and Nutritional Implications. Front. Physiol. 2017, 8, 902. [Google Scholar] [CrossRef] [PubMed]
- Roopashree, P.G.; Shetty, S.S.; Suchetha Kumari, N. Effect of Medium Chain Fatty Acid in Human Health and Disease. J. Funct. Foods 2021, 87, 104724. [Google Scholar] [CrossRef]
- Dou, X.; Lu, X.-H.; Lu, M.-Z.; Yu, L.-S.; Xue, R.; Ji, J.-B. The Effects of Trace Elements on the Lipid Productivity and Fatty Acid Composition of Nannochloropis oculata. J. Renew. Energy 2013, 2013, 671545. [Google Scholar] [CrossRef]
- Polat, E.; Yüksel, E.; Altınbaş, M. Effect of Different Iron Sources on Sustainable Microalgae-Based Biodiesel Production Using Auxenochlorella Protothecoides. Renew. Energy 2020, 162, 1970–1978. [Google Scholar] [CrossRef]
- Concas, A.; Steriti, A.; Pisu, M.; Cao, G. Experimental and Theoretical Investigation of the Effects of Iron on Growth and Lipid Synthesis of Microalgae in View of Their Use to Produce Biofuels. J. Environ. Chem. Eng. 2021, 9, 105349. [Google Scholar] [CrossRef]
- Shiels, K.; Tsoupras, A.; Lordan, R.; Zabetakis, I.; Murray, P.; Kumar Saha, S. Anti-Inflammatory and Antithrombotic Properties of Polar Lipid Extracts, Rich in Unsaturated Fatty Acids, from the Irish Marine Cyanobacterium Spirulina Subsalsa. J. Funct. Foods 2022, 94, 105124. [Google Scholar] [CrossRef]
- Sheng, J.; Vannela, R.; Rittmann, B.E. Evaluation of Methods to Extract and Quantify Lipids from Synechocystis PCC 6803. Bioresour. Technol. 2011, 102, 1697–1703. [Google Scholar] [CrossRef]
- Fais, G.; Malavasi, V.; Scano, P.; Soru, S.; Caboni, P.; Cao, G. Metabolomics and Lipid Profile Analysis of Coccomyxa Melkonianii SCCA 048. Extremophiles 2021, 25, 357–368. [Google Scholar] [CrossRef]
- Wada, H.; Murata, N. Lipids in Photosynthesis: Essential and Regulatory Functions; Springer Science & Business Media: New York City, NY, USA, 2009; Volume 30, ISBN 9048128633. [Google Scholar]
- Jones, M.R. Lipids in Photosynthetic Reaction Centres: Structural Roles and Functional Holes. Prog. Lipid Res. 2007, 46, 56–87. [Google Scholar] [CrossRef] [PubMed]
- Loll, B.; Kern, J.; Saenger, W.; Zouni, A.; Biesiadka, J. Towards Complete Cofactor Arrangement in the 3.0 Å Resolution Structure of Photosystem II. Nature 2005, 438, 1040–1044. [Google Scholar] [CrossRef]
- Endo, K.; Kobayashi, K.; Wada, H. Sulfoquinovosyldiacylglycerol Has an Essential Role in Thermosynechococcus elongatus BP-1 under Phosphate-Deficient Conditions. Plant Cell Physiol. 2016, 57, 2461–2471. [Google Scholar] [CrossRef] [PubMed]
- Ibañez, E.; Cifuentes, A. Benefits of Using Algae as Natural Sources of Functional Ingredients. J. Sci. Food Agric. 2013, 93, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Plouguerné, E.; De Souza, L.M.; Sassaki, G.L.; Cavalcanti, J.F.; Villela Romanos, M.T.; Da Gama, B.A.P.; Pereira, R.C.; Barreto-Bergter, E. Antiviral Sulfoquinovosyldiacylglycerols (SQDGs) from the Brazilian Brown Seaweed Sargassum Vulgare. Mar. Drugs 2013, 11, 4628–4640. [Google Scholar] [CrossRef] [PubMed]
- Stengel, D.B.; Connan, S.; Popper, Z.A. Algal Chemodiversity and Bioactivity: Sources of Natural Variability and Implications for Commercial Application. Biotechnol. Adv. 2011, 29, 483–501. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M.S.; Rodríguez, A.D.; Berlinck, R.G.S.; Fusetani, N. Marine Pharmacology in 2007-8: Marine Compounds with Antibacterial, Anticoagulant, Antifungal, Anti-Inflammatory, Antimalarial, Antiprotozoal, Antituberculosis, and Antiviral Activities; Affecting the Immune and Nervous System, and Other Miscellaneous Me. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2011, 153, 191–222. [Google Scholar] [CrossRef]
- Mueller, M.; Hobiger, S.; Jungbauer, A. Anti-Inflammatory Activity of Extracts from Fruits, Herbs and Spices. Food Chem. 2010, 122, 987–996. [Google Scholar] [CrossRef]
- Du, B.; Lin, C.; Bian, Z.; Xu, B. An Insight into Anti-Inflammatory Effects of Fungal Beta-Glucans. Trends Food Sci. Technol. 2015, 41, 49–59. [Google Scholar] [CrossRef]
- Bruno, A.; Rossi, C.; Marcolongo, G.; Di Lena, A.; Venzo, A.; Berrie, C.P.; Corda, D. Selective in Vivo Anti-Inflammatory Action of the Galactolipid Monogalactosyldiacylglycerol. Eur. J. Pharmacol. 2005, 524, 159–168. [Google Scholar] [CrossRef] [PubMed]
- de Los Reyes, C.; Ortega, M.J.; Rodriguez-Luna, A.; Talero, E.; Motilva, V.; Zubia, E. Molecular Characterization and Anti-Inflammatory Activity of Galactosylglycerides and Galactosylceramides from the Microalga Isochrysis Galbana. J. Agric. Food Chem. 2016, 64, 8783–8794. [Google Scholar] [CrossRef]
- Ulivi, V.; Lenti, M.; Gentili, C.; Marcolongo, G.; Cancedda, R.; Descalzi Cancedda, F. Anti-Inflammatory Activity of Monogalactosyldiacylglycerol in Human Articular Cartilage in Vitro: Activation of an Anti-Inflammatory Cyclooxygenase-2 (COX-2) Pathway. Arthritis Res. Ther. 2011, 13, R92. [Google Scholar] [CrossRef]
- Van Kiem, P.; Van Minh, C.; Nhiem, N.X.; Cuong, N.X.; Tai, B.H.; Quang, T.H.; Anh, H.L.T.; Yen, P.H.; Ban, N.K.; Kim, S.H. Inhibitory Effect on TNF-α-Induced IL-8 Secretion in HT-29 Cell Line by Glyceroglycolipids from the Leaves of Ficus Microcarpa. Arch. Pharm. Res. 2012, 35, 2135–2142. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Lee, J.-B.; Atsumi, K.; Kanazashi, M.; Shibayama, T.; Okamoto, K.; Kawahara, T.; Hayashi, T. In Vitro and in Vivo Anti-Herpes Simplex Virus Activity of Monogalactosyl Diacylglyceride from Coccomyxa Sp. KJ (IPOD FERM BP-22254), a Green Microalga. PLoS ONE 2019, 14, e0219305. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Ding, D.; Li, J.; He, L.; Xu, X.; Zhao, Y.; Yan, B.; Li, Z.; Xu, J. Characterisation of Genes Involved in Galactolipids and Sulfolipids Metabolism in Maize and Arabidopsis and Their Differential Responses to Phosphate Deficiency. Funct. Plant Biol. 2020, 47, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Sanina, N.; Davydova, L.; Chopenko, N.; Kostetsky, E.; Shnyrov, V. Modulation of Immunogenicity and Conformation of HA1 Subunit of Influenza A Virus H1/N1 Hemagglutinin in Tubular Immunostimulating Complexes. Int. J. Mol. Sci. 2017, 18, 1895. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, K.R.; Cardellina, J.H.; Fuller, R.W.; Weislow, O.S.; Kiser, R.F.; Snader, K.M.; Patterson, G.M.L.; Boyd, M.R. AIDS-Antiviral Sulfolipids from Cyanobacteria (Blue-Green Algae). JNCI J. Natl. Cancer Inst. 1989, 81, 1254–1258. [Google Scholar] [CrossRef] [PubMed]
- Plouguerné, E.; da Gama, B.A.P.; Pereira, R.C.; Barreto-Bergter, E. Glycolipids from Seaweeds and Their Potential Biotechnological Applications. Front. Cell. Infect. Microbiol. 2014, 4, 174. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, T.; Nishida, M.; Hada, T.; Kuramochi, K.; Sugawara, F.; Kobayashi, S.; Iijima, H.; Shimada, H.; Yoshida, H.; Mizushina, Y. Inhibitory Effect of Sulfoquinovosyl Diacylglycerol on Prokaryotic DNA Polymerase I Activity and Cell Growth of Escherichia Coli. J. Oleo Sci. 2007, 56, 43–47. [Google Scholar] [CrossRef]
- Cassier-Chauvat, C.; Chauvat, F. Responses to Oxidative and Heavy Metal Stresses in Cyanobacteria: Recent Advances. Int. J. Mol. Sci. 2014, 16, 871–886. [Google Scholar] [CrossRef]
- Monteiro, C.M.; Castro, P.M.L.; Malcata, F.X. Metal Uptake by Microalgae: Underlying Mechanisms and Practical Applications. Biotechnol. Prog. 2012, 28, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Concas, A.; Pisu, M.; Cao, G. Microalgal Cell Disruption Through Fenton Reaction: Experiments, Modeling and Remarks on Its Effect on the Extracted Lipids Composition. Chem. Eng. Trans. 2015, 43, 367–372. [Google Scholar] [CrossRef]
- Ajayan, K.V.; Selvaraju, M.; Thirugnanamoorthy, K. Enrichment of Chlorophyll and Phycobiliproteins in Spirulina Platensis by the Use of Reflector Light and Nitrogen Sources: An in-Vitro Study. Biomass Bioenergy 2012, 47, 436–441. [Google Scholar] [CrossRef]
- Gladfelter, M.F.; Buley, R.P.; Belfiore, A.P.; Fernandez-Figueroa, E.G.; Gerovac, B.L.; Baker, N.D.; Wilson, A.E. Dissolved Nitrogen Form Mediates Phycocyanin Content in Cyanobacteria. Freshw. Biol. 2022, 67, 954–964. [Google Scholar] [CrossRef]
- Goiris, K.; Muylaert, K.; Fraeye, I.; Foubert, I.; De Brabanter, J.; De Cooman, L. Antioxidant Potential of Microalgae in Relation to Their Phenolic and Carotenoid Content. J. Appl. Phycol. 2012, 24, 1477–1486. [Google Scholar] [CrossRef]
- Faraloni, C.; Torzillo, G. Synthesis of Antioxidant Carotenoids in Microalgae in Response to Physiological Stress. In Carotenoids; IntechOpen: London, UK, 2017. [Google Scholar]
- Soru, S.; Malavasi, V.; Concas, A.; Caboni, P.; Cao, G. A Novel Investigation of the Growth and Lipid Production of the Extremophile Microalga Coccomyxa Melkonianii SCCA 048 under the Effect of Different Cultivation Conditions: Experiments and Modeling. Chem. Eng. J. 2019, 377, 120589. [Google Scholar] [CrossRef]
- Soru, S.; Malavasi, V.; Caboni, P.; Concas, A.; Cao, G. Behavior of the Extremophile Green Alga Coccomyxa Melkonianii SCCA 048 in Terms of Lipids Production and Morphology at Different PH Values. Extremophiles 2019, 23, 79–89. [Google Scholar] [CrossRef]










| Macronutrients | Micronutrients | ||
|---|---|---|---|
| Component | (g × L−1) | Component | (mg × L−1) |
| Na2SO4 | 0.085 | Al | 2.4 |
| C5H4N4O3 | 0.012 | Ca | 4.06 |
| Na3C6H5O7 × 2H2O | 0.036 | Fe | 3.205 |
| C4H7N3O | 0.044 | K | 4.16 |
| CH4N2O | 0.750 | Mg | 0.74 |
| KCl | 0.115 | Mn | 0.095 |
| NaCl | 0.087 | Na | 2.33 |
| CaCl2 | 0.009 | P | 0.125 |
| NH4Cl | 0.063 | Si | 5.14 |
| K2C2O4 × H2O | 0.002 | Ti | 0.635 |
| MgSO4 × 7H2O | 0.054 | ||
| NaH2PO4 × 7H2O | 0.146 | ||
| Na2HPO4 × 2H2O | 0.041 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fais, G.; Casula, M.; Sidorowicz, A.; Manca, A.; Margarita, V.; Fiori, P.L.; Pantaleo, A.; Caboni, P.; Cao, G.; Concas, A. Cultivation of Chroococcidiopsis thermalis Using Available In Situ Resources to Sustain Life on Mars. Life 2024, 14, 251. https://doi.org/10.3390/life14020251
Fais G, Casula M, Sidorowicz A, Manca A, Margarita V, Fiori PL, Pantaleo A, Caboni P, Cao G, Concas A. Cultivation of Chroococcidiopsis thermalis Using Available In Situ Resources to Sustain Life on Mars. Life. 2024; 14(2):251. https://doi.org/10.3390/life14020251
Chicago/Turabian StyleFais, Giacomo, Mattia Casula, Agnieszka Sidorowicz, Alessia Manca, Valentina Margarita, Pier Luigi Fiori, Antonella Pantaleo, Pierluigi Caboni, Giacomo Cao, and Alessandro Concas. 2024. "Cultivation of Chroococcidiopsis thermalis Using Available In Situ Resources to Sustain Life on Mars" Life 14, no. 2: 251. https://doi.org/10.3390/life14020251
APA StyleFais, G., Casula, M., Sidorowicz, A., Manca, A., Margarita, V., Fiori, P. L., Pantaleo, A., Caboni, P., Cao, G., & Concas, A. (2024). Cultivation of Chroococcidiopsis thermalis Using Available In Situ Resources to Sustain Life on Mars. Life, 14(2), 251. https://doi.org/10.3390/life14020251