Fluorine-Rich Planetary Environments as Possible Habitats for Life
Abstract
:1. Introduction
2. Hydrofluoric Acid as a Solvent for Life?
3. Fluorine-Containing Organic Compounds as Possible Building Blocks of Life
3.1. Fluorine-Containing Organic Compounds
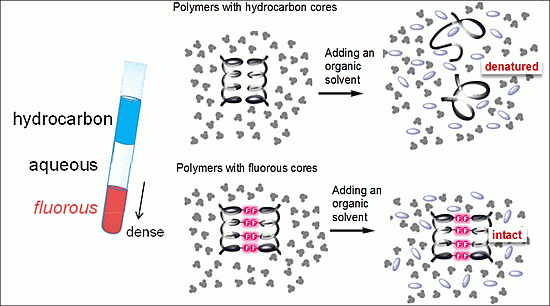
3.2. Fluorous Effect and Folding of Highly Fluorinated Bio-Molecules

3.3. From Fluorinated Amino Acids to Proteins and Peptides

4. Life Based on Fluorine Chemistry?
5. Summary
Author Contributions
Conflicts of Interest
References
- Lide, D.R. Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Goldsmith, D.; Owen, T. The Search for Life in the Universe; University Science Books: Sausalito, CA, USA, 2003. [Google Scholar]
- Kirk, K.L. Biochemistry of Halogenated Organic Compounds; Plenum: New York, NY, USA, 1991; Volume 9B, pp. 127–150. [Google Scholar]
- O’Hagan, D.; Schaffrath, C.; Cobb, S.L.; Hamilton, J.T.G.; Murphy, C.D. Biochemistry: Biosynthesis of an organofluorine molecule. Nature 2002, 416, 279. [Google Scholar] [CrossRef]
- Gribble, G.W. The diversity of naturally occurring organobromine compounds. Chem. Soc. Rev. 1999, 28, 335–346. [Google Scholar] [CrossRef]
- Gribble, G.W. Naturally Occurring Organofluorines. In Organofluorines; Neilson, A.H., Ed.; Springer-Verlag: Berlin/Heidelberg, Germany, 2002; pp. 121–136. [Google Scholar]
- O’Hagan, D.; Rzepa, H.S. Some influences of fluorine in bioorganic chemistry. Chem. Commun. 1997, 645–652. [Google Scholar]
- Harper, D.B.; O’Hagan, D. The fluorinated natural products. Nat. Prod. Rep. 1994, 11, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Schulze-Makuch, D.; Irwin, L.N. Life in the Universe: Expectations and Constraints, 2nd ed.; Springer-Verlag: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Firsoff, V.A. Life beyond the Earth; Basic Books: New York, NY, USA, 1963. [Google Scholar]
- Olah, G.A. Crossing conventional boundaries in half a century of research. J. Org. Chem. 2005, 70, 2413–2429. [Google Scholar] [CrossRef] [PubMed]
- Vanderborgh, N.E. Evaluation of the lanthanum fluoride membrane electrode response in acidic solutions. Talanta 1968, 15, 1009–1013. [Google Scholar] [CrossRef] [PubMed]
- Heidelberger, C.; Chaudhuri, N.K.; Danneberg, P.; Mooren, D.; Griesbach, L.; Duschinsky, R.; Schnitzer, R.J.; Pleven, E.; Scheiner, J. Fluorinated pyrimidines, a new class of tumour-inhibitory compounds. Nature 1957, 179, 663–666. [Google Scholar] [CrossRef] [PubMed]
- Kirk, K.L. Fluorine in medicinal chemistry: recent therapeutic application of fluorinated small molecules. J. Fluor. Chem. 2006, 127, 1013–1029. [Google Scholar] [CrossRef]
- Purcer, S.; Moore, P.R.; Swallow, S.; Gouverneur, V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 2008, 37, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Gung, B.W.; Patel, M.; Xue, X. A threshold for charge transfer in aromatic interactions? A quantitative study of π-stacking interactions. J. Org. Chem. 2005, 70, 10532–10537. [Google Scholar] [CrossRef] [PubMed]
- Cejas, M.A.; Kinney, W.A.; Chen, C.; Vinter, J.G.; Almond, H.R.; Balss, K.M.; Maryanoff, C.A.; Schmidt, U.; Breaslav, M.; Mahan, A.; Lacy, E.; Maryanoff, B.E. Thrombogenic collagen-mimetic peptides: Self-assembly of triple helix-based fibrils driven by hydrophobic interactions. Proc. Natl. Acad. Sci. USA 2008, 105, 8513–8518. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.S.; Kool, E.T. Selective pairing of Polyfluorinated DNA bases. J. Am. Chem. Soc. 2004, 126, 3040–3041. [Google Scholar] [CrossRef] [PubMed]
- Cametti, M.; Crousse, B.; Metrangolo, P.; Milani, R.; Resnati, G. The fluorous effect in biomolecular applications. Chem. Soc. Rev. 2012, 41, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Gladysz, J.A.; Jurisch, M. Structural, physical, and chemical properties of fluorous compounds. In Luorous Chemistry; Series Topics in Current Chemistry; Springer-Verlag: Berlin/Heidelberg, Germany, 2012; Volume 308, pp. 1–23. [Google Scholar]
- Santaella, C.; Vierling, P.; Riess, J.G. High stable liposomes derived from perfluoroalkylated glycerophosphocholines. Angew. Chem. Int. Ed. 1991, 30, 567–568. [Google Scholar] [CrossRef]
- Santaella, C.; Vierling, P.; Riess, J.G.; Gulik-Krzywicki, T.; Gulik, A.; Monasse, B. Polymorphic phase behavior of perfluoroalkylated phosphatidylcholines. Biochim. Biophys. Acta 1994, 1190, 25–39. [Google Scholar] [CrossRef]
- Santaella, C.; Vierling, P. Molecular order and mobility within liposomal membrane made from highly fluorinated phospholipids. Chem. Phys. Lipids 1995, 77, 173–177. [Google Scholar]
- Gege, C.; Schneider, M.F.; Schumacher, G.; Limozin, L.; Rothe, U.; Bendas, G.; Tanaka, M.; Schmidt, R.R. Functional microdomains of glycolipids with partially fluorinated membrane anchors: impact on cell adhesion. ChemPhysChem 2004, 5, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Marsh, E.N.G. Fluorinated Proteins: From Design and Synthesis to Structure and Stability. Acc. Chem. Res. 2014. [Google Scholar] [CrossRef]
- Yoder, N.C.; Kumar, K. Fluorinated amino acids in protein design. Chem. Soc. Rev. 2002, 31, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Biffinger, J.C.; Kim, H.W.; DiMagno, S.G. The polar hydrophobicity of fluorinated compounds. ChemBioChem 2004, 5, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Budisa, N. Engineering the Genetic Code-Expanding the Amino Acid Repertoire for the Design of Novel Proteins; Wiley: Weinheim, Germany, 2005. [Google Scholar]
- Merkel, L.; Budisa, N. Organic fluorine as a polypeptide building element: In vivo expression of fluorinated peptides, proteins and proteomes. Org. Biomol. Chem. 2012, 10, 7241–7261. [Google Scholar] [CrossRef] [PubMed]
- Dobbs, A.P.; Kimberley, M.R. Fluorous phase chemistry: A new industrial technology. J. Fluor. Chem. 2002, 118, 3–17. [Google Scholar] [CrossRef]
- Marsh, E.N.G. Towards the nonstick egg: Designing fluorous proteins. Chem. Biol. 2000, 7, R153–R157. [Google Scholar] [CrossRef] [PubMed]
- Schulze-Makuch, D.; Haque, S.; Antonio, M.R.S.; Ali, D.; Hosein, R.; Song, Y.C.; Yang, J.; Zaikova, E.; Beckles, D.M.; Guinan, E.; Lehto, H.J.; Hallam, S.J. Microbial life in a liquid asphalt desert. Astrobiology 2011, 11, 241–258. [Google Scholar]
- Biava, H.; Budisa, N. Evolution of fluorinated enzymes: An emerging trend for biocatalyst stabilization. Eng. Life Sci. 2014, 14, 340–351. [Google Scholar] [CrossRef]
- Rennert, O.M.; Anker, H.S. On the Incorporation of 5',5',5'-Trifluoroleucine into Proteins of E. coli. Biochemistry 1963, 2, 471–476. [Google Scholar] [CrossRef]
- O’Hagan, D. Understanding organofluorine chemistry. An introduction to the C–F bond. Chem. Soc. Rev. 2008, 37, 308–319. [Google Scholar]
- Salwiczek, M.; Nyakatura, E.K.; Gerling, U.I.M.; Ye, S.; Koksch, B. Fluorinated amino acids: compatibility with native protein structures and effects on protein–protein interactions. Chem. Soc. Rev. 2012, 41, 2135–2171. [Google Scholar] [CrossRef] [PubMed]
- Jäckel, C.; Salwiczek, M.; Koksch, B. Fluorine in a native protein environment- how the spatial demand and polarity of fluoroalkyl groups affect protein folding. Angew. Chem. Int. Ed. 2006, 45, 4198–4203. [Google Scholar]
- Pratt, E.A.; Ho, C. Incorporation of fluorotryptophans into proteins of Escherichia coli. Biochemistry 1975, 14, 3035–3040. [Google Scholar] [CrossRef] [PubMed]
- Renner, C.; Alefelder, S.; Bae, J.H.; Budisa, N.; Huber, R.; Moroder, L. Fluoroprolines as tools for protein design and engineering. Angew. Chem. Int. Ed. 2001, 40, 923–925. [Google Scholar] [CrossRef]
- Kitevski-LeBlanc, J.L.; Prosser, R.S. Current applications of 19F NMR to studies of protein structure and dynamics. Prog. Nuc. Mag. Res. 2012, 62, 1–33. [Google Scholar]
- Buer, B.C.; Levin, B.J.; Marsh, E.N.G. Influence of fluorination on the thermodynamic of protein folding. J. Am. Chem. Soc. 2012, 134, 13027–13034. [Google Scholar] [CrossRef] [PubMed]
- Duewel, H.S.; Daub, E.; Robinson, V.; Honek, J.F. Elucidation of solvent exposure, side-chain reactivity, and steric demands of the trifluoromethionine residue in a recombinant protein. Biochemistry 2001, 40, 13167–13176. [Google Scholar] [CrossRef] [PubMed]
- Dalvi, V.H.; Rossky, P.J. Molecular origins of fluorocarbon hydrophobicity. Proc. Natl. Acad. Sci. USA 2010, 107, 13603–13607. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-H.; Lee, H.-Y.; Slutsky, M.M.; Anderson, J.T.; Marsch, E.N.G. Fluorous effect in proteins: de novo design and characterization of a four-α-helix bundle protein containing hexafluoroleucine. Biochemistry 2004, 43, 16277–16284. [Google Scholar] [CrossRef] [PubMed]
- Bilgiçer, B.; Fichera, A.; Kumar, K. A coiled coil with a fluorous core. J. Am. Chem. Soc. 2001, 123, 4393–4399. [Google Scholar]
- Buer, B.C.; Meagher, J.L.; Stuckey, J.A.; Marsh, E.N.G. Structural basis for the enhanced stability of highly fluorinated proteins. Proc. Natl. Acad. Sci. USA 2012, 109, 4810–4815. [Google Scholar] [CrossRef] [PubMed]
- Budisa, N. Prolegomena to future efforts on genetic code engineering by expanding its amino acid repertoire. Angew. Chem. Int. Ed. 2004, 43, 6426–6463. [Google Scholar] [CrossRef]
- Johnson, J.A.; Lu, Y.Y.; van Deventer, J.A.; Tirrell, D.A. Residue-specific incorporation of noncanonical amino acids into proteins: recent developments and applications. Curr. Opin. Chem. Biol. 2010, 14, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Budisa, N.; Pipitone, O.; Siwanowicz, I.; Rubini, M.; Pal, P.P.; Holak, T.A.; Gelmi, M.L. Efforts towards the design of “Teflon” proteins: in vivo translation with trifluorinated leucine and methionine analogs. Chem. Biodivers. 2004, 1, 1465–1475. [Google Scholar] [CrossRef] [PubMed]
- Schulze-Makuch, D.; Irwin, L.N. Exotic forms of life in the universe. Naturwissenschaften 2006, 93, 155–172. [Google Scholar]
- Wong, J.T.F. Evolution of the genetic code. Microbiol. Sci. 1988, 5, 174–181. [Google Scholar] [PubMed]
- Kasting, J.F.; Siefert, J.L. Life and the evolution of Earth’s atmosphere. Science 2002, 296, 1066–1068. [Google Scholar] [CrossRef] [PubMed]
- Budisa, N. Xenobiology, new-to-nature synthetic cells and genetic firewall. Curr. Org. Chem. 2014, 18, 936–943. [Google Scholar] [CrossRef]
- Marlière, P. The farther, the safer: a manifesto for securely navigating synthetic species away from the old living world. Syst. Synth. Biol. 2009, 3, 77–84. [Google Scholar]
- Marlière, P.; Patrouix, J.; Döring, V.; Herdewijn, P.; Tricot, S.; Cruveiller, S.; Bouzon, M.; Mutzel, R. Chemical evolution of a bacterial genome. Angew. Chem. Int. Ed. 2011, 50, 7109–7114. [Google Scholar]
- Acevedo-Rocha, C.G.; Budisa, N. On the road towards chemically modified organisms endowed with a genetic firewall. Angew. Chem. Int. Ed. 2011, 50, 6960–6962. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Budisa, N.; Kubyshkin, V.; Schulze-Makuch, D. Fluorine-Rich Planetary Environments as Possible Habitats for Life. Life 2014, 4, 374-385. https://doi.org/10.3390/life4030374
Budisa N, Kubyshkin V, Schulze-Makuch D. Fluorine-Rich Planetary Environments as Possible Habitats for Life. Life. 2014; 4(3):374-385. https://doi.org/10.3390/life4030374
Chicago/Turabian StyleBudisa, Nediljko, Vladimir Kubyshkin, and Dirk Schulze-Makuch. 2014. "Fluorine-Rich Planetary Environments as Possible Habitats for Life" Life 4, no. 3: 374-385. https://doi.org/10.3390/life4030374
APA StyleBudisa, N., Kubyshkin, V., & Schulze-Makuch, D. (2014). Fluorine-Rich Planetary Environments as Possible Habitats for Life. Life, 4(3), 374-385. https://doi.org/10.3390/life4030374