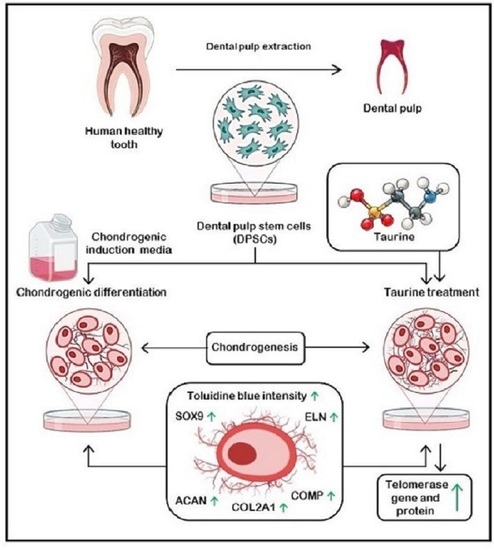
Taurine Augments Telomerase Activity and Promotes Chondrogenesis in Dental Pulp Stem Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Source and Cell Culture Protocol for Dental Pulp Stem Cells
2.2. Flow Cytometric Characterization of DPSCs
2.3. Osteogenic Differentiation
2.4. Adipogenic Differentiation
2.5. Chondrogenic Differentiation
2.6. Cytotoxicity Assay for Determination of Effect of Taurine on DPSCs
2.7. Determination of the Effect of Taurine on Chondrogenic Differentiation of DPSCs
2.8. Real-Time Quantitative PCR for Analysis of Gene Expression
2.9. Western Blot Analysis
2.10. Immunofluorescence Assay
2.11. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Buckwalter, J.A.; Mankin, H.J. Articular cartilage repair and transplantation. Arthritis Rheum. 1998, 41, 1331–1342. [Google Scholar] [CrossRef]
- Bentley, G.; Biant, L.C.; Carrington, R.W.J.; Akmal, M.; Goldberg, A.; Williams, A.M.; Skinner, J.A.; Pringle, J. A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J. Bone Jt. Surg. Br. 2003, 85, 223–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knutsen, G.; Engebretsen, L.; Ludvigsen, T.C.; Drogset, J.O.; Grøntvedt, T.; Solheim, E.; Strand, T.; Roberts, S.; Isaksen, V.; Johansen, O. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J. Bone Jt. Surg. Am. 2004, 86, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Tatullo, M.; Spagnuolo, G.; Codispoti, B.; Zamparini, F.; Zhang, A.; Esposti, M.; Aparicio, C.; Rengo, C.; Nuzzolese, M.; Manzoli, L.; et al. PLA-Based Mineral-Doped Scaffolds Seeded with Human Periapical Cyst-Derived MSCs: A Promising Tool for Regenerative Healing in Dentistry. Materials 2019, 12, 597. [Google Scholar] [CrossRef] [Green Version]
- Scheibe, F.; Ladhoff, J.; Huck, J.; Grohmann, M.; Blazej, K.; Oersal, A.; Baeva, N.; Seifert, M.; Priller, J. Immune effects of mesenchymal stromal cells in experimental stroke. J. Cereb. Blood Flow Metab. 2012, 32, 1578–1588. [Google Scholar] [CrossRef] [Green Version]
- Trounson, A.; McDonald, C. Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell Stem Cell 2015, 17, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Boese, A.C.; Le, Q.-S.E.; Pham, D.; Hamblin, M.H.; Lee, J.-P. Neural stem cell therapy for subacute and chronic ischemic stroke. Stem Cell Res. Ther. 2018, 9, 154. [Google Scholar] [CrossRef]
- Leong, W.K.; Lewis, M.D.; Koblar, S.A. Concise review: Preclinical studies on human cell-based therapy in rodent ischemic stroke models: Where are we now after a decade? Stem Cells 2013, 31, 1040–1043. [Google Scholar] [CrossRef] [PubMed]
- Prockop, D.J.; Brenner, M.; Fibbe, W.E.; Horwitz, E.; Le Blanc, K.; Phinney, D.G.; Simmons, P.J.; Sensebe, L.; Keating, A. Defining the risks of mesenchymal stromal cell therapy. Cytotherapy 2010, 12, 576–578. [Google Scholar] [CrossRef]
- Lees, J.S.; Sena, E.S.; Egan, K.J.; Antonic, A.; Koblar, S.A.; Howells, D.W.; Macleod, M.R. Stem cell-based therapy for experimental stroke: A systematic review and meta-analysis. Int. J. Stroke 2012, 7, 582–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savitz, S.I. Developing Cellular Therapies for Stroke. Stroke 2015, 46, 2026–2031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalladka, D.; Muir, K.W. Brain repair: Cell therapy in stroke. Stem Cells Cloning 2014, 7, 31–44. [Google Scholar] [CrossRef] [Green Version]
- Meng, H.; Hu, L.; Zhou, Y.; Ge, Z.; Wang, H.; Wu, C.; Jin, J. A Sandwich Structure of Human Dental Pulp Stem Cell Sheet, Treated Dentin Matrix, and Matrigel for Tooth Root Regeneration. Stem Cells Dev. 2020, 29, 521–532. [Google Scholar] [CrossRef]
- Fujii, Y.; Kawase-Koga, Y.; Hojo, H.; Yano, F.; Sato, M.; Chung, U.; Ohba, S.; Chikazu, D. Bone regeneration by human dental pulp stem cells using a helioxanthin derivative and cell-sheet technology. Stem Cell Res. Ther. 2018, 9, 24. [Google Scholar] [CrossRef] [Green Version]
- Marrelli, M.; Codispoti, B.; Shelton, R.M.; Scheven, B.A.; Cooper, P.R.; Tatullo, M.; Paduano, F. Dental Pulp Stem Cell Mechanoresponsiveness: Effects of Mechanical Stimuli on Dental Pulp Stem Cell Behavior. Front. Physiol. 2018, 9. [Google Scholar] [CrossRef]
- Pedroni, A.C.F.; Sarra, G.; de Oliveira, N.K.; Moreira, M.S.; Deboni, M.C.Z.; Marques, M.M. Cell sheets of human dental pulp stem cells for future application in bone replacement. Clin. Oral Investig. 2019, 23, 2713–2721. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, N.; Smith, E.E.; Angstadt, S.; Zhang, W.; Khademhosseini, A.; Yelick, P.C. Dental cell sheet biomimetic tooth bud model. Biomaterials 2016, 106, 167–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casado-Díaz, A.; Anter, J.; Dorado, G.; Quesada-Gómez, J.M. Effects of quercetin, a natural phenolic compound, in the differentiation of human mesenchymal stem cells (MSC) into adipocytes and osteoblasts. J. Nutr. Biochem. 2016, 32, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Ballini, A.; Boccaccio, A.; Saini, R.; Van Pham, P.; Tatullo, M. Dental-Derived Stem Cells and Their Secretome and Interactions with Bioscaffolds/Biomaterials in Regenerative Medicine: From the In Vitro Research to Translational Applications. Stem Cells Int. 2017, 2017, 1–3. [Google Scholar] [CrossRef]
- Bouckenooghe, T.; Remacle, C.; Reusens, B. Is taurine a functional nutrient? Curr. Opin. Clin. Nutr. Metab. Care 2006, 9, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Birdsall, T.C. Therapeutic applications of taurine. Altern. Med. Rev. 1998, 3, 128–136. [Google Scholar]
- Warskulat, U.; Heller-Stilb, B.; Oermann, E.; Zilles, K.; Haas, H.; Lang, F.; Häussinger, D. Phenotype of the taurine transporter knockout mouse. Methods Enzymol. 2007, 428, 439–458. [Google Scholar] [CrossRef]
- Klusa, V.; Klimaviciusa, L.; Duburs, G.; Poikans, J.; Zharkovsky, A. Anti-neurotoxic effects of tauropyrone, a taurine analogue. Adv. Exp. Med. Biol. 2006, 583, 499–508. [Google Scholar] [CrossRef]
- Petrovic, L.; Schlegel, K.A.; Ries, J.; Park, J.; Diebel, E.; Schultze-Mosgau, S.; Wiltfang, J. In Vitro effect of taurolidine on squamous cell carcinoma in the oral cavity. Mund. Kiefer. Gesichtschir. 2003, 7, 102–107. [Google Scholar] [CrossRef]
- Marcinkiewicz, J.; Kurnyta, M.; Biedroń, R.; Bobek, M.; Kontny, E.; Maśliński, W. Anti-inflammatory effects of taurine derivatives (taurine chloramine, taurine bromamine, and taurolidine) are mediated by different mechanisms. Adv. Exp. Med. Biol. 2006, 583, 481–492. [Google Scholar] [CrossRef]
- Zeybek, A.; Sağlam, B.; Cikler, E.; Cetinel, S.; Ercan, F.; Sener, G. Taurine ameliorates stress-induced degeneration of the urinary bladder. Acta Histochem. 2007, 109, 208–214. [Google Scholar] [CrossRef]
- Zeybek, A.; Ercan, F.; Cetinel, S.; Cikler, E.; Sağlam, B.; Sener, G. Taurine ameliorates water avoidance stress-induced degenerations of gastrointestinal tract and liver. Dig. Dis. Sci. 2006, 51, 1853–1861. [Google Scholar] [CrossRef]
- Zeybek, A.; Sağlam, B.; Cikler, E.; Cetinel, S.; Ercan, F.; Sener, G. Protective effects of taurine on protamine sulfate induced bladder damage. World J. Urol. 2006, 24, 438–444. [Google Scholar] [CrossRef]
- Liao, X.-B.; Zhou, X.-M.; Li, J.-M.; Tan, Z.-P.; Liu, L.-M.; Zhang, W.; Tan, H.; Lu, Y.; Yuan, L.-Q. Taurine transporter is expressed in vascular smooth muscle cells. Amino Acids 2007, 33, 639–643. [Google Scholar] [CrossRef]
- Allard, M.L.; Jeejeebhoy, K.N.; Sole, M.J. The management of conditioned nutritional requirements in heart failure. Heart Fail. Rev. 2006, 11, 75–82. [Google Scholar] [CrossRef]
- Gupta, R.C.; Win, T.; Bittner, S. Taurine analogues; a new class of therapeutics: Retrospect and prospects. Curr. Med. Chem. 2005, 12, 2021–2039. [Google Scholar] [CrossRef]
- Parcell, S. Sulfur in human nutrition and applications in medicine. Altern. Med. Rev. 2002, 7, 22–44. [Google Scholar]
- Guizouarn, H.; Motais, R.; Garcia-Romeu, F.; Borgese, F. Cell volume regulation: The role of taurine loss in maintaining membrane potential and cell pH. J. Physiol. 2000, 523 Pt 1, 147–154. [Google Scholar] [CrossRef]
- Kong, W.X.; Chen, S.W.; Li, Y.L.; Zhang, Y.J.; Wang, R.; Min, L.; Mi, X. Effects of taurine on rat behaviors in three anxiety models. Pharmacol. Biochem. Behav. 2006, 83, 271–276. [Google Scholar] [CrossRef]
- Soyka, M.; Roesner, S. New pharmacological approaches for the treatment of alcoholism. Expert Opin. Pharmacother. 2006, 7, 2341–2353. [Google Scholar] [CrossRef]
- Trip, J.; Drost, G.; van Engelen, B.G.M.; Faber, C.G. Drug treatment for myotonia. Cochrane Database Syst. Rev. 2006, CD004762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.; Xu, Z.; Mi, M.; Xu, H.; Zhu, J.; Wei, N.; Chen, K.; Zhang, Q.; Zeng, K.; Wang, J.; et al. Dietary taurine supplementation ameliorates diabetic retinopathy via anti-excitotoxicity of glutamate in streptozotocin-induced Sprague-Dawley rats. Neurochem. Res. 2008, 33, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Grimble, R.F. The effects of sulfur amino acid intake on immune function in humans. J. Nutr. 2006, 136, 1660S–1665S. [Google Scholar] [CrossRef] [Green Version]
- Tan, B.; Jiang, D.-J.; Huang, H.; Jia, S.-J.; Jiang, J.-L.; Hu, C.-P.; Li, Y.-J. Taurine protects against low-density lipoprotein-induced endothelial dysfunction by the DDAH/ADMA pathway. Vascul. Pharmacol. 2007, 46, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Lu, Z.; Wu, H.; Zheng, L. Chondroprotective effects of taurine in primary cultures of human articular chondrocytes. Tohoku J. Exp. Med. 2015, 235, 201–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, F.; Qu, C.; Song, T.; Ding, G.; Fan, Z.; Liu, D.; Liu, Y.; Zhang, C.; Shi, S.; Wang, S. Vitamin C treatment promotes mesenchymal stem cell sheet formation and tissue regeneration by elevating telomerase activity. J. Cell. Physiol. 2012, 227, 3216–3224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patil, V.R.; Kharat, A.H.; Kulkarni, D.G.; Kheur, S.M.; Bhonde, R.R. Long term explant culture for harvesting homogeneous population of human dental pulp stem cells. Cell Biol. Int. 2018, 42, 1602–1610. [Google Scholar] [CrossRef] [PubMed]
- Bergholt, N.L.; Lysdahl, H.; Lind, M.; Foldager, C.B. A Standardized Method of Applying Toluidine Blue Metachromatic Staining for Assessment of Chondrogenesis. Cartilage 2019, 10, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, V.; Rattan, V.; Jha, V.; Pal, A.; Bhattacharyya, S. Molecular spectrum of secretome regulates the relative hepatogenic potential of mesenchymal stem cells from bone marrow and dental tissue. Sci. Rep. 2017, 7, 15015. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Huang, H.; Li, Z.; Liu, X.; Fan, W.; Wang, X.; Sun, X.; Zhu, J.; Zhou, H.; Wei, H. Taurine Promotes the Cartilaginous Differentiation of Human Umbilical Cord-Derived Mesenchymal Stem Cells in Vitro. Neurochem. Res. 2017, 42, 2344–2353. [Google Scholar] [CrossRef]
- Pellegrino, L.; Cocchiola, R.; Francolini, I.; Lopreiato, M.; Piozzi, A.; Zanoni, R.; Scotto d’Abusco, A.; Martinelli, A. Taurine grafting and collagen adsorption on PLLA films improve human primary chondrocyte adhesion and growth. Colloids Surf. B Biointerfaces 2017, 158, 643–649. [Google Scholar] [CrossRef]
- Jeon, S.-H.; Lee, M.-Y.; Kim, S.-J.; Joe, S.-G.; Kim, G.-B.; Kim, I.-S.; Kim, N.-S.; Hong, C.-U.; Kim, S.-Z.; Kim, J.-S.; et al. Taurine increases cell proliferation and generates an increase in [Mg2+]i accompanied by ERK 1/2 activation in human osteoblast cells. FEBS Lett. 2007, 581, 5929–5934. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Benítez, R.; Pasantes-Morales, H.; Saldaña, I.T.; Ramos-Mandujano, G. Taurine stimulates proliferation of mice embryonic cultured neural progenitor cells. J. Neurosci. Res. 2010, 88, 1673–1681. [Google Scholar] [CrossRef]
- Hernández-Benítez, R.; Ramos-Mandujano, G.; Pasantes-Morales, H. Taurine stimulates proliferation and promotes neurogenesis of mouse adult cultured neural stem/progenitor cells. Stem Cell Res. 2012, 9, 24–34. [Google Scholar] [CrossRef] [Green Version]






| Experimental Group | Treatment |
|---|---|
| Control | - |
| Induction | Chondrogenic induction media treatment |
| TI-III + Induction | Telomerase inhibitor + Chondrogenic induction media treatment |
| 10 µM Tau | 10 µM taurine treatment |
| Experimental Group | Treatment |
|---|---|
| Control | - |
| TI-III | Telomerase inhibitor treatment |
| 10 µM Tau | 10 µM taurine treatment |
| TI-III + 10 µM Tau | Telomerase inhibitor + 10 µM taurine treatment |
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| TERT | 5′-GCC GAT TGT GAA CAT GGA CTA CG-3′ | 5′-GCT CGT AGT TGA GCA CGC TGA A-3′ |
| SOX9 | 5′-GCC GAA AGC GGG CTC GAA AC-3′ | 5′-AAA AGT GGG GGC GCT TGC ACC-3′ |
| COL2A1 | 5′-CCT CCA GGT CTT CAG GGA AT-3′ | 5′-AGG AGG TCC AAC TTC TCC CT-3′ |
| ACAN | 5′-GCG AGT TGT CAT GGT CTG AA-3′ | 5′-TTC TTG GAG AAG GGA GTC CA-3′ |
| ELN | 5′-GGT TGT GTC ACC AGA AGC AGC T-3′ | 5′-CCG TAA GTA GGA ATG CCT CCA AC-3′ |
| COMP | 5′-GGA GAT GCT TGT GAC AGC GAT C-3′ | 5′-TGA GTC CTC CTG GGC ACT GTT A-3′ |
| GAPDH | 5′-GTC TCC TCT GAC TTC AAC AGC G-3′ | 5′-ACC ACC CTG TTG CTG TAG CCA A-3′ |
| Concentration | MTT—48 h | MTT—7 Days |
|---|---|---|
| Control | 0.46 ± 0.029 | 1.28 ± 0.42 |
| 1 µM Tau | 0.48 ± 0.073 | 1.63 ± 0.10 |
| 2.5 µM Tau | 0.41 ± 0.09 | 1.62 ± 0.17 |
| 5 µM Tau | 0.53 ± 0.03 | 1.59 ± 0.31 |
| 10 µM Tau | 0.57 ± 0.034 | 1.30 ± 0.29 |
| 25 µM Tau | 0.34 ± 0.10 | 0.27 ± 0.09 |
| 50 µM Tau | 0.25 ± 0.15 | 0.09 ± 0.02 |
| Gene | Control | TI-III | 10 µM Tau | TI-III + 10 µM Tau |
|---|---|---|---|---|
| TERT | 1.00 ± 0.05 | 0.57 ± 0.15 | 3.33 ± 0.36 | 1.95 ± 0.47 |
| Gene | Control | TI-III | 10 µM Tau | TI-III + 10 µM Tau |
|---|---|---|---|---|
| hTERT | 3.42 ± 0.14 | 1.61 ± 0.26 | 4.63 ± 0.36 | 2.50 ± 0.26 |
| Concentration | TB Intensity—Day 21 |
|---|---|
| Control | 0.35 ± 0.15 |
| Induction | 2.83 ± 0.21 |
| TI-III + Induction | 1.16 ± 0.34 |
| 10 µM Tau | 4.14 ± 0.25 |
| Gene | Control | Induction | TI-III + Induction | 10 µM Tau |
|---|---|---|---|---|
| SOX9 | 1.00 ± 0.48 | 5.38 ± 1.43 | 2.37 ± 0.34 | 13.15 ± 4.11 |
| COL2A1 | 1.00 ± 0.39 | 3.65 ± 1.18 | 2.13 ± 0.28 | 6.58 ± 0.51 |
| ACAN | 1.00 ± 0.48 | 14.34 ± 1.45 | 2.03 ± 0.35 | 63.96 ± 4.72 |
| ELN | 1.00 ± 0.62 | 8.09 ± 1.86 | 2.96 ± 0.45 | 10.05 ± 1.21 |
| COMP | 1.00 ± 0.31 | 10.33 ± 4.74 | 7.21 ± 4.10 | 8.47 ± 3.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mashyakhy, M.; Alkahtani, A.; Abumelha, A.S.; Sharroufna, R.J.; Alkahtany, M.F.; Jamal, M.; Robaian, A.; Binalrimal, S.; Chohan, H.; Patil, V.R.; et al. Taurine Augments Telomerase Activity and Promotes Chondrogenesis in Dental Pulp Stem Cells. J. Pers. Med. 2021, 11, 491. https://doi.org/10.3390/jpm11060491
Mashyakhy M, Alkahtani A, Abumelha AS, Sharroufna RJ, Alkahtany MF, Jamal M, Robaian A, Binalrimal S, Chohan H, Patil VR, et al. Taurine Augments Telomerase Activity and Promotes Chondrogenesis in Dental Pulp Stem Cells. Journal of Personalized Medicine. 2021; 11(6):491. https://doi.org/10.3390/jpm11060491
Chicago/Turabian StyleMashyakhy, Mohammed, Ahmed Alkahtani, Abdulaziz S. Abumelha, Reham Jamal Sharroufna, Mazen F. Alkahtany, Mohamed Jamal, Ali Robaian, Sultan Binalrimal, Hitesh Chohan, Vikrant R. Patil, and et al. 2021. "Taurine Augments Telomerase Activity and Promotes Chondrogenesis in Dental Pulp Stem Cells" Journal of Personalized Medicine 11, no. 6: 491. https://doi.org/10.3390/jpm11060491
APA StyleMashyakhy, M., Alkahtani, A., Abumelha, A. S., Sharroufna, R. J., Alkahtany, M. F., Jamal, M., Robaian, A., Binalrimal, S., Chohan, H., Patil, V. R., Raj, A. T., Bhandi, S., Reda, R., Testarelli, L., & Patil, S. (2021). Taurine Augments Telomerase Activity and Promotes Chondrogenesis in Dental Pulp Stem Cells. Journal of Personalized Medicine, 11(6), 491. https://doi.org/10.3390/jpm11060491