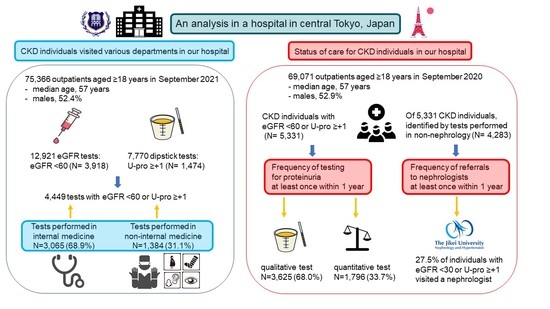
Chronic Kidney Disease Patients Visiting Various Hospital Departments: An Analysis in a Hospital in Central Tokyo, Japan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Clinical Departments Visited by CKD Patients
2.2. Status of Care for CKD Individuals
2.2.1. Frequency of Testing for Proteinuria or Albuminuria
2.2.2. Frequency of Referral to Nephrologists
3. Results
3.1. Clinical Departments Visited by CKD Patients
3.1.1. Frequency of Testing for Proteinuria or Albuminuria
3.1.2. Frequency of Referral to Nephrologists
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Luyckx, V.A.; Tonelli, M.; Stanifer, J.W. The global burden of kidney disease and the sustainable development goals. Bull. World Health Organ. 2018, 96, 414–422D. [Google Scholar] [CrossRef]
- GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef] [Green Version]
- Wakasugi, M.; Kazama, J.J.; Narita, I. Use of Japanese Society for Dialysis Therapy dialysis tables to compare the local and national incidence of dialysis. Ther. Apher. Dial. 2012, 16, 63–67. [Google Scholar] [CrossRef]
- Section 3. Measures against Lifestyle-Related Diseases through “Health Japan 21” and Promotion of “Shokuiku (Food and Nutrition Education)”. Available online: https://www.mhlw.go.jp/english/wp/wp-hw2/part2/p2c1s3.pdf (accessed on 7 November 2021).
- Chapter 3. Health Check-Ups in Japan. Available online: https://www.oecd-ilibrary.org/sites/9789264311602-7-en/index.html?itemId=/content/component/9789264311602-7-en (accessed on 15 November 2021).
- Fukui, A.; Yokoo, T.; Nangaku, M.; Kashihara, N. New measures against chronic kidney diseases in Japan since 2018. Clin. Exp. Nephrol. 2019, 23, 1263–1271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plantinga, L.C.; Tuot, D.S.; Powe, N.R. Awareness of chronic kidney disease among patients and providers. Adv. Chronic Kidney Dis. 2010, 17, 225–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, M.; Wanner, C.; Schich, M.; Kotseva, K.; Wood, D.; Hartmann, K.; Fette, G.; Rücker, V.; Oezkur, M.; Störk, S.; et al. Patient’s and physician’s awareness of kidney disease in coronary heart disease patients—A cross-sectional analysis of the German subset of the EUROASPIRE IV survey. BMC Nephrol. 2017, 18, 321. [Google Scholar] [CrossRef] [Green Version]
- Wei, C.J.; Shih, C.L.; Hsu, Y.J.; Chen, Y.C.; Yeh, J.Z.; Shih, J.H.; Chiu, C.H. Development and application of a chronic kidney disease-specific health literacy, knowledge and disease awareness assessment tool for patients with chronic kidney disease in Taiwan. BMJ Open 2021, 11, e052597. [Google Scholar] [CrossRef] [PubMed]
- Coresh, J.; Selvin, E.; Stevens, L.A.; Manzi, J.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Levey, A.S. Prevalence of chronic kidney disease in the United States. JAMA 2007, 298, 2038–2047. [Google Scholar] [CrossRef] [Green Version]
- Brück, K.; Stel, V.S.; Gambaro, G.; Hallan, S.; Völzke, H.; Ärnlöv, J.; Kastarinen, M.; Guessous, I.; Vinhas, J.; Stengel, B.; et al. European CKD Burden Consortium. CKD prevalence varies across the European general population. J. Am. Soc. Nephrol. 2016, 27, 2135–2147. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.; Hayen, A.; Chapman, J.R.; Webster, A.C.; Wang, J.J.; Mitchell, P.; Craig, J.C. Association of CKD and cancer risk in older people. J. Am. Soc. Nephrol. 2009, 20, 1341–1350. [Google Scholar] [CrossRef] [Green Version]
- Kidoguchi, S.; Sugano, N.; Tokudome, G.; Yokoo, T.; Yano, Y.; Hatake, K.; Nishiyama, A. New concept of onco-hypertension and future perspectives. Hypertension 2021, 77, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.D.; Targher, G. NAFLD as a driver of chronic kidney disease. J. Hepatol. 2020, 72, 785–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, A.S.; Forman, J.P.; Orav, E.J.; Bates, D.W.; Denker, B.M.; Sequist, T.D. Primary care management of chronic kidney disease. J. Gen. Intern. Med. 2011, 26, 386–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravera, M.; Noberasco, G.; Weiss, U.; Re, M.; Gallina, A.M.; Filippi, A.; Cannavò, R.; Ravera, G.; Cricelli, C.; Deferrari, G. CKD awareness and blood pressure control in the primary care hypertensive population. Am. J. Kidney Dis. 2011, 57, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Gasparini, A.; Evans, M.; Coresh, J.; Grams, M.E.; Norin, O.; Qureshi, A.R.; Runesson, B.; Barany, P.; Ärnlöv, J.; Jernberg, T.; et al. Prevalence and recognition of chronic kidney disease in Stockholm healthcare. Nephrol. Dial. Transplant. 2016, 31, 2086–2094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Shamsi, S.; Al Dhanhani, A.; Sheek-Hussein, M.M.; Bakoush, O. Provision of care for chronic kidney disease by non-nephrologists in a developing nation: A national survey. BMJ Open 2016, 6, e010832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolide, A.D.; Kumela, K.; Kerga, F.; Debalke, S.; Seboka, M.; Edilu, B.; Gashe, F.; Bobassa, E.M. Knowledge, attitude, and practices toward chronic kidney disease among care providers in Jimma town: Cross-sectional study. BMC Public Health 2020, 20, 1079. [Google Scholar] [CrossRef] [PubMed]
- Ueda, A.; Kurahashi, M.; Yokoi, M.; Nakao, Y.; Hamaguchi, Y. Construction and Verification of a System to Prevent Hepatitis B Virus Reactivation—Consideration during and after using chemotherapy, immunosuppressant drugs and steroids-. Jpn. J. Pharm. Health Care Sci. 2017, 43, 394–400. [Google Scholar] [CrossRef]
- Levin, A.; Stevens, P.E.; Bilous, R.W.; Coresh, J.; De Francisco, A.L.; De Jong, P.E.; Griffith, K.E.; Hemmelgarn, B.R.; Iseki, K.; Lamb, E.J.; et al. Kidney disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 2013, 3, 1–150. [Google Scholar]
- Japanese Society of Nephrology. Essential points from Evidence-based Clinical Practice Guidelines for Chronic Kidney Disease 2018. Clin. Exp. Nephrol. 2019, 23, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Iseki, K.; Ikemiya, Y.; Iseki, C.; Takishita, S. Proteinuria and the risk of developing end-stage renal disease. Kidney Int. 2003, 63, 1468–1474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chronic Kidney Disease Prognosis Consortium; Matsushita, K.; van der Velde, M.; Astor, B.C.; Woodward, M.; Levey, A.S.; de Jong, P.E.; Coresh, J.; Gansevoort, R.T. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 2010, 375, 2073–2081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukui, A.; Kaneko, H.; Okada, A.; Yano, Y.; Itoh, H.; Matsuoka, S.; Morita, K.; Kiriyama, H.; Kamon, T.; Fujiu, K.; et al. Semiquantitative assessed proteinuria and risk of heart failure: Analysis of a nationwide epidemiological database. Nephrol. Dial. Transplant. 2021, gfab248. [Google Scholar] [CrossRef] [PubMed]
- Couser, W.G.; Remuzzi, G.; Mendis, S.; Tonelli, M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2011, 80, 1258–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, C.; Roderick, P.; Harris, S.; Rogerson, M. Decline in kidney function before and after nephrology referral and the effect on survival in moderate to advanced chronic kidney disease. Nephrol. Dial. Transplant. 2006, 21, 2133–2143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.C.; Chang, J.M.; Chou, M.C.; Lin, M.Y.; Chen, J.H.; Sun, J.H.; Guh, J.Y.; Hwang, S.J.; Chen, H.C. Slowing renal function decline in chronic kidney disease patients after nephrology referral. Nephrology 2008, 13, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Lonnemann, G.; Duttlinger, J.; Hohmann, D.; Hickstein, L.; Reichel, H. Timely referral to outpatient nephrology care slows progression and reduces treatment costs of chronic kidney diseases. Kidney Int. Rep. 2016, 2, 142–151. [Google Scholar] [CrossRef] [Green Version]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A.; et al. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef]
- Van den Bulck, S.A.; Vankrunkelsven, P.; Goderis, G.; Van Pottelbergh, G.; Swerts, J.; Panis, K.; Hermens, R. Developing quality indicators for Chronic Kidney Disease in primary care, extractable from the Electronic Medical Record. A Rand-modified Delphi method. BMC Nephrol. 2020, 21, 161. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Snijder, R.; Nozaki, K. Diagnosis Patterns of CKD and Anemia in the Japanese Population. Kidney Int. Rep. 2020, 5, 694–705. [Google Scholar] [CrossRef]
- Nagai, K.; Asahi, K.; Iseki, K.; Yamagata, K. Estimating the prevalence of definitive chronic kidney disease in the Japanese general population. Clin. Exp. Nephrol. 2021, 25, 885–892. [Google Scholar] [CrossRef] [PubMed]
- ERA-EDTA Council; ERACODA Working Group. Chronic kidney disease is a key risk factor for severe COVID-19: A call to action by the ERA-EDTA. Nephrol. Dial. Transplant. 2021, 36, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M.; Shinkawa, K.; Yanagita, M.; Kawakami, K. Prevalence, recognition and management of chronic kidney disease in Japan: Population-based estimate using a healthcare database with routine health checkup data. Clin. Kidney J. 2021, 14, 2197–2202. [Google Scholar] [CrossRef]
- Yoshimura, R.; Yamamoto, R.; Shinzawa, M.; Kataoka, R.; Ahn, M.; Ikeguchi, N.; Wakida, N.; Toki, H.; Moriyama, T. Associations of kidney tests at medical facilities and health checkups with incidence of end-stage kidney disease: A retrospective cohort study. Sci. Rep. 2021, 11, 20717. [Google Scholar] [CrossRef] [PubMed]
- White, S.L.; Yu, R.; Craig, J.C.; Polkinghorne, K.R.; Atkins, R.C.; Chadban, S.J. Diagnostic accuracy of urine dipsticks for detection of albuminuria in the general community. Am. J. Kidney Dis. 2011, 58, 19–28. [Google Scholar] [CrossRef]
- Samal, L.; Linder, J.A. The primary care perspective on routine urine dipstick screening to identify patients with albuminuria. Clin. J. Am. Soc. Nephrol. 2013, 8, 131–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.I.; Baek, H.; Kim, B.R.; Jung, H.H. Comparison of urine dipstick and albumin: Creatinine ratio for chronic kidney disease screening: A population-based study. PLoS ONE 2017, 12, e0171106. [Google Scholar] [CrossRef]
- Usui, T.; Yoshida, Y.; Nishi, H.; Yanagimoto, S.; Matsuyama, Y.; Nangaku, M. Diagnostic accuracy of urine dipstick for proteinuria category in Japanese workers. Clin. Exp. Nephrol. 2020, 24, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Sumida, K.; Nadkarni, G.N.; Grams, M.E.; Sang, Y.; Ballew, S.H.; Coresh, J.; Matsushita, K.; Surapaneni, A.; Brunskill, N.; Chadban, S.J.; et al. Conversion of urine protein-creatinine ratio or urine dipstick protein to urine albumin-creatinine ratio for use in chronic kidney disease screening and prognosis: An individual participant-based meta-analysis. Ann. Intern. Med. 2020, 173, 426–435. [Google Scholar] [CrossRef]
- Sugiyama, T.; Imai, K.; Ihana-Sugiyama, N.; Tanaka, H.; Yanagisawa-Sugita, A.; Sasako, T.; Higashi, T.; Okamura, T.; Yamauchi, T.; Ueki, K.; et al. Variation in process quality measures of diabetes care by region and institution in Japan during 2015-2016: An observational study of nationwide claims data. Diabetes Res. Clin. Pract. 2019, 155, 107750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bello, A.K.; Ronksley, P.E.; Tangri, N.; Kurzawa, J.; Osman, M.A.; Singer, A.; Grill, A.K.; Nitsch, D.; Queenan, J.A.; Wick, J.; et al. Quality of chronic kidney disease management in Canadian primary care. JAMA Netw. Open 2019, 2, e1910704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Xiong, J.; Chen, Y.; Deng, J.; Peng, H.; Zhao, J.; He, J. The effectiveness of multidisciplinary care models for patients with chronic kidney disease: A systematic review and meta-analysis. Int. Urol. Nephrol. 2018, 50, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Delanaye, P.; Glassock, R.J.; De Broe, M.E. Epidemiology of chronic kidney disease: Think (at least) twice! Clin. Kidney J. 2017, 10, 370–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Characteristic | CKD Identification in September 2021 Used for Method 2.1 | CKD Identification in September 2020 Used for Method 2.2 |
|---|---|---|
| Total patients (≥18 years) | 75,366 | 69,071 |
| Median age (years) | 60 | 60 |
| Sex (% male) | 52.4% | 52.9% |
| Number of tests for eGFR Results with eGFR < 60 | 12,921 3918 (30.3%) | 15,945 5351 (33.6%) |
| Number of tests for U-pro Results with U-pro ≥ +1 | 7770 1474 (19.0%) | 7377 1368 (19.0%) |
| Number of CKD patients (eGFR < 60 or U-pro ≥ +1) | N/A | 5331 |
| Follow-up period | N/A | 1 year (Until 30 September 2021) |
| Clinical Department | Number of Tests for CKD by Category | ||||||
|---|---|---|---|---|---|---|---|
| Total Tests for CKD | G1–G2 eGFR ≥ 60 and U-pro ≥ +1 | G3–G5 eGFR <60 | G3a eGFR 45–59 | G3b eGFR 30–44 | G4 eGFR 15–29 | G5 eGFR < 15 | |
| Overall | 4449 | 531 | 3918 | 2401 | 913 | 350 | 254 |
| Internal Medicine | 3065 | 387 | 2678 | 1572 | 645 | 270 | 191 |
| Nephrology and Hypertension | 928 | 132 | 796 | 295 | 208 | 155 | 138 |
| Diabetes, Metabolism, and Endocrinology | 580 | 145 | 435 | 279 | 112 | 32 | 12 |
| Cardiology | 408 | 6 | 402 | 244 | 103 | 40 | 15 |
| Clinical Oncology and Hematology | 395 | 12 | 383 | 259 | 94 | 18 | 12 |
| Gastroenterology and Hepatology | 344 | 42 | 302 | 228 | 55 | 10 | 9 |
| Rheumatology | 202 | 35 | 167 | 122 | 33 | 10 | 2 |
| Respiratory Medicine | 100 | 6 | 94 | 68 | 20 | 3 | 3 |
| Neurology | 56 | 3 | 53 | 40 | 11 | 2 | 0 |
| General Medicine | 52 | 6 | 46 | 37 | 9 | 0 | 0 |
| Surgery | 396 | 24 | 372 | 254 | 75 | 19 | 24 |
| Urology | 348 | 39 | 309 | 188 | 87 | 27 | 7 |
| Obstetrics and Gynecology | 107 | 13 | 94 | 61 | 20 | 10 | 3 |
| Emergency Medicine | 90 | 17 | 73 | 31 | 19 | 9 | 14 |
| Otorhinolaryngology | 73 | 6 | 67 | 59 | 6 | 0 | 2 |
| Dermatology | 73 | 15 | 58 | 32 | 16 | 6 | 4 |
| Orthopedic Surgery | 71 | 4 | 67 | 49 | 13 | 1 | 4 |
| Cardiac Surgery | 46 | 1 | 45 | 26 | 14 | 5 | 0 |
| Neurosurgery | 45 | 12 | 33 | 26 | 6 | 0 | 1 |
| Infectious Diseases and Infection Control | 37 | 3 | 34 | 33 | 1 | 0 | 0 |
| Ophthalmology | 34 | 0 | 34 | 24 | 4 | 2 | 4 |
| Radiology | 22 | 3 | 19 | 16 | 3 | 0 | 0 |
| Psychiatry | 10 | 0 | 10 | 9 | 0 | 1 | 0 |
| Other departments | 32 | 7 | 25 | 21 | 4 | 0 | 0 |
| Number of CKD Patients | Number of CKD Patients Who Had Each Test and Its Frequency | |||
|---|---|---|---|---|
| CKD category | Dipstick test | PCR | ACR | PCR or ACR |
| eGFR < 60 or U-pro ≥ +1 (n = 5331) | 3625 (68.0%) | 1431 (26.8%) | 553 (10.4%) | 1796 (33.7%) |
| G1-G2: eGFR ≥ 60 and U-pro ≥ +1 (n = 451) | 451 (100%) | 170 (37.7%) | 87 (19.3%) | 231 (51.2%) |
| G3a: eGFR 45–59 (n = 3017) | 1884 (62.4%) | 490 (16.2%) | 289 (9.6%) | 703 (23.3%) |
| G3b: eGFR 30–44 (n = 1144) | 836 (73.1%) | 364 (31.8%) | 130 (11.4%) | 444 (38.8%) |
| G4: eGFR 15–29 (n = 417) | 364 (87.3%) | 250 (60.0%) | 39 (9.4%) | 260 (62.4%) |
| G5: eGFR < 15 (n = 302) | 90 (70.2%) | 157 (52.0%) | 8 (2.6%) | 158 (52.3%) |
| Number of CKD Patients Who Visited the Nephrology Department and Its Frequency | ||||
|---|---|---|---|---|
| CKD category | U-pro (−) or no test | U-pro (±) | U-pro ≥ +1 | Total |
| G1: eGFR ≥ 90 | N/A | 26/353 (7.4%) | 26/353 (7.4%) | |
| G2: eGFR 60–89 | N/A | |||
| G3a: eGFR 45–59 | N/A | 15/198 (7.6%) | 32/176 (18.2%) | 47/374 (12.6%) |
| G3b: eGFR 30–44 | 97/772 (12.6%) | 37/127 (29.1%) | 134/899 (14.9%) | |
| G4: eGFR 15–29 | 68/172 (39.5%) | 35/64 (54.7%) | 103/236 (43.6%) | |
| G5: eGFR < 15 | 60/97 (61.9%) | 20/22 (90.9%) | 80/119 (67.2%) | |
| Total | 240/1239 (19.4%) | 150/742 (20.2%) | 390/1981 (19.7%) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukui, A.; Takeshita, K.; Nakashima, A.; Maruyama, Y.; Yokoo, T. Chronic Kidney Disease Patients Visiting Various Hospital Departments: An Analysis in a Hospital in Central Tokyo, Japan. J. Pers. Med. 2022, 12, 39. https://doi.org/10.3390/jpm12010039
Fukui A, Takeshita K, Nakashima A, Maruyama Y, Yokoo T. Chronic Kidney Disease Patients Visiting Various Hospital Departments: An Analysis in a Hospital in Central Tokyo, Japan. Journal of Personalized Medicine. 2022; 12(1):39. https://doi.org/10.3390/jpm12010039
Chicago/Turabian StyleFukui, Akira, Kohei Takeshita, Akio Nakashima, Yukio Maruyama, and Takashi Yokoo. 2022. "Chronic Kidney Disease Patients Visiting Various Hospital Departments: An Analysis in a Hospital in Central Tokyo, Japan" Journal of Personalized Medicine 12, no. 1: 39. https://doi.org/10.3390/jpm12010039
APA StyleFukui, A., Takeshita, K., Nakashima, A., Maruyama, Y., & Yokoo, T. (2022). Chronic Kidney Disease Patients Visiting Various Hospital Departments: An Analysis in a Hospital in Central Tokyo, Japan. Journal of Personalized Medicine, 12(1), 39. https://doi.org/10.3390/jpm12010039