Effect of Different Combinations of Phosphorus and Nitrogen Fertilization on Arbuscular Mycorrhizal Fungi and Aphids in Wheat
Abstract
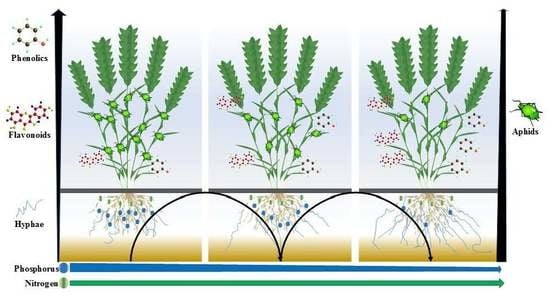
:1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Soil Preparation
2.3. Study Materials
2.4. Experimental Design
2.5. Arbuscular Mycorrhizal Fungi (AMF) Colonization Rate
2.6. Chemical Analysis
2.7. Data Analysis
3. Results
3.1. Effect of P and N Fertilizer Amounts on AMF
3.2. Effect of P and N Fertilizer Amounts on Wheat Aphids
3.3. Effect of P and N Fertilizer Amounts on Secondary Metabolites in Wheat Leaves
3.4. Effect of P and N Fertilizer Amounts on Plant Traits
3.5. Structural Equation Modeling (SEM)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Montenegro, J.D.; Golicz, A.A.; Bayer, P.E.; Hurgobin, B.; Lee, H.; Chan, C.K.; Visendi, P.; Lai, K.; Doležel, J.; Batley, J. The pangenome of hexaploid bread wheat. Plant J. 2017, 90, 1007–1013. [Google Scholar] [CrossRef] [Green Version]
- Tian, Z.; Li, Y.; Liang, Z.; Guo, H.; Cai, J.; Jiang, D.; Cao, W.; Li, X. Genetic improvement of nitrogen uptake and utilization of winter wheat in the Yangtze River Basin of China. Field Crop. Res. 2016, 196, 251–260. [Google Scholar] [CrossRef]
- Wang, Z.; Sadras, V.O.; Hoogmoed, M.; Yang, X.; Fang, H.; Han, X.; Zhang, S. Shifts in nitrogen and phosphorus uptake and allocation in response to selection for yield in Chinese winter wheat. Crop Pasture Sci. 2017, 68, 807–816. [Google Scholar] [CrossRef]
- El Khattabi, J.; Louche, B.; Darwishe, H.; Chaaban, F.; Carlier, E. Impact of Fertilizer Application and Agricultural Crops on the Quality of Groundwater in the Alluvial Aquifer, Northern France. Water Air Soil Pollut. 2018, 229, 128. [Google Scholar] [CrossRef]
- Chakraborty, S.; Tiwari, P.K.; Sasmal, S.K.; Misra, A.K.; Chattopadhyay, J. Effects of fertilizers used in agricultural fields on algal blooms. Eur. Phys. J. Spec. Top. 2017, 226, 2119–2133. [Google Scholar] [CrossRef]
- Mao, Q.; Lu, X.; Zhou, K.; Chen, H.; Zhu, X.; Mori, T.; Mo, J. Effects of long-term nitrogen and phosphorus additions on soil acidification in an N-rich tropical forest. Geoderma 2017, 285, 57–63. [Google Scholar] [CrossRef]
- Dunwell, J.M. Transgenic cereals: Current status and future prospects. J. Cereal Sci. 2014, 59, 419–434. [Google Scholar] [CrossRef]
- Schutz, K.; Bonkowski, M.; Scheu, S. Effects of Collembola and fertilizers on plant performance (Triticum aestivum) and aphid reproduction (Rhopalosiphum padi). Basic Appl. Ecol. 2008, 9, 182–188. [Google Scholar] [CrossRef]
- Shen, F.; Wu, J.; Fan, H.; Liu, W.; Guo, X.; Duan, H.; Hu, L.; Lei, X.; Wei, X. Soil N/P and C/P ratio regulate the responses of soil microbial community composition and enzyme activities in a long-term nitrogen loaded Chinese fir forest. Plant Soil 2018, 436, 91–107. [Google Scholar] [CrossRef]
- Zhu, C.; Tian, G.; Luo, G.; Kong, Y.; Guo, J.; Wang, M.; Guo, S.; Ling, N.; Shen, Q. N-fertilizer-driven association between the arbuscular mycorrhizal fungal community and diazotrophic community impacts wheat yield. Agric. Ecosyst. Environ. 2018, 254, 191–201. [Google Scholar] [CrossRef]
- Babikova, Z.; Gilbert, L.; Bruce, T.; Dewhirst, S.Y.; Pickett, J.A.; Johnson, D. Arbuscular mycorrhizal fungi and aphids interact by changing host plant quality and volatile emission. Funct. Ecol. 2014, 28, 375–385. [Google Scholar] [CrossRef] [Green Version]
- Catherine, G.; Alison, B. Mycorrhizal fungal-plant-insect interactions: The importance of a community approach. Env. Entomol. 2009, 38, 93–102. [Google Scholar]
- Chen, J.; Ni, H.; Sun, J. The resistance threshold and interactions of several plant secondary metabolites to wheat aphids. J. Plant Prot. 2002, 1, 7–12. [Google Scholar]
- Jiang, Y.; Xie, Q.; Wang, W.X.; Yang, J.; Zhang, X.; Yu, N.; Zhou, Y.; Wang, E. Medicago AP2-domain transcription factor WRI5a is a master regulator of lipid biosynthesis and transfer during mycorrhizal symbiosis. Mol Plant. 2018, 11, 1344–1359. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.E.; Smith, F.A.; Jakobsen, I. Functional diversity in arbuscular mycorrhizal (AM) symbioses: The contribution of the mycorrhizal P uptake pathway is not correlated with mycorrhizal responses in growth or total P uptake. New Phytol. 2004, 162, 511–524. [Google Scholar] [CrossRef]
- Elbon, A.; Whalen, J.K. Phosphorus supply to vegetable crops from arbuscular mycorrhizal fungi: A review. Biol. Agric. Hortic. 2015, 31, 73–90. [Google Scholar] [CrossRef]
- Marschner, H.; Dell, B. Nutrient uptake in mycorrhizal symbiosis. Plant Soil 1994, 159, 89–102. [Google Scholar] [CrossRef]
- Bennett, A.E.; Bever, J.D.; Deane Bowers, M. Arbuscular mycorrhizal fungal species suppress inducible plant responses and alter defensive strategies following herbivory. Oecologia 2009, 160, 771–779. [Google Scholar] [CrossRef]
- Goławska, S. Antifeedant activity of luteolin and genistein against the pea aphid, Acyrthosiphon pisum. J. Pest Sci. 2012, 85, 443–450. [Google Scholar] [CrossRef] [Green Version]
- Goławska, S.; Łukasik, I.; Leszczyński, B. Effect of Alfalfa saponins and flavonoids on pea aphid. Entomol. Exp. Appl. 2010, 128, 147–153. [Google Scholar] [CrossRef]
- Li, J.; Tian, B. Peppermint essential oil toxicity to the Pear psylla (Hemiptera: Psyllidae) and potential applications in the field. J. Econ. Entomol. 2020. [Google Scholar] [CrossRef]
- Aliferis, K.A.; Chamoun, R.; Jabaji, S. Metabolic responses of willow (Salix purpurea L.) leaves to mycorrhization as revealed by mass spectrometry and 1H NMR spectroscopy metabolite profiling. Front. Plant Sci. 2015, 6, 344. [Google Scholar] [CrossRef] [Green Version]
- Sunitha, V.; Rao, G.V.R.; Lakshmi, K.V.; Saxena, K.B.; Rao, V.R.; Reddy, Y.V.R. Morphological and biochemical factors associated with resistance to Maruca vitrata (Lepidoptera: Pyralidae) in short duration pigeonpea. Int. J. Trop. Insect Sci. 2018, 28, 45–52. [Google Scholar] [CrossRef]
- Woźniak, A.; Drzewiecka, K.; Kęsy, J.; Marczak, Ł.; Narożna, D.; Grobela, M.; Motała, R.; Bocianowski, J.; Morkunas, I. The influence of lead on generation of signaling molecules and accumulation of flavonoids in pea seedlings in response to pea aphid infestation. Molecules 2017, 22, 1404. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Elsevier Academic Press Inc.: San Diego, CA, USA, 2008; pp. 13–15. [Google Scholar]
- Zitlalpopoca-hernandez, G.; Najera-Rincon, M.B.; del-Val, E.; Alarcon, A.; Jackson, T.; Larsen, J. Multitrophic interactions between maize mycorrhizas, the root feeding insect Phyllophaga vetula and the entomopathogenic fungus Beauveria bassiana. Appl. Soil Ecol. 2017, 115, 38–43. [Google Scholar] [CrossRef]
- Choudhury, N.K.; Biswal, U.C. Changes in the content of chlorophyll, protein and nucleic acids and in the efficiency of photoelectron transport of chloroplasts during growth of maize seedlings. Plant Sci. Let. 1979, 16, 95–99. [Google Scholar] [CrossRef]
- Evans, J.; Condon, J.; Cornish, P.S. New fertiliser options for managing phosphorus for organic and low-input farming systems. Crop Pasture Sci. 2009, 60, 152–162. [Google Scholar] [CrossRef]
- Dzida, K.; Jarosz, Z.; Michałojc´, Z. Effect of nitrogen fertilization on the yield and nutritive value of Beta vulgaris L. J. Elem. 2012, 17, 19–29. [Google Scholar] [CrossRef] [Green Version]
- Kurdish, I.; Roy, A.; Skorochod, I.; Chobotarov, A.; Herasimenko, I.; Plotnikov, V.; Gylchuk, V.; Korniychuk, A. Free-flowing complex bacterial preparation for crop and efficiency of its use in agroecosystems. J. Microbiol. Biotechnol. Food Sci. 2015, 4, 527–531. [Google Scholar] [CrossRef]
- Dordas, C. Nitrogen nutrition index and leaf chlorophyll concentration and its relationship with nitrogen use efficiency in barley (Hordeum vulgare L.). J. Plant Nutr. 2017, 40, 1190–1203. [Google Scholar] [CrossRef]
- Winter, G.; Todd, C.D.; Trovato, M.; Forlani, G.; Funck, D. Physiological implications of arginine metabolism in plants. Front. Plant Sci. 2015, 6, 534. [Google Scholar] [CrossRef] [Green Version]
- Yoneyama, T.; Ito, O.; Engelaar, W.M.H.G. Uptake, metabolism and distribution of nitrogen in crop plants traced by enriched and natural 15N: Progress over the last 30 years. Phytochem. Rev. 2003, 2, 121–132. [Google Scholar] [CrossRef]
- Thapa, S.; Prasanna, R.; Ramakrishnan, B.; Sheoran, N.; Kumar, A.; Velmourougane, K.; Kumar, A. Interactive effects of Magnaporthe inoculation and nitrogen doses on the plant enzyme machinery and phyllosphere microbiome of resistant and susceptible rice cultivars. Arch. Microbiol. 2018, 200, 1287–1295. [Google Scholar] [CrossRef]
- Lu, N.; Wang, W.; Zhang, Q.; Li, D.; Yao, X.; Tian, Y.; Zhu, Y.; Cao, W.; Baret, F.; Liu, S.; et al. Estimation of nitrogen nutrition status in winter wheat from unmanned aerial vehicle based multi-angular multispectral imagery. Front. Plant Sci. 2019, 10, 1601. [Google Scholar] [CrossRef] [Green Version]
- Akar, T.; Cengiz, M.F.; Tekin, M. A comparative study of protein and free amino acid contents in some important ancient wheat lines. Qual. Assur. Saf. Crop 2019, 11, 191–200. [Google Scholar] [CrossRef]
- Kiba, T.; Kudo, T.; Kojima, M.; Sakakibara, H. Hormonal control of nitrogen acquisition: Roles of auxin, abscisic acid, and cytokinin. JExB 2011, 62, 1399–1409. [Google Scholar] [CrossRef]
- Pavlíková, D.; Neuberg, M.; Žižková, E.; Motyka, V.; Pavlík, M. Interactions between nitrogen nutrition and phytohormone levels in Festulolium plants. Plant Soil Environ. 2012, 58, 367–372. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Gamboa, G.; Garde-Cerdán, T.; Portu, J.; Moreno-Simunovic, Y.; Martínez-Gil, A.M. Foliar nitrogen application in Cabernet Sauvignon vines: Effects on wine flavonoid and amino acid content. Food Res. Int. 2017, 96, 46–53. [Google Scholar] [CrossRef]
- Shan, L.; Song, C.; Zhang, X.; Ren, J. Effects of long-term nitrogen and phosphorus addition on plant defence compounds in a freshwater wetland. Ecol. Indic. 2018, 94, 1–6. [Google Scholar] [CrossRef]
- Kang, Z.; Liu, F.; Tan, X.; Zhang, Z.; Zhu, J.; Tian, H.; Liu, T. Infection of powdery mildew reduces the fitness of grain aphids (Sitobion avenae) through restricted nutrition and induced defense response in wheat. Front. Plant Sci. 2018, 9, 778. [Google Scholar] [CrossRef] [Green Version]
- Kuhlmann, F.; Mueller, C. UV-B impact on aphid performance mediated by plant quality and plant changes induced by aphids. Plant Biol. 2010, 12, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.Y.; Sun, D.X.; Li, Y.G.; Wang, C.Y.; Xie, Y.X.; Guo, T.C. Effect of nitrogen fertilisation and irrigation on phenolic content, phenolic acid composition, and antioxidant activity of winter wheat grain. J. Sci. Food Agric. 2015, 95, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Engert, N.; John, A.; Henning, W.; Honermeier, B. Effect of sprouting on the concentration of phenolic acids and antioxidative capacity in wheat cultivars (Triticum aestivum ssp aestivum L.) in dependency of nitrogen fertilization. J. Appl. Bot. Food Qual. 2011, 84, 111–118. [Google Scholar]
- Saito, M.; Oba, H.; Kojima, T. Effect of nitrogen on the sporulation of arbuscular mycorrhizal fungi colonizing several gramineous plant species. Soil Sci. Plant Nutr. 2011, 57, 29–34. [Google Scholar] [CrossRef]
- Zhao, A.; Liu, L.; Xu, T.; Shi, L.; Xie, W.; Zhang, W.; Fu, S.; Feng, H.; Chen, B.D. Influences of canopy nitrogen and water addition on AM fungal biodiversity and community composition in a mixed deciduous forest of China. Front Plant Sci. 2018, 9, 1842. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.H.; Han, T.; Qian, L.; Li, L.K.; Chen, F.J. The effect of fertilizer-N on the inter-specific competition among three wheat aphids under elevated CO2. J. Appl. Entomol. 2019, 143, 1032–1042. [Google Scholar]
- Aqueel, M.A.; Leather, S.R. Effect of nitrogen fertilizer on the growth and survival of Rhopalosiphum padi (L.) and Sitobion avenae (F.) (Homoptera: Aphididae) on different wheat cultivars. Crop Prot. 2011, 30, 216–221. [Google Scholar] [CrossRef]
- Yadesa, W.; Tadesse, A.; Kibret, K.; Dechassa, N. Effect of liming and applied phosphorus on growth and P uptake of maize (Zea mays subsp.) plant grown in acid soils of West Wollega, Ethiopia. J. Plant Nutr. 2019, 42, 477–490. [Google Scholar] [CrossRef]
- Battini, F.; Gronlund, M.; Agnolucci, M.; Giovannetti, M.; Jakobsen, I. Facilitation of phosphorus uptake in maize plants by mycorrhizosphere bacteria. Sci. Rep. 2017, 7, 4686. [Google Scholar] [CrossRef]
- Rempelos, L.; Almuayrifi, A.M.; Baranski, M.; Tetard-Jones, C.; Eyre, M.; Shotton, P.; Cakrnak, I.; Ozturk, L.; Cooper, J.; Volakakis, N.; et al. Effects of agronomic management and climate on leaf phenolic profiles, disease severity, and grain yield in organic and conventional wheat production systems. J. Agric. Food Chem. 2018, 66, 10369–10379. [Google Scholar] [CrossRef]
- Wang, C.; Yin, G.; Xia, X.; He, Z.; Zhang, P.; Yao, Z.; Qin, J.; Li, Z.; Liu, D. Molecular mapping of a new temperature-sensitive gene LrZH22 for leaf rust resistance in Chinese wheat cultivar Zhoumai 22. Mol. Breed. 2016, 36, 18. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Y.; Feng, X.; Xia, B.; Zeng, J.; Liu, Y. Occurring trends of major crop pests in national significances in 2009. China Plant Prot. 2009, 29, 33–36. [Google Scholar]
- Yu, X.; Wang, G.; Huang, S.; Ma, Y.; Xia, L. Engineering plants for aphid resistance: Current status and future perspectives. Appl. Genet. 2014, 127, 2065–2083. [Google Scholar] [CrossRef]
- Giovannetti, M.; Sbrana, C.; Logi, C. Early processes involved in host recognition by arbuscular mycorrhizal fungi. New Phytol. 2010, 127, 703–709. [Google Scholar] [CrossRef]
- Kim, Y.-C.; Gao, C.; Zheng, Y.; He, X.-H.; Yang, W.; Chen, L.; Wan, S.-Q.; Guo, L.-D. Arbuscular mycorrhizal fungal community response to warming and nitrogen addition in a semiarid steppe ecosystem. Mycorrhiza 2015, 25, 267–276. [Google Scholar] [CrossRef]
- Gao, C.; Kim, Y.-C.; Zheng, Y.; Yang, W.; Chen, L.; Ji, N.-N.; Wan, S.-Q.; Guo, L.-D. Increased precipitation, rather than warming, exerts a strong influence on arbuscular mycorrhizal fungal community in a semiarid steppe ecosystem. Botany 2016, 94, 459–469. [Google Scholar] [CrossRef]
- Pei, Y.; Siemann, E.; Tian, B.; Ding, J. Root flavonoids are related to enhanced AMF colonization of an invasive tree. AoB Plants 2020, 12, plaa002. [Google Scholar] [CrossRef] [Green Version]
- Lester, G.E.; Lewers, K.S.; Medina, M.B.; Saftner, R.A. Comparative analysis of strawberry total phenolics via Fast Blue BB vs. Folin–Ciocalteu: Assay interference by ascorbic acid. J. Food Compos. Anal. 2012, 27, 102–107. [Google Scholar] [CrossRef] [Green Version]
- Liwani, U.; Magwaza, L.S.; Odindo, A.O.; Sithole, N.J. Growth, morphological and yield responses of irrigated wheat (Triticum aestivum L.) genotypes to water stress. Acta Agric. Scand. B Soil Plant Sci. 2019, 69, 369–376. [Google Scholar] [CrossRef]
- Sokolova, O.; Vorozhtsov, D. Development of rapid method for determining the total carbon in boron carbide samples with elemental analyzer. Russ. J. Appl. Chem. 2014, 87, 1640–1643. [Google Scholar] [CrossRef]
- Zhang, C.J.; Shen, J.P.; Sun, Y.F.; Wang, J.T.; Zhang, L.M.; Yang, Z.L.; Han, H.Y.; Wan, S.Q.; He, J.Z. Interactive effects of multiple climate change factors on ammonia oxidizers and denitrifiers in a temperate steppe. FEMS Microbiol. Ecol. 2017, 93, fix037s. [Google Scholar] [CrossRef]
- Schroeder, J.I.; Emmanuel, D.; Frommer, W.B.; Mary Lou, G.; Harrison, M.J.; Luis, H.E.; Tomoaki, H.; Kochian, L.V.; Rana, M.; Nishizawa, N.K. Using membrane transporters to improve crops for sustainable food production. Nature 2013, 497, 60–66. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.; Zhang, L.; He, X.; Chen, X.; Cui, Z. Long-term optimization of crop yield while concurrently improving soil quality. LDD 2019, 30, 897–909. [Google Scholar] [CrossRef]
- Zhang, L.; Du, Y.; Li, X.G. Modern wheat cultivars have greater root nitrogen uptake efficiency than old cultivars. J. Plant Nutr. Soil Sci. 2020, 183, 192–199. [Google Scholar] [CrossRef]
- Mansour, E.; Merwad, A.M.A.; Yasin, M.A.T.; Abdul-Hamid, M.I.E.; El-Sobky, E.E.A.; Oraby, H.F. Nitrogen use efficiency in spring wheat: Genotypic variation and grain yield response under sandy soil conditions. J. Agric. Sci. 2017, 155, 1407–1423. [Google Scholar] [CrossRef]
- Smith, S.; Read, D. Mineral nutrition, toxic element accumulation and water relations of arbuscular mycorrhizal plants. Mycor. Symbiosis 2008, 3, 145-VI. [Google Scholar]
- Koch, M.; Tanami, Z.; Bodani, H.; Wininger, S.; Kapulnik, Y. Field application of vesicular-arbuscular mycorrhizal fungi improved garlic yield in disinfected soil. Mycorrhiza 1997, 7, 47–50. [Google Scholar] [CrossRef]
- Daei, G.; Ardekani, M.R.; Rejali, F.; Teimuri, S.; Miransari, M. Alleviation of salinity stress on wheat yield, yield components, and nutrient uptake using arbuscular mycorrhizal fungi under field conditions. J. Plant Physiol. 2009, 166, 617–625. [Google Scholar] [CrossRef]
- Luo, W.; Li, J.; Ma, X.; Niu, H.; Hou, S.; Wu, F. Effect of arbuscular mycorrhizal fungi on uptake of selenate, selenite, and selenomethionine by roots of winter wheat. Plant Soil 2019, 438, 71–83. [Google Scholar] [CrossRef]
- Roe, M.A.; Faulks, R.M.; Belsten, J.L. Role of reducing sugars and amino acids in fry colour of chips from potatoes grown under different nitrogen regimes. J. Sci. Food Agric. 2010, 52, 207–214. [Google Scholar] [CrossRef]
- Anderson, T.; Boersma, M.; Raubenheimer, D. Stoichiometry: Linking elements to biochemicals. Ecology 2004, 85, 1193–1202. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, T.D.; Ferrari, J.; Hartley, S.E.; Hodge, A. Aphids can acquire the nitrogen delivered to plants by arbuscular mycorrhizal fungi. Funct. Ecol. 2019, 33, 576–586. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Yan, X. Effects of arbuscular mycorrhizal fungi on plant secondary metabolism. Acta Phytoecol. Sin. 2006, 30, 514–521. [Google Scholar]
- Chen, J.; Ni, H.; Sun, J. The resistance threshold and interactions of several plant secondary metabolites to wheat aphids. Acta Phytoecol. Sin. 2002, 29, 7–12. [Google Scholar]
- Niemeyer, H.M.; Copaja, S.V.; Barria, B.N. The Triticeae as sources of hydroxamic acids, secondary metabolites in wheat conferring resistance against aphids. Hereditas 2010, 116, 295–299. [Google Scholar] [CrossRef]
- Onkokesung, N.; Reichelt, M.; Van, D.A.; Schuurink, R.C.; van Loon, J.J.; Dicke, M. Modulation of flavonoid metabolites in Arabidopsis thaliana through overexpression of the MYB75 transcription factor: Role of kaempferol-3,7-dirhamnoside in resistance to the specialist insect herbivore Pieris brassicae. JExB 2014, 65, 2203. [Google Scholar] [CrossRef] [Green Version]
- Coronado, C.; Zuanazzi, J.A.S.; Sallaud, C.; Quirion, J.C.; Esnault, R.; Husson, H.P.; Kondorosi, A.; Ratet, P. Alfalfa root flavonoid production is nitrogen regulated. Plant Physiol. 1995, 108, 533–542. [Google Scholar] [CrossRef] [Green Version]
- Løvdal, T.; Olsen, K.M.; Slimestad, R.; Verheul, M.; Lillo, C. Synergetic effects of nitrogen depletion, temperature, and light on the content of phenolic compounds and gene expression in leaves of tomato. Phytochemistry 2010, 71, 605–613. [Google Scholar] [CrossRef]
- Chandler, S.F.; Dodds, J.H. The effect of phosphate, nitrogen and sucrose on the production of phenolics and solasodine in callus cultures of Solanum laciniatum. Plant Cell Rep. 1983, 2, 205. [Google Scholar] [CrossRef] [PubMed]
- Otálora, G.; Piñero, M.C.; López-Marín, J.; Varó, P.; Amor, F.M.D. Effects of foliar nitrogen fertilization on the phenolic, mineral, and amino acid composition of escarole (Cichorium endivia L. var. Latifolium). Sci. Hortic-Amst. 2018, 239, 87–92. [Google Scholar]
- Murali, N.S.; Teramura, A.H. Effects of ultraviolet-B irradiance on soybean. VI. Influence of phosphorus nutrition on growth and flavonoid content. Physiol. Plant 2010, 63, 413–416. [Google Scholar] [CrossRef]
- Yu, M.M.; Chen, Y.H.; Zhu, Z.B.; Liu, L.; Zhang, L.X.; Guo, Q.S. Effect of phosphorus supply on plant productivity, photosynthetic efficiency and bioactive-component production in Prunella vulgaris L. under hydroponic condition. J. Plant Nutr. 2016, 39, 1672–1680. [Google Scholar] [CrossRef]
- Zarina, Z.; Ghazali, C.M.R.; Sam, S.T. Characterization analysis for leaves of Leucaena leucocephala by using phytochemical screening assay. In Proceedings of the 3rd Electronic & Green Materials International Conference, Aonang Krabi, Thailand, 29–30 April 2017; American Institute of Physics: Melville, NY, USA, 2017. [Google Scholar]
- He, G.; Zhang, J.; Hu, X.; Wu, J. Effect of aluminum toxicity and phosphorus deficiency on the growth and photosynthesis of oil tea (Camellia oleifera Abel.) seedlings in acidic red soils. Acta Physiol. Plant 2011, 33, 1285–1292. [Google Scholar] [CrossRef]
- Carine, T.N.; Germaine-Alice, W.; Desire, T.V.; Judith, M.T.; Neacute, O.A.; Emmanuel, Y.; Godswill, N.N. Effect of phosphorus fertilization on arbuscular mycorrhizal fungi in the Bambara groundnut rhizosphere. Afr. J. Microbiol. Res. 2017, 11, 1399–1410. [Google Scholar]
- Valentine, A.; Osborne, B.; Mitchell, D. Interactions between phosphorus supply and total nutrient availability on mycorrhizal colonization, growth and photosynthesis of cucumber. Sci. Hortic-Amst. 2001, 88, 177–189. [Google Scholar] [CrossRef]
- Monokrousos, N.; Papatheodorou, E.M.; Orfanoudakis, M.; Jones, D.G.; Scullion, J.; Stamou, G.P. The effects of plant type, AMF inoculation and water regime on rhizosphere microbial communities. Eur. J. Soil. Sci. 2020, 71, 265–278. [Google Scholar] [CrossRef]
- Kempel, A.; Schmidt, A.K.; Brandl, R.; Schädler, M. Support from the underground: Induced plant resistance depends on arbuscular mycorrhizal fungi. Funct. Ecol. 2010, 24, 293–300. [Google Scholar] [CrossRef]
- Paloma, S.B.; Pilar, T.; Jordi, G.; Pozo, M.J.; Gemma, C.E.; Miguel, C.; Víctor, F. The nitrogen availability interferes with mycorrhiza-induced resistance against Botrytis cinerea in tomato. Front. Microbiol. 2016, 7, 1598. [Google Scholar]
- Talaat, N.B.; Shawky, B.T. Protective effects of arbuscular mycorrhizal fungi on wheat (Triticum aestivum L.) plants exposed to salinity. Env. Exp. Bot. 2014, 98, 20–31. [Google Scholar] [CrossRef]
- Huang, T.M.; Zheng, X.F.; Hou, Y.Y.; Xiao, L.I.; Wang, Z.H. Yield and N, P and K uptake and utilization of winter wheat affected by straw return to soil. J. Plant Nutr. Fertil. 2015, 21, 853–863. [Google Scholar]
- Sang, J.K.; Eo, J.K.; Lee, E.H.; Park, H.; Eom, A.H. Effects of arbuscular mycorrhizal fungi and soil conditions on crop plant growth. Mycobiology 2017, 45, 20. [Google Scholar]
- Symanczik, S.; Gisler, M.; Thonar, C.; Schlaeppi, K.; Van der Heijden, M.; Kahmen, A.; Boller, T.; Mäder, P. Application of mycorrhiza and soil from a permaculture system improved phosphorus acquisition in Naranjilla. Front. Plant Sci. 2017, 8, 1263. [Google Scholar] [CrossRef] [PubMed]





| Nutrient Source | Levels | Fertilizer Amount | |
|---|---|---|---|
| In the Garden (g/pot) | In the Field (g/plot) | ||
| Monobasic potassium Phosphate (P) | P0 | 0 | 0 |
| P1 | 0.8172 | 187.67 | |
| P2 | 1.6344 | 375.34 | |
| Urea (N) | N0 | 0 | 0 |
| N1 | 1.3083 | 299.84 | |
| N2 | 2.6166 | 599.68 | |
| Effects | df | In the Garden | In the Field | ||
|---|---|---|---|---|---|
| F | P | F | P | ||
| Phosphorus (P) | 2 | 3.21 | 0.0499 | 1.96 | 0.1492 |
| Nitrogen (N) | 2 | 3.29 | 0.3160 | 2.94 | 0.0605 |
| P*N | 4 | 1.13 | 0.3564 | 0.23 | 0.9187 |
| Effects | df | F | P |
|---|---|---|---|
| Phosphorus (P) | 2 | 2.65 | 0.0770 |
| Nitrogen (N) | 2 | 5.18 | 0.0076 |
| P *N | 4 | 1.88 | 0.1211 |
| Effects | df | Total Phenolics | Total Flavonoids | ||
|---|---|---|---|---|---|
| F | P | F | P | ||
| Phosphorus (P) | 2 | 6.58 | 0.0072 | 29.63 | <0.0001 |
| Nitrogen (N) | 2 | 3.78 | 0.0425 | 7.95 | 0.0034 |
| P*N | 4 | 0.38 | 0.8216 | 4.45 | 0.0113 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Tian, B.; Yu, Z.; Ding, J. Effect of Different Combinations of Phosphorus and Nitrogen Fertilization on Arbuscular Mycorrhizal Fungi and Aphids in Wheat. Insects 2020, 11, 365. https://doi.org/10.3390/insects11060365
Wang C, Tian B, Yu Z, Ding J. Effect of Different Combinations of Phosphorus and Nitrogen Fertilization on Arbuscular Mycorrhizal Fungi and Aphids in Wheat. Insects. 2020; 11(6):365. https://doi.org/10.3390/insects11060365
Chicago/Turabian StyleWang, Chao, Baoliang Tian, Zhenzhen Yu, and Jianqing Ding. 2020. "Effect of Different Combinations of Phosphorus and Nitrogen Fertilization on Arbuscular Mycorrhizal Fungi and Aphids in Wheat" Insects 11, no. 6: 365. https://doi.org/10.3390/insects11060365
APA StyleWang, C., Tian, B., Yu, Z., & Ding, J. (2020). Effect of Different Combinations of Phosphorus and Nitrogen Fertilization on Arbuscular Mycorrhizal Fungi and Aphids in Wheat. Insects, 11(6), 365. https://doi.org/10.3390/insects11060365