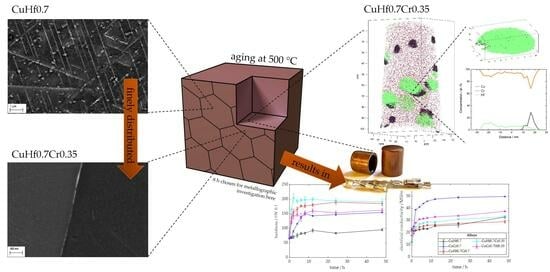
Analyzing the Precipitation Effects in Low-Alloyed Copper Alloys Containing Hafnium and Chromium
Abstract
:1. Introduction
1.1. Precipitation of Binary Alloys: CuCr
1.2. Ternary Alloys: CuCrZr
1.3. Precipitation of Binary Alloys: CuHf
1.4. Ternary Alloys: CuCrHf
2. Materials and Methods
3. Results
3.1. Binary Alloys
3.1.1. CuCr0.7
3.1.2. CuHf0.7
3.1.3. Comparison
3.2. Ternary Alloys
3.2.1. Hardness and Electrical Conductivity
3.2.2. Differential Scanning Calorimetry (DSC)
3.2.3. Atom Probe Tomography (APT)
4. Discussion
4.1. Discussion of Binary Concepts and the General Utilization of Prior Cold Rolling
4.2. DSC Investigations with Binary Alloys Containing Hafnium or Chromium
4.3. DSC Investigations with Ternary Alloys Containing Hafnium and Chromium
4.4. APT with CuHf0.7Cr0.35
4.5. Perspectives of Ternary Copper Alloys Containing Hafnium and Chromium
5. Conclusions
- Binary copper alloys with low hafnium concentration needed diffusion-facilitating influences to promote following precipitation reactions due to hafnium’s larger atom radius and therefore lowered diffusivity. Cold working prior to aging treatment introduced paths of higher diffusion, which became visible in peak shifts of conducted DSC measurements and reflected in resulting material properties such as hardness and electrical conductivity.
- Electrical conductivity reacted very sensitively regarding solute atoms in the copper matrix and increased significantly within place-taking precipitation processes of the supersaturated quenched metals. In direct comparison, CuCr0.7 had the highest electrical conductivity with maximum 49.7 MS/m and 53.6 MS/m after 24 h of aging at 400 °C (without and with 75% thickness reduction by cold rolling), whereas CuHf0.7 reached only 26.4 MS/m and 44.8 MS/m. Ternary alloys aged without cold rolling reached, in the case of CuHf0.7Cr0.7, 28.7 MS/m, and in the case of CuHf0.7Cr0.35, it was 31.0 MS/m.
- Regarding hardness, the ternary alloys significantly expanded the range of mechanical properties and appeared to be beneficial in comparison to binary alloys. Best hardness values without cold rolling prior to the aging process were obtained at 400 °C aging temperature with CuHf0.7Cr0.35 (204 HV 0.1) and CuHf0.7Cr0.7 (194 HV 0.1), whereas binary alloys such as CuCr0.7 reached only 154 HV 0.1. In the case of aged binary alloys cold rolling with 75% thickness reduction increased the reachable peak hardness at this aging temperature to 169 HV 0.1 (CuCr0.7) and 195 HV 0.1 (CuHf0.7). Ternary alloys showed beneficial hardening potential with accelerated reactions without the need of prior cold rolling.
- Precipitates containing mainly chromium were visible in the ternary CuHfCr alloys. During the conducted experimental investigations after 8 h of aging at 500 °C, these precipitates had a diameter of about 5 nm to 15 nm.
- Hafnium atoms segregated at the precipitate–matrix interface of Cr-containing particles. Increased hafnium concentrations next to these chromium phases lowered the necessary effort for intermetallic CuHf phases to precipitate, acting like a potent heterogeneous nucleation site. As a result, many intermetallic CuHf precipitates saddled on top of the existing chromium particles. The finely distributed precipitates resulted in excellent mechanical properties with less overaging and good electrical conductivities.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Davis, J.R. Metals Handbook: Non-Ferrous Alloys and Special-Purpose Materials, 10th ed.; ASM International: Materials Park, OH, USA, 1990; ISBN 0871703785. [Google Scholar]
- Dies, K. Kupfer und Kupferlegierungen in der Technik; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 978-3-642-48932-7. [Google Scholar]
- Gottstein, G. Materialwissenschaft und Werkstofftechnik: Physikalische Grundlagen, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 978-3-642-36602-4. [Google Scholar]
- Porter, D.A.; Easterling, K.E.; Sherif, M.Y. (Eds.) Phase Transformations in Metals and Alloys, 4th ed.; CRC Press: Boca Raton, FL, USA, 2021; ISBN 9781003011804. [Google Scholar]
- Kupferinstitut, D. Niedriglegierte Kupferwerkstoffe: Eigenschaften-Verarbeitung-Verwendung. TechnologieForum Kupfer 2012, 8, 1–36. [Google Scholar]
- Dölling, J.; Kracun, S.F.; Prahl, U.; Fehlbier, M.; Zilly, A. A Comparative Differential Scanning Calorimetry Study of Precipitation Hardenable Copper-Based Alloys with Optimized Strength and High Conductivity. Metals 2023, 13, 150. [Google Scholar] [CrossRef]
- Li, M.; Zhang, L.; Zhu, M.; Wang, H.; Wei, H. Physical Properties and Precipitate Microstructures of Cu-Hf Alloys at Different Processing Stages. Scanning 2018, 2018, 3653987. [Google Scholar] [CrossRef] [PubMed]
- Freudenberger, J.; Heilmaier, M. Materialkunde der Nichteisenmetalle und -Legierungen, 1st ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2020; ISBN 9783527822546. [Google Scholar]
- Chakrabarti, D.J.; Laughlin, D.E. The Cr-Cu (Chromium-Copper) system. Bull. Alloy Phase Diagr. 1984, 5, 59–68. [Google Scholar] [CrossRef]
- Li, H.; Xie, S.; Wu, P.; Mi, X. Study on improvement of conductivity of Cu-Cr-Zr alloys. Rare Met. 2007, 26, 124–130. [Google Scholar] [CrossRef]
- Peng, L.; Xie, H.; Huang, G.; Xu, G.; Yin, X.; Feng, X.; Mi, X.; Yang, Z. The phase transformation and strengthening of a Cu-0.71 wt% Cr alloy. J. Alloys Compd. 2017, 708, 1096–1102. [Google Scholar] [CrossRef]
- Chbihi, A.; Sauvage, X.; Blavette, D. Atomic scale investigation of Cr precipitation in copper. Acta Mater. 2012, 60, 4575–4585. [Google Scholar] [CrossRef]
- Liu, J.; Hou, M.; Yang, H.; Xie, H.; Yang, C.; Zhang, J.; Feng, Q.; Wang, L.; Meng, L.; Wang, H. In-situ TEM study of the dynamic interactions between dislocations and precipitates in a Cu-Cr-Zr alloy. J. Alloys Compd. 2018, 765, 560–568. [Google Scholar] [CrossRef]
- Wan, X.; Xie, W.; Chen, H.; Tian, F.; Wang, H.; Yang, B. First-principles study of phase transformations in Cu–Cr alloys. J. Alloys Compd. 2021, 862, 158531. [Google Scholar] [CrossRef]
- Bodyakova, A.; Mishnev, R.; Belyakov, A.; Kaibyshev, R. Effect of chromium content on precipitation in Cu–Cr–Zr alloys. J. Mater. Sci. 2022, 57, 13043–13059. [Google Scholar] [CrossRef]
- Holzwarth, U.; Stamm, H. The precipitation behaviour of ITER-grade Cu–Cr–Zr alloy after simulating the thermal cycle of hot isostatic pressing. J. Nucl. Mater. 2000, 279, 31–45. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, X.; Ge, Y.; Wang, J.; Cui, J.-Z. Effect of processing and heat treatment on behavior of Cu-Cr-Zr alloys to railway contact wire. Metall. Mater. Trans. A 2006, 37, 3233–3238. [Google Scholar] [CrossRef]
- Abib, K.; Larbi, F.H.; Rabahi, L.; Alili, B.; Bradai, D. DSC analysis of commercial Cu–Cr–Zr alloy processed by equal channel angular pressing. Trans. Nonferrous Met. Soc. China 2015, 25, 838–843. [Google Scholar] [CrossRef]
- Bourezg, Y.I.; Abib, K.; Azzeddine, H.; Bradai, D. Kinetics of Cr clustering in a Cu-Cr-Zr alloy processed by equal-channel angular pressing: A DSC study. Thermochim. Acta 2020, 686, 178550. [Google Scholar] [CrossRef]
- Caron, R.N.; Sharif, A. Copper Alloys: Properties and Applications. In Reference Module in Materials Science and Materials Engineering; Hashmi, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Zeiger, H. Stand und Entwicklung auf dem Gebiet der Kupferwerkstoffe. Mater. Werkst. 1986, 17, 75–78. [Google Scholar] [CrossRef]
- Zhang, S.; Li, R.; Kang, H.; Chen, Z.; Wang, W.; Zou, C.; Li, T.; Wang, T. A high strength and high electrical conductivity Cu-Cr-Zr alloy fabricated by cryorolling and intermediate aging treatment. Mater. Sci. Eng. A 2017, 680, 108–114. [Google Scholar] [CrossRef]
- Wang, H.; Gong, L.; Liao, J.; Chen, H.; Xie, W.; Yang, B. Retaining meta-stable fcc-Cr phase by restraining nucleation of equilibrium bcc-Cr phase in CuCrZrTi alloys during ageing. J. Alloys Compd. 2018, 749, 140–145. [Google Scholar] [CrossRef]
- Hatakeyama, M.; Toyama, T.; Yang, J.; Nagai, Y.; Hasegawa, M.; Ohkubo, T.; Eldrup, M.; Singh, B.N. 3D-AP and positron annihilation study of precipitation behavior in Cu–Cr–Zr alloy. J. Nucl. Mater. 2009, 386–388, 852–855. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, X.; Cai, P.; Li, P.; Cao, F.; Gao, F.; Liang, S. Precipitation behavior and microstructural evolution during thermo-mechanical processing of precipitation hardened Cu-Hf based alloys. Acta Mater. 2023, 245, 118659. [Google Scholar] [CrossRef]
- Okamoto, H. Cu-Hf (Copper-Hafnium). J. Phase Equilib. Diffus. 2007, 28, 583–584. [Google Scholar] [CrossRef]
- Watanabe, R. Study on Copper-Rich Copper-Hafnium Alloys. J. Jpn. Inst. Met. 1966, 30, 754–759. [Google Scholar] [CrossRef]
- Saarivirta, M.J. High conductivity copper alloys—Part 2. Met. Ind. 1963, 103, 716–718. [Google Scholar]
- Saarivirta, M.J. High conductivity copper alloys—Part 3. Met. Ind. 1963, 103, 758–760. [Google Scholar]
- Ghosh, G. First-principles calculations of structural energetics of Cu–TM (TM = Ti, Zr, Hf) intermetallics. Acta Mater. 2007, 55, 3347–3374. [Google Scholar] [CrossRef]
- Bever, M.B.; Holt, D.L.; Titchener, A.L. The stored energy of cold work. Prog. Mater. Sci. 1973, 17, 5–177. [Google Scholar] [CrossRef]
- Kodetová, V.; Vlach, M.; Čížek, J.; Cieslar, M.; Bajtošová, L.; Kudrnová, H.; Leibner, M.; Šíma, V. Early Stages of Precipitation in Mould-Cast, Cold-Rolled and Heat-Treated Aluminium Alloy AA7075 with Sc,Zr-Addition. Acta Phys. Pol. A 2020, 137, 250–254. [Google Scholar] [CrossRef]
- Quainoo, G.K.; Yannacopoulos, S. The effect of cold work on the precipitation kinetics of AA6111 aluminum. J. Mater. Sci. 2004, 39, 6495–6502. [Google Scholar] [CrossRef]
- Long, D.C.; Ohmori, Y.; Nakai, K. Effects of Cold Rolling on the Aging Kinetics in an Al–Mg–Si Based Commercial Alloy. Mater. Trans. JIM 2000, 41, 690–695. [Google Scholar] [CrossRef]
- Naimi, A.; Yousfi, H.; Trari, M. Influence of cold rolling degree and ageing treatments on the precipitation hardening of 2024 and 7075 alloys. Mech. Time-Depend. Mater. 2013, 17, 285–296. [Google Scholar] [CrossRef]
- Panigrahi, S.K.; Jayaganthan, R.; Pancholi, V.; Gupta, M. A DSC study on the precipitation kinetics of cryorolled Al 6063 alloy. Mater. Chem. Phys. 2010, 122, 188–193. [Google Scholar] [CrossRef]
- Saarivirta, M.J. High conductivity copper alloys—Part 1. Met. Ind. 1963, 103, 685–688. [Google Scholar]
- Yang, Y.; Kuang, G.; Li, R. Optimizing the Electrical and Mechanical Properties of Cu-Cr Alloys by Hf Microalloying. Metals 2022, 12, 485. [Google Scholar] [CrossRef]
- Dobatkin, S.V.; Shangina, D.V.; Bochvar, N.R.; Terent’ev, V.F.; Prosvirnin, D.V.; Putinceva, M.N.; Purcek, G.; Yanar, H.; Alsaran, A.; Raab, G.I. Enhanced mechanical and service properties of ultrafinegrained copper-based alloys with Cu, Zr and Hf additives. Mater. Sci. Non-Equilib. Phase Transform. 2017, 3, 3–5. [Google Scholar]
- Shangina, D.V.; Bochvar, N.R.; Dobatkin, S.V. The effect of alloying with hafnium on the thermal stability of chromium bronze after severe plastic deformation. J. Mater. Sci. 2012, 47, 7764–7769. [Google Scholar] [CrossRef]
- Shangina, D.; Maksimenkova, Y.; Bochvar, N.; Serebryany, V.; Raab, G.; Vinogradov, A.; Skrotzki, W.; Dobatkin, S. Influence of alloying with hafnium on the microstructure, texture, and properties of Cu–Cr alloy after equal channel angular pressing. J. Mater. Sci. 2016, 51, 5493–5501. [Google Scholar] [CrossRef]
- Shangina, D.; Maksimenkova, Y.; Bochvar, N.; Serebryany, V.; Raab, G.; Vinogradov, A.; Skrotzki, W.; Dobatkin, S. Structure and Properties of Cu Alloys Alloying with Cr and Hf after Equal Channel Angular Pressing. Adv. Mater. Res. 2014, 922, 651–656. [Google Scholar] [CrossRef]
- Shangina, D.V.; Terent’ev, V.F.; Prosvirnin, D.V.; Antonova, O.V.; Bochvar, N.R.; Gorshenkov, M.V.; Raab, G.I.; Dobatkin, S.V. Mechanical Properties, Fatigue Life, and Electrical Conductivity of Cu-Cr-Hf Alloy after Equal Channel Angular Pressing. Adv. Eng. Mater. 2018, 20, 1700536. [Google Scholar] [CrossRef]
- Bochvar, N.R.; Rybalchenko, O.V.; Shangina, D.V.; Dobatkin, S.V. Effect of equal-channel angular pressing on the precipitation kinetics in Cu-Cr-Hf alloys. Mater. Sci. Eng. A 2019, 757, 84–87. [Google Scholar] [CrossRef]
- Falahutdinov, R.M.; Popov, V.V.; Popova, E.N.; Stolbovsky, A.V.; Shorokhov, E.V.; Gaan, K.V. The Effect of the Initial State on the Structure Evolution of Hafnium Bronze under Annealing. Phys. Metals Metallogr. 2022, 123, 900–907. [Google Scholar] [CrossRef]
- Rybalchenko, O.V.; Bochvar, N.R.; Rybalchenko, G.V.; Martynenko, N.S.; Tabachkova, N.Y.; Dobatkin, S.V. Comparative analysis of the aging kinetics in low-alloyed Cu–Cr–Hf and Cu–Cr–Zr alloys after high pressure torsion. J. Alloys Compd. 2023, 955, 170246. [Google Scholar] [CrossRef]
- Dölling, J.; Henle, R.; Prahl, U.; Zilly, A.; Nandi, G. Copper-Based Alloys with Optimized Hardness and High Conductivity: Research on Precipitation Hardening of Low-Alloyed Binary CuSc Alloys. Metals 2022, 12, 902. [Google Scholar] [CrossRef]
- Dölling, J.; Zilly, A. Niedriglegierte festigkeitsoptimierte Kupferbasislegierungen mit hohen Leitfähigkeitseigenschaften: Untersuchung des Potentials binärer CuSc-Legierungen. In Proceedings of the Kupfer-Symposium 2021 Tagungsbeiträge, Werkstofftagung. Kupfer-Symposium, Jena, Germany, 24–25 November 2021; Deutsches Kupferinstitut, E.V., Ed.; 2021; pp. 16–19. [Google Scholar]
- Henle, R.; Dölling, J.; Prahl, U.; Nandi, G.; Zilly, A. DSC Analysis of the Effect of Cold Deformation on the Precipitation Kinetics of a Binary Cu-Sc Alloy. Materials 2023, 16, 3462. [Google Scholar] [CrossRef] [PubMed]
- Birol, Y. DSC analysis of the precipitation reaction in AA6005 alloy. J. Therm. Anal. Calorim. 2008, 93, 977–981. [Google Scholar] [CrossRef]
- Li, X.; Starink, M.J. Analysis of Precipitation and Dissolution in Overaged 7xxx Aluminium Alloys Using DSC. Mater. Sci. Forum 2000, 2000, 1071–1076. [Google Scholar] [CrossRef]
- Vončina, M.; Medved, J.; Kores, S.; Xie, P.; Schumacher, P.; Li, J. Precipitation microstructure in Al-Si-Mg-Mn alloy with Zr additions. Mater. Charact. 2019, 155, 109820. [Google Scholar] [CrossRef]
- Ceguerra, A.V.; Breen, A.J.; Stephenson, L.T.; Felfer, P.J.; Araullo-Peters, V.J.; Liddicoat, P.V.; Cui, X.; Yao, L.; Haley, D.; Moody, M.P.; et al. The rise of computational techniques in atom probe microscopy. Curr. Opin. Solid State Mater. Sci. 2013, 17, 224–235. [Google Scholar] [CrossRef]
- Yoo, S.-H.; Kim, S.-H.; Woods, E.; Gault, B.; Todorova, M.; Neugebauer, J. Origins of the hydrogen signal in atom probe tomography: Case studies of alkali and noble metals. New J. Phys. 2022, 24, 13008. [Google Scholar] [CrossRef]
- Felfer, P. Atom-Probe-Toolbox; Github; FAU Erlangen: Erlangen-Nürnberg, Germany, 2023. [Google Scholar]
- Felfer, P.; Ceguerra, A.V.; Ringer, S.P.; Cairney, J.M. Detecting and extracting clusters in atom probe data: A simple, automated method using Voronoi cells. Ultramicroscopy 2015, 150, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Miyake, J.; Fine, M.E. Electrical conductivity versus strength in a precipitation hardened alloy. Acta Metall. Mater. 1992, 40, 733–741. [Google Scholar] [CrossRef]
- Naboka, M.; Giordano, J. (Eds.) High Strengh Copper-Based Conductor Materials; Nova Science Publishers: Hauppauge, NY, USA, 2011; ISBN 9781612095042. [Google Scholar]
- Dalan, F.C.; de Lima Andreani, G.F.; Travessa, D.N.; Faizova, S.; Fiazov, I.A.; Cardoso, K.R. Effect of ECAP Processing on Hardness, Electrical Conductivity, and Precipitation Kinetics of the Cu-0.81Cr-0.07Zr Alloy. J. Electron. Mater. 2021, 50, 6171–6182. [Google Scholar] [CrossRef]
- Füssel, U.; Jüttner, S. Lebensdauererhöhung von Widerstandspunktschweißelektroden durch Einsatz verschleißabhängiger Fräsintervalle und dispersionsgehärteter Kupferwerkstoffe: Schlussbericht zu IGF-Vorhaben Nr. 18.456: Berichtszeitraum: 01.07.2015–30.09.2017; Industrielle Gemeinschaftsforschung: Magdeburg, Germany, 2018. [Google Scholar]
- Fukumoto, S.; Lum, I.; Biro, E.; Boomer, D.R.; Zhou, Y. Effects of Electrode Degradation on Electrode Life in Resistance Spot Welding of Aluminum Alloy 5182. Weld. J. 2003, 82, 307s–312s. [Google Scholar]
- Shangina, D.V.; Ivanov, N.I.; Bochvar, N.R.; Dobatkin, S.V. Resistance of the Contact Welding Electrodes Made of a Cu–0.7% Cr–0.9% Hf Alloy with an Ultrafine-Grained Structure. Russ. Metal. 2018, 2018, 815–819. [Google Scholar] [CrossRef]
- AMI Doduco. Datenbuch der Elektrischen Kontakte, 3rd ed.; AMI Doduco GmbH: Pforzheim, Germany, 2009. [Google Scholar]
- Mroczkowski, R.S.; Jugy, R.; Gerfer, A. (Eds.) Triologie der Steckverbinder: Applikationshandbuch zur Optimierten Steckverbinderauswahl, 3rd ed.; Swiridoff: Künzelsau, Germany, 2016; ISBN 978-3-89929-200-8. [Google Scholar]
- Katzier, H. Elektrische Steckverbinder: Technologien, Anwendungen und Anforderungen; mit 60 Tabellen, 1. Auflage; Leuze: Bad Saulgau, Germany, 2012; ISBN 9783874802734. [Google Scholar]
- KME Germany GmbH & Co., K.G. KME Kupferpulver: Materialdatenblatt; KME Germany GmbH & Co. KG: Osnabrück, Germany, 2022. [Google Scholar]
- Poltz, I.; Jürgens, P.; Blüm, M.; Weber, S. Additive Fertigung von CuSn11 Werkstoffen mit selektivem Laserschmelzen (SLM). Metall 2016, 70, 438–442. [Google Scholar]
- Fraunhofer Gesellschaft e.V. Fraunhofer-Institut für Lasertechnik ILT. Schlussbericht zu IGF-Vorhaben Nr. 19.549 N: Additive Fertigung von Bauteilen aus Rein-Kupfer mittels SLM und “grüner” Laserstrahlung; Industrielle Gemeinschaftsforschung: Aachen, Germany, 2020. [Google Scholar]
- Tiberto, D.; Klotz, U.E.; Held, F.; Wolf, G. Additive manufacturing of copper alloys: Influence of process parameters and alloying elements. Mater. Sci. Technol. 2019, 35, 969–977. [Google Scholar] [CrossRef]
- Popovich, A.; Sufiiarov, V.; Polozov, I.; Borisov, E.; Masaylo, D.; Orlov, A. Microstructure and mechanical properties of additive manufactured copper alloy. Mater. Lett. 2016, 179, 38–41. [Google Scholar] [CrossRef]
- Silbernagel, C.; Gargalis, L.; Ashcroft, I.; Hague, R.; Galea, M.; Dickens, P. Electrical resistivity of pure copper processed by medium-powered laser powder bed fusion additive manufacturing for use in electromagnetic applications. Addit. Manuf. 2019, 29, 100831. [Google Scholar] [CrossRef]
- Ellis, D.L. GRCop-84: A High-Temperature Copper Alloy for High-Heat-Flux Applications: 213566; Glenn Research Center: Cleveland, OH, USA, 2005. [Google Scholar]
- Tang, X.; Chen, X.; Sun, F.; Liu, P.; Zhou, H.; Fu, S. The current state of CuCrZr and CuCrNb alloys manufactured by additive manufacturing: A review. Mater. Des. 2022, 224, 111419. [Google Scholar] [CrossRef]
- Uchida, S.; Kimura, T.; Nakamoto, T.; Ozaki, T.; Miki, T.; Takemura, M.; Oka, Y.; Tsubota, R. Microstructures and electrical and mechanical properties of Cu-Cr alloys fabricated by selective laser melting. Mater. Des. 2019, 175, 107815. [Google Scholar] [CrossRef]














Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dölling, J.; Kuglstatter, M.; Prahl, U.; Höppel, H.W.; Ortner, P.; Ott, B.; Kracun, S.F.; Fehlbier, M.; Zilly, A. Analyzing the Precipitation Effects in Low-Alloyed Copper Alloys Containing Hafnium and Chromium. Metals 2024, 14, 258. https://doi.org/10.3390/met14030258
Dölling J, Kuglstatter M, Prahl U, Höppel HW, Ortner P, Ott B, Kracun SF, Fehlbier M, Zilly A. Analyzing the Precipitation Effects in Low-Alloyed Copper Alloys Containing Hafnium and Chromium. Metals. 2024; 14(3):258. https://doi.org/10.3390/met14030258
Chicago/Turabian StyleDölling, Julia, Moritz Kuglstatter, Ulrich Prahl, Heinz Werner Höppel, Patrick Ortner, Benedict Ott, Stefanie Felicia Kracun, Martin Fehlbier, and Andreas Zilly. 2024. "Analyzing the Precipitation Effects in Low-Alloyed Copper Alloys Containing Hafnium and Chromium" Metals 14, no. 3: 258. https://doi.org/10.3390/met14030258
APA StyleDölling, J., Kuglstatter, M., Prahl, U., Höppel, H. W., Ortner, P., Ott, B., Kracun, S. F., Fehlbier, M., & Zilly, A. (2024). Analyzing the Precipitation Effects in Low-Alloyed Copper Alloys Containing Hafnium and Chromium. Metals, 14(3), 258. https://doi.org/10.3390/met14030258