Improved Plasticity of Ti-Based Bulk Metallic Glass at Room Temperature by Electroless Thin Nickel Coating
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of BMG Rods
2.2. Preparation of Electroless Ni Coating
2.3. Compression Test
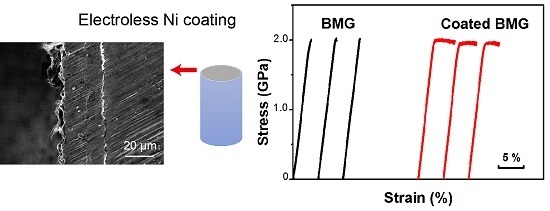
3. Results
3.1. Preparation of Electroless Ni Coating
3.2. Effect of Ni-Coating on the Mechanical Behavior of Ti-Based BMG
4. Discussion
4.1. Role of Thin Ni-Coating on the Shear Banding and Fracture Behavior of Ti-Based BMG
4.2. Peeling Mechnsium of the Ni-Coating
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kim, Y.C.; Kim, W.T.; Kim, D.H. A development of Ti-based bulk metallic glass. Mater. Sci. Eng. A 2004, 375–377, 127–135. [Google Scholar] [CrossRef]
- Duan, G.; Wiest, A.; Lind, M.L.; Kahl, A.; Johnson, W.L. Lightweight Ti-based bulk metallic glasses excluding late transition metals. Scr. Mater. 2008, 58, 465–468. [Google Scholar] [CrossRef]
- Park, J.M.; Wang, G.; Pauly, S.; Mattern, N.; Kim, D.H.; Eckert, J. Ductile Ti-Based Bulk Metallic Glasses with High Specific Strength. Metall. Mater. Trans. A 2011, 42, 1456–1462. [Google Scholar] [CrossRef]
- Gong, P.; Yao, K.F.; Wang, X.; Shao, Y. Centimeter-sized Ti-based bulk metallic glass with high specific strength. Prog. Nat. Sci. Mater. 2012, 22, 401–406. [Google Scholar] [CrossRef]
- Gong, P.; Yao, K.; Wang, X.; Shao, Y. A New Centimeter-Sized Ti-Based Quaternary Bulk Metallic Glass with Good Mechanical Properties. Adv. Eng. Mater. 2013, 15, 691–696. [Google Scholar] [CrossRef]
- Morrison, M.L.; Buchanan, R.A.; Peker, A.; Liaw, P.K.; Horton, J.A. Electrochemical behavior of a Ti-based bulk metallic glass. J. Non-Cryst. Solids 2007, 353, 2115–2124. [Google Scholar] [CrossRef]
- Gu, J.-L.; Shao, Y.; Zhao, S.-F.; Lu, S.-Y.; Yang, G.-N.; Chen, S.-Q.; Yao, K.-F. Effects of Cu addition on the glass forming ability and corrosion resistance of Ti-Zr-Be-Ni alloys. J. Alloys Compd. 2017, 725, 573–579. [Google Scholar] [CrossRef]
- Zhu, S.L.; Wang, X.M.; Qin, F.X.; Yoshimura, M.; Inoue, A. New TiZrCuPd Quaternary Bulk Glassy Alloys with Potential of Biomedical Applications. Mater. Trans. 2007, 48, 2445–2448. [Google Scholar] [CrossRef]
- Wang, G.; Fan, H.B.; Huang, Y.J.; Shen, J.; Chen, Z.H. A new TiCuHfSi bulk metallic glass with potential for biomedical applications. Mater. Des. 2014, 54, 251–255. [Google Scholar] [CrossRef]
- Pang, S.; Liu, Y.; Li, H.; Sun, L.; Li, Y.; Zhang, T. New Ti-based Ti–Cu–Zr–Fe–Sn–Si–Ag bulk metallic glass for biomedical applications. J. Alloys Compd. 2015, 625, 323–327. [Google Scholar] [CrossRef]
- Tang, M.Q.; Zhang, H.F.; Zhu, Z.W.; Fu, H.M.; Wang, A.M.; Li, H.; Hu, Z.Q. TiZr-base Bulk Metallic Glass with over 50 mm in Diameter. J. Mater. Sci. Technol. 2010, 26, 481–486. [Google Scholar] [CrossRef]
- Gong, P.; Wang, X.; Shao, Y.; Chen, N.; Liu, X.; Yao, K. A Ti–Zr–Be–Fe–Cu bulk metallic glass with superior glass-forming ability and high specific strength. Intermetallics 2013, 43, 177–181. [Google Scholar] [CrossRef]
- Telford, M. The case for bulk metallic glasses. Mater. Today 2004, 7, 36–43. [Google Scholar] [CrossRef]
- Nishiyama, N.; Amiya, K.; Inoue, A. Novel applications of bulk metallic glass for industrial products. J. Non-Cryst. Solids 2007, 353, 3615–3621. [Google Scholar] [CrossRef]
- Gong, P.; Wang, X.; Shao, Y.; Chen, N.; Yao, K.F. Ti-Zr-Be-Fe quaternary bulk metallic glasses designed by Fe alloying. Sci. China Phys. Mech. 2013, 56, 2090–2097. [Google Scholar] [CrossRef]
- Gong, P.; Wang, X.; Yao, K.F. Effects of alloying elements on crystallization kinetics of Ti-Zr-Be bulk metallic glass. J. Mater. Sci. 2016, 51, 5321–5329. [Google Scholar] [CrossRef]
- Wang, X.; Shao, Y.; Gong, P.; Yao, K.F. The effect of simulated thermal cycling on thermal and mechanical stability of a Ti-based bulk metallic glass. J. Alloys Compd. 2013, 575, 449–454. [Google Scholar] [CrossRef]
- Nieh, T.; Yang, Y.; Lu, J.; Liu, C. Effect of surface modifications on shear banding and plasticity in metallic glasses: An overview. Prog. Nat. Sci. Mater. 2012, 22, 355–363. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, W.H.; Greer, A.L. Making metallic glasses plastic by control of residual stress. Nat. Mater. 2006, 5, 857–860. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, Y.; Jiang, H.; Liu, C.; Ruan, H.; Lu, J. Superior Tensile Ductility in Bulk Metallic Glass with Gradient Amorphous Structure. Sci. Rep. 2014, 4, 4757. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.P.; Greene, J.E.; Jang, J.S.C.; Huang, J.C.; Shen, Y.-L.; Liaw, P.K.; Yokoyama, Y.; Inoue, A.; Nieh, T.G. Bendable bulk metallic glass: Effects of a thin, adhesive, strong, and ductile coating. Acta Mater. 2012, 60, 3226–3238. [Google Scholar] [CrossRef]
- Chen, B.; Li, Y.; Yi, M.; Li, R.; Pang, S.; Wang, H.; Zhang, T. Optimization of mechanical properties of bulk metallic glasses by residual stress adjustment using laser surface melting. Scr. Mater. 2012, 66, 1057–1060. [Google Scholar] [CrossRef]
- Li, H.; Li, L.; Fan, C.; Choo, H.; Liaw, P.K. Nanocrystalline coating enhanced ductility in a Zr-based bulk metallic glass. J. Mater. Res. 2007, 22, 508–513. [Google Scholar] [CrossRef]
- Li, J.-F.; Wang, X.; Yang, G.-N.; Chen, N.; Liu, X.; Yao, K.-F. Enhanced plasticity of a Fe-based bulk amorphous alloy by thin Ni coating. Mater. Sci. Eng. A 2015, 645, 318–322. [Google Scholar] [CrossRef]
- Greer, A.L.; Cheng, Y.Q.; Ma, E. Shear bands in metallic glasses. Mater. Sci. Eng. R 2013, 74, 71–132. [Google Scholar] [CrossRef]
- Steif, P.S.; Spaepen, F.; Hutchinson, J.W. Strain localization in amorphous metals. Acta. Metall. 1982, 30, 447–455. [Google Scholar] [CrossRef]
- Yu, P.; Liu, Y.H.; Wang, G.; Bai, H.Y.; Wang, W.H. Enhance plasticity of bulk metallic glasses by geometric confinement. J. Mater. Res. 2007, 22, 2384–2388. [Google Scholar] [CrossRef]
- Qiu, S.B.; Yao, K.F. Novel application of the electrodeposition on bulk metallic glasses. Appl. Surf. Sci. 2008, 255, 3454–3458. [Google Scholar] [CrossRef]
- Chen, W.; Chan, K.C.; Chen, S.H.; Guo, S.F.; Li, W.H.; Wang, G. Plasticity enhancement of a Zr-based bulk metallic glass by an electroplated Cu/Ni bilayered coating. Mater. Sci. Eng. A 2012, 552, 199–203. [Google Scholar] [CrossRef]
- Ren, L.W.; Meng, M.M.; Wang, Z.; Yang, F.Q.; Yang, H.J.; Zhang, T.; Qiao, J.W. Enhancement of plasticity in Zr-based bulk metallic glasses electroplated with copper coatings. Intermetallics 2015, 57, 121–126. [Google Scholar] [CrossRef]
- Balaraju, J.N.; Kalavati; Rajam, K.S. Influence of particle size on the microstructure, hardness and corrosion resistance of electroless Ni–P–Al2O3 composite coatings. Surf. Coat. Technol. 2006, 200, 3933–3941. [Google Scholar] [CrossRef]
- Krishnan, K.H.; John, S.; Srinivasan, K.N.; Praveen, J.; Ganesan, M.; Kavimani, P.M. An overall aspect of electroless Ni-P depositions—A review article. Metall. Mater. Trans. A 2006, 37, 1917–1926. [Google Scholar] [CrossRef]
- Ren, L.W.; Wang, Z.; Meng, M.M.; Tian, H.; Yang, H.J.; Qiao, J.W. Plasticity enhancement in bulk metallic glasses by electroless plating with Ni-P amorphous films. J. Non-Cryst. Solids 2015, 430, 115–119. [Google Scholar] [CrossRef]
- Cao, J.W.; Han, J.G.; Guo, Z.H.; Zhao, W.B.; Guo, Y.Q.; Xia, Z.H.; Qiao, J.W. Plasticity enhancement of high-entropy bulk metallic glasses by electroless plating with Ni-P amorphous films. Mater. Sci. Eng. A 2016, 673, 141–147. [Google Scholar] [CrossRef]
- Wang, X.; Gong, P.; Yao, K.F. Mechanical behavior of bulk metallic glass prepared by copper mold casting with reversed pressure. J. Mater. Proc. Technol. 2016, 237, 270–276. [Google Scholar] [CrossRef]
- Lu, G.J.; Zangari, G. Study of the electroless deposition process of Ni-P-based ternary alloys. J. Electrochem. Soc. 2003, 150, 777–786. [Google Scholar] [CrossRef]
- Torre, F.H.D.; Klaumünzer, D.; Maaß, R.; Löffler, J.F. Stick–slip behavior of serrated flow during inhomogeneous deformation of bulk metallic glasses. Acta Mater. 2010, 58, 3742–3750. [Google Scholar] [CrossRef]
- Sun, B.A.; Pauly, S.; Tan, J.; Stoica, M.; Wang, W.H.; Kühn, U.; Eckert, J. Serrated flow and stick–slip deformation dynamics in the presence of shear band interaction in a Zr-based bulk metallic glass. Acta Mater. 2013, 60, 4160–4171, Corrigendum in 2013, 61, 2281. [Google Scholar] [CrossRef]
- Yao, K.F.; Ruan, F.; Yang, Y.Q.; Chen, N. Superductile bulk metallic glass. Appl. Phys. Lett. 2006, 88, 122106. [Google Scholar] [CrossRef]
- Wang, G.; Feng, Q.; Yang, B.; Jiang, W.; Liaw, P.K.; Liu, C.T. Thermographic studies of temperature evolutions in bulk metallic glasses: An overview. Intermetallics 2012, 30, 1–11. [Google Scholar] [CrossRef]
- Lewandowski, J.J.; Greer, A.L. Temperature rise at shear bands in metallic glasses. Nat. Mater. 2006, 5, 15–18. [Google Scholar] [CrossRef]








| Status | Number | σ0.2 (MPa) | σy (MPa) | εp (%) | E (GPa) |
|---|---|---|---|---|---|
| As-cast | 1 | 2014 | 2014 | 0.46 | 68 |
| 2 | 2028 | 2030 | 0.52 | 60 | |
| 3 | 2023 | 2023 | 0 | 62 | |
| average | 2021 ± 7 | 2022 ± 8 | 0.33 ± 0.28 | 63.3 ± 4.2 | |
| Coated | 4 | 1995 | 1995 | 4.31 | 66 |
| 5 | 1959 | 1981 | 3.71 | 63 | |
| 6 | 1953 | 1978 | 3.08 | 62 | |
| average | 1969 ± 22 | 1984 ± 9 | 3.70 ± 0.62 | 63.2 ± 2.1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Hu, X.; Zhao, L.; Jiang, D.; Chen, P.; Wang, P.; Zhang, Z.; Liu, S.; Cui, C. Improved Plasticity of Ti-Based Bulk Metallic Glass at Room Temperature by Electroless Thin Nickel Coating. Metals 2017, 7, 562. https://doi.org/10.3390/met7120562
Wang X, Hu X, Zhao L, Jiang D, Chen P, Wang P, Zhang Z, Liu S, Cui C. Improved Plasticity of Ti-Based Bulk Metallic Glass at Room Temperature by Electroless Thin Nickel Coating. Metals. 2017; 7(12):562. https://doi.org/10.3390/met7120562
Chicago/Turabian StyleWang, Xin, Ximei Hu, Lichen Zhao, Dongxu Jiang, Peng Chen, Pengdong Wang, Zhipeng Zhang, Shuiqing Liu, and Chunxiang Cui. 2017. "Improved Plasticity of Ti-Based Bulk Metallic Glass at Room Temperature by Electroless Thin Nickel Coating" Metals 7, no. 12: 562. https://doi.org/10.3390/met7120562
APA StyleWang, X., Hu, X., Zhao, L., Jiang, D., Chen, P., Wang, P., Zhang, Z., Liu, S., & Cui, C. (2017). Improved Plasticity of Ti-Based Bulk Metallic Glass at Room Temperature by Electroless Thin Nickel Coating. Metals, 7(12), 562. https://doi.org/10.3390/met7120562