Investigation on Microstructural Evolution and Properties of an Al-Cu-Li Alloy with Mg and Zn Microalloying during Homogenization
Abstract
:1. Introduction
2. Materials and Methods
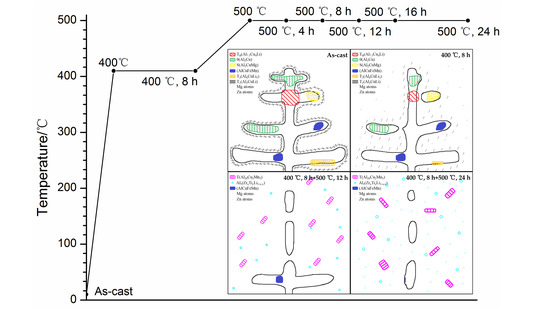
3. Results
3.1. Optical Microstructure
3.2. Segregation Evolution
3.3. Constituent Particles
3.4. Dispersoids
4. Discussion
4.1. Microstructural Evolution Analysis
4.2. Properties Evolution Analysis
5. Conclusions
- (1)
- Low-melting eutectic phases such as S, T2, TB and coarse T1 phases were nucleated in and around the dendrites of the as-cast alloy, in which the early enrichment of Mg-Zn atoms clusters was revealed.
- (2)
- The Li-containing phases dissolves preferentially during homogenization followed by the diffusion of Mg, Zn, Zr, and Mn, leading to the dissolution of S and AlCuFeMn phases and the precipitation of T phases as well as Al3Zr particles.
- (3)
- Mg-Zn atom clusters was easily bound to the vacancies together thus promoted the nucleation Al3(ZrxTiyLi1−x−y) dispersoid particles, which resulted in the non-recrystallization characteristic of the alloy.
- (4)
- After homogenization, the yield ratio was decreased from 0.81 to 0.52, displaying better plastic deformation ability, in which the formation of Al3(ZrxTiyLi1−x−y) dispersoids resulted in the significant improvement on thermal stability of the alloy.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abd El-Aty, A.; Xu, Y.; Guo, X.; Zhang, S.; Ma, Y.; Chen, D. Strengthening mechanisms, deformation behavior, and anisotropic mechanical properties of Al-Li alloys: A review. J. Mater. Res. 2018, 10, 49–67. [Google Scholar] [CrossRef]
- Mogucheva, A.; Kaibyshev, R. Microstructure and Mechanical Properties of an Al-Li-Mg-Sc-Zr Alloy Subjected to ECAP. Metals 2016, 6, 254. [Google Scholar] [CrossRef]
- Peng, Z.; Li, J.; Sang, F.; Chen, Y.; Zhang, X.; Zheng, Z.; Pan, Q. Structures and tensile properties of Sc-containing 1445 Al-Li alloy sheet. J. Alloy. Compd. 2018, 747, 471–483. [Google Scholar] [CrossRef]
- Xiang, S.; Liu, D.; Zhu, R.; Li, J.; Chen, Y.; Zhang, X. Hot deformation behavior and microstructure evolution of 1460 Al–Li alloy. Trans. Nonferrous Met. Soc. China 2015, 25, 3855–3864. [Google Scholar] [CrossRef]
- Ma, J.; Yan, D.; Rong, L.; Li, Y. Effect of Sc addition on microstructure and mechanical properties of 1460 alloy. Prog. Nat. Sci. Mater. 2014, 24, 13–18. [Google Scholar] [CrossRef]
- Nayan, N.; Murty, S.V.S.N.; Jha, A.K.; Pant, B.; Sharma, S.C.; George, K.M.; Sastry, G.V.S. Processing and characterization of Al–Cu–Li alloy AA2195 undergoing scale up production through the vacuum induction melting technique. Mater. Sci. Eng. A 2013, 576, 21–28. [Google Scholar] [CrossRef]
- Tsivoulas, D.; Prangnell, P.B. The effect of Mn and Zr dispersoid-forming additions on recrystallization resistance in Al–Cu–Li AA2198 sheet. Acta Mater. 2014, 77, 1–16. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, T.; Yang, W.; Xiao, R.; Liu, Z.; Li, L. Microstructure and mechanical properties of laser beam-welded AA2060 Al-Li alloy. J. Mater. Process. Tech. 2016, 237, 301–308. [Google Scholar] [CrossRef]
- Goebel, J.; Ghidini, T.; Graham, A.J. Stress-corrosion cracking characterisation of the advanced aerospace Al–Li 2099-T86 alloy. Mater. Sci. Eng. A 2016, 673, 16–23. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, X.; Hashimoto, T.; Liu, B.; Luo, C.; Sun, Z.; Tang, Z.; Lu, F.; Ma, Y. Corrosion behaviour of 2A97-T6 Al-Cu-Li alloy: The influence of non-uniform precipitation. Corros. Sci. 2018, 132, 1–8. [Google Scholar] [CrossRef]
- Li, H.; Tang, Y.; Zeng, Z.; Zheng, Z.; Zheng, F. Effect of ageing time on strength and microstructures of an Al–Cu–Li–Zn–Mg–Mn–Zr alloy. Mater. Sci. Eng. A 2008, 498, 314–320. [Google Scholar] [CrossRef]
- Sha, G.; Cerezo, A. Early-stage precipitation in Al–Zn–Mg–Cu alloy (7050). Acta Mater. 2004, 52, 4503–4516. [Google Scholar] [CrossRef]
- Xu, D.; Li, Z.; Wang, G.; Li, X.; Lv, X.; Zhang, Y.; Fan, Y.; Xiong, B. Phase transformation and microstructure evolution of an ultra-high strength Al-Zn-Mg-Cu alloy during homogenization. Mater. Charact. 2017, 131, 285–297. [Google Scholar] [CrossRef]
- Zhang, S.; Zeng, W.; Yang, W.; Shi, C.; Wang, H. Ageing response of a Al–Cu–Li 2198 alloy. Mater. Des. 2014, 63, 368–374. [Google Scholar] [CrossRef]
- Gao, C.; Ma, Y.; Tang, L.; Wang, P.; Zhang, X. Microstructural evolution and mechanical behavior of friction spot welded 2198-T8 Al-Li alloy during aging treatment. Mater. Des. 2017, 115, 224–230. [Google Scholar] [CrossRef]
- Yu, X.; Yin, D.; Yu, Z.; Zhang, Y.; Li, S. Microstructure Evolution of Novel Al-Cu-Li-Ce Alloys during Homogenization. Rare. Met. Mater. Eng. 2016, 45, 1687–1694. [Google Scholar]
- Jia, M.; Zheng, Z.; Gong, Z. Microstructure evolution of the 1469 Al–Cu–Li–Sc alloy during homogenization. J. Alloy. Compd. 2014, 614, 131–139. [Google Scholar] [CrossRef]
- Gumbmann, E.; Geuser, F.D.; Sigli, C.; Deschamps, A. Influence of Mg, Ag and Zn minor solute additions on the precipitation kinetics and strengthening of an Al-Cu-Li alloy. Acta Mater. 2017, 133, 172–185. [Google Scholar] [CrossRef]
- Sidhar, H.; Mishra, R.S. Aging kinetics of friction stir welded Al-Cu-Li-Mg-Ag and Al-Cu-Li-Mg alloys. Mater. Des. 2016, 110, 60–71. [Google Scholar] [CrossRef]
- Liu, Q.; Zhu, R.; Li, J.; Chen, Y.; Zhang, X.; Zhang, L.; Zheng, Z. Microstructural evolution of Mg, Ag and Zn micro-alloyed Al–Cu–Li alloy during homogenization. Trans. Nonferrous Met. Soc. China 2016, 26, 607–619. [Google Scholar] [CrossRef]
- Riestra, M.; Ghassemali, E.; Bogdanoff, T.; Seifeddine, S. Interactive effects of grain refinement, eutectic modification and solidification rate on tensile properties of Al-10Si alloy. Mater. Sci. Eng. A 2017, 703, 270–279. [Google Scholar] [CrossRef]
- Chen, Y.; Pan, S.; Liu, W.; Liu, X.; Tang, C. Morphologies, orientation relationships, and evolution of the T-phase in an Al-Cu-Mg-Mn alloy during homogenization. J. Alloy. Compd. 2017, 709, 213–226. [Google Scholar] [CrossRef]
- Yoshimura, R.; Konno, T.; Abe, E.; Hiraga, K. Transmission electron microscopy study of the evolution of precipitates in aged Al–Li–Cu alloys: The θ′ and T1 phases. Acta Mater. 2003, 51, 4251–4266. [Google Scholar] [CrossRef]
- Wang, S.; Starink, M. Two types of S phase precipitates in Al–Cu–Mg alloys. Acta Mater. 2007, 55, 933–941. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Li, C.; Peng, Z.; Chen, W.; Zheng, Z. Corrosion mechanism associated with T1 and T2 precipitates of Al–Cu–Li alloys in NaCl solution. J. Alloy. Compd. 2008, 460, 688–693. [Google Scholar] [CrossRef]
- Huang, B.; Zheng, Z. Independent and combined roles of trace Mg and Ag additions in properties precipitation process and precipitation kinetics of Al–Cu–Li–(Mg)–(Ag)–Zr–Ti alloys. Acta Mater. 1998, 46, 4381–4393. [Google Scholar] [CrossRef]
- Tsivoulas, D.; Robson, J.D. Heterogeneous Zr solute segregation and Al3Zr dispersoid distributions in Al–Cu–Li alloys. Acta Mater. 2015, 93, 73–86. [Google Scholar] [CrossRef]
- Yang, S.; Shen, J.; Yan, X.; Li, X.; Zhang, F.; Sun, B. Homogenization Treatment Parameter Optimization and Microstructural Evolution of Al-Cu-Li Alloy. Rare. Met. Mater. Eng. 2017, 46, 28–34. [Google Scholar]
- Medjahed, A.; Henniche, A.; Derradji, M.; Yu, T.; Wang, Y.; Wu, R.; Hou, L.; Zhang, J.; Li, X.; Zhang, M. Effects of Cu/Mg ratio on the microstructure, mechanical and corrosion properties of Al-Li-Cu-Mg-X alloys. Mater. Sci. Eng. A 2018, 718, 241–249. [Google Scholar] [CrossRef]
- Ivanov, R.; Deschamps, A.; Geuser, F.D. High throughput evaluation of the effect of Mg concentration on natural ageing of Al-Cu-Li-(Mg) alloys. Scr. Mater. 2018, 150, 156–159. [Google Scholar] [CrossRef] [Green Version]
- Gumbmann, E.; Geuser, F.D.; Deschamps, A.; Lefebvre, W.; Robaut, F.; Sigli, C. A combinatorial approach for studying the effect of Mg concentration on precipitation in an Al–Cu–Li alloy. Scr. Mater. 2016, 110, 44–47. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Yin, D.; Yu, Z. Effects of Cerium and Zirconium Microalloying Addition on the Microstructures and Tensile Properties of Novel Al-Cu-Li Alloys. Rare. Met. Mater. Eng. 2016, 45, 1917–1923. [Google Scholar]
- Jia, Z.; Hu, G.; Forbord, B.; Solberg, J.K. Enhancement of recrystallization resistance of Al–Zr–Mn by two-step precipitation annealing. Mater. Sci. Eng. A 2008, 483–484, 195–198. [Google Scholar] [CrossRef]
- Guo, Z.; Zhao, G.; Chen, X. Effects of two-step homogenization on precipitation behavior of Al3Zr dispersoids and recrystallization resistance in 7150 aluminum alloy. Mater. Charact. 2015, 102, 122–130. [Google Scholar] [CrossRef]
- Decreus, B.; Deschamps, A.; Geuser, F.D.; Donnadieu, P.; Sigli, C.; Weyland, M. The influence of Cu/Li ratio on precipitation in Al–Cu–Li–x alloys. Acta Mater. 2013, 61, 2207–2218. [Google Scholar] [CrossRef]
- Bacca, M.; Hayhurst, D.R.; Mcmeeking, R.M. Continuous dynamic recrystallization during severe plastic deformation. Mech. Mater. 2015, 90, 148–156. [Google Scholar] [CrossRef] [Green Version]














| Cu | Li | Mg | Zn | Mn | Zr | Ti | Fe | Si | Al |
|---|---|---|---|---|---|---|---|---|---|
| 3.5 | 1.5 | 0.5 | 0.4 | 0.3 | 0.12 | 0.06 | ≤0.10 | ≤0.08 | Bal |
| Area | Cu | Mg | Zn | Mn | Zr | Fe |
|---|---|---|---|---|---|---|
| A | 45.6 | 0.5 | 2.2 | - | - | - |
| B | 27.5 | 0.4 | 1.2 | - | ~0.5 | - |
| C | 20.9 | - | 0.7 | 7.5 | - | 4.6 |
| Area | Al | Cu | Mg | Zn | Mn | Fe | Zr | Closet Phase |
|---|---|---|---|---|---|---|---|---|
| A | 67.6 | 29.3 | 1.5 | 1.2 | 0.3 | 0.2 | - | Al2Cu |
| B | 70.1 | 15.1 | 13.6 | 0.9 | 0.2 | 0.2 | - | Al2CuMg |
| C | 61.7 | 34.6 | 1.4 | 1.3 | 0.4 | 0.6 | - | Al7.5Cu4Li |
| D | 80.1 | 13.4 | 5.1 | 0.5 | 0.1 | 0.2 | - | Al6CuLi3 |
| E | 79.7 | 11.3 | 1.6 | 0.4 | 2.4 | 4.6 | - | AlCuFeMn |
| F | 61.4 | 30.7 | 3.3 | 3.2 | 0.5 | 0.7 | - | Al2CuLi |
| G | 64.8 | 31.8 | 1.4 | 1.0 | 0.1 | 0.2 | ~0.5 | Al2Cu |
| H | 64.0 | 32.0 | 0.9 | 1.2 | 0.3 | 0.4 | ~0.5 | Al2Cu |
| I | 65.9 | 30.3 | 1.9 | 1.0 | 0.1 | 0.2 | - | Al2Cu |
| J | 75.2 | 11.9 | 0.3 | 0.2 | 5.5 | 6.7 | - | AlCuFeMn |
| K | 67.1 | 29.6 | 1.3 | 0.8 | 0.1 | 0.3 | ~0.5 | Al2Cu |
| Homogenization Time (h) | UTS (MPa) | YS (MPa) | Yield Ratio (YS/UTS) | Conductivity (MS/m) |
|---|---|---|---|---|
| 0 | 212.5 | 172.1 | 0.81 | 6.52 |
| 8 | 143.5 | 101.9 | 0.71 | 7.31 |
| 12 | 137.4 | 93.4 | 0.68 | 7.78 |
| 16 | 133.5 | 89.4 | 0.67 | 8.05 |
| 20 | 129.6 | 81.6 | 0.63 | 8.27 |
| 24 | 124.8 | 76.1 | 0.61 | 8.48 |
| 28 | 119.6 | 67.0 | 0.56 | 8.56 |
| 32 | 116.5 | 60.6 | 0.52 | 8.66 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Yu, W.; Wang, X.; Du, R.; You, W. Investigation on Microstructural Evolution and Properties of an Al-Cu-Li Alloy with Mg and Zn Microalloying during Homogenization. Metals 2018, 8, 1010. https://doi.org/10.3390/met8121010
Li H, Yu W, Wang X, Du R, You W. Investigation on Microstructural Evolution and Properties of an Al-Cu-Li Alloy with Mg and Zn Microalloying during Homogenization. Metals. 2018; 8(12):1010. https://doi.org/10.3390/met8121010
Chicago/Turabian StyleLi, Hongying, Weichen Yu, Xiaoyu Wang, Rong Du, and Wen You. 2018. "Investigation on Microstructural Evolution and Properties of an Al-Cu-Li Alloy with Mg and Zn Microalloying during Homogenization" Metals 8, no. 12: 1010. https://doi.org/10.3390/met8121010
APA StyleLi, H., Yu, W., Wang, X., Du, R., & You, W. (2018). Investigation on Microstructural Evolution and Properties of an Al-Cu-Li Alloy with Mg and Zn Microalloying during Homogenization. Metals, 8(12), 1010. https://doi.org/10.3390/met8121010