Characterization of Porous Phosphate Coatings Enriched with Calcium, Magnesium, Zinc and Copper Created on CP Titanium Grade 2 by Plasma Electrolytic Oxidation
Abstract
:1. Introduction
2. Materials and Methods
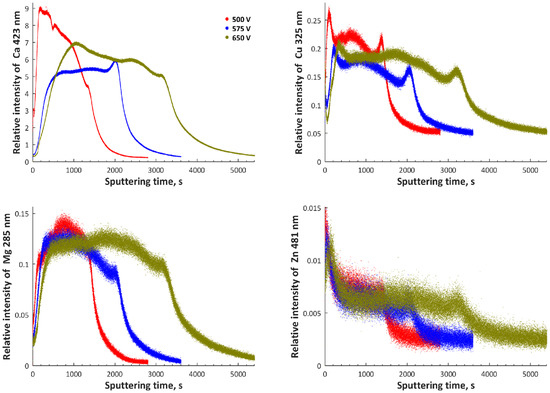
3. Results and Discussion
4. Summary
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Jiang, B.; Lei, T.; Guo, L. Dependence of growth features of microarc oxidation coatings of titanium alloy on control modes of alternate pulse. Mater. Lett. 2004, 58, 1907–1911. [Google Scholar] [CrossRef]
- Sowa, M.; Lastowka, D.; Kukharenko, A.I.; Korotin, D.M.; Kurmaev, E.Z.; Cholakh, S.O.; Simka, W. Characterisation of anodic oxide films on zirconium formed in sulphuric acid: XPS and corrosion resistance investigations. J. Solid State Electrochem. 2017, 21, 203–210. [Google Scholar] [CrossRef]
- Sowa, M.; Kazek-Kęsik, A.; Socha, R.P.; Dercz, G.; Michalska, J.; Simka, W. Modification of tantalum surface via plasma electrolytic oxidation in silicate solutions. Electrochim. Acta 2013, 114, 627–636. [Google Scholar] [CrossRef]
- Pereira, B.L.; Lepienski, C.M.; Mazzaro, I.; Kuromoto, N.K. Apatite grown in niobium by two-step plasma electrolytic oxidation. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, K. The effect of Micro-arc Oxidation treatment on the microstructure and properties of open cell Ti6Al4V alloy foams. Surf. Coat. Technol. 2015, 272, 72–78. [Google Scholar] [CrossRef]
- Simka, W.; Nawrat, G.; Chlode, J.; Maciej, A.; Winiarski, A.; Szade, J.; Radwanski, K.; Gazdowicz, J. Electropolishing and anodic passivation of Ti6Al7Nb alloy. Przem. Chem. 2011, 90, 84–90. [Google Scholar]
- Rokosz, K.; Hryniewicz, T.; Raaen, S.; Chapon, P. Investigation of porous coatings obtained on Ti-Nb-Zr-Sn alloy biomaterial by Plasma Electrolytic Oxidation: Characterisation and Modelling. Int. J. Adv. Manuf. Technol. 2016, 87, 3497–3512. [Google Scholar] [CrossRef]
- Siu, H.T.; Man, H.C. Fabrication of bioactive titania coating on nitinol by plasma electrolytic oxidation. Appl. Surf. Sci. 2014, 274, 181–187. [Google Scholar] [CrossRef]
- Sowa, M.; Piotrowska, M.; Widziołek, M.; Dercz, G.; Tylko, G.; Gorewoda, T.; Osyczka, A.M.; Simka, W. Bioactivity of coatings formed on Ti13Nb13Zr alloy using plasma electrolytic oxidation. Mater. Sci. Eng. C 2015, 49, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Simka, W.; Sadowski, A.; Warczak, M.; Iwaniak, A.; Dercz, G.; Michalska, J.; Maciej, A. Modification of titanium oxide layer by calcium and phosphorus. Electrochim. Acta 2011, 56, 8962–8968. [Google Scholar] [CrossRef]
- Villapún, V.M.; Dover, L.G.; Cross, A.; González, S. Antibacterial Metallic Touch Surfaces. Materials 2016, 9, 736. [Google Scholar] [CrossRef] [PubMed]
- Jansson, T.; Clare-Salzler, Z.J.; Zaveri, T.D.; Mehta, S.; Dolgova, N.V.; Chu, B.H.; Ren, F.; Keselowsky, B.G. Antibacterial effects of zinc oxide nanorod surfaces. J. Nanosci. Nanotechnol. 2012, 12, 7132–7138. [Google Scholar] [CrossRef] [PubMed]
- Rokosz, K.; Hryniewicz, T.; Gaiaschi, S.; Chapon, P.; Raaen, S.; Pietrzak, K.; Malorny, W.; Fernandes, J.S. Characterization of Porous Phosphate Coatings Enriched with Magnesium or Zinc on CP Titanium Grade 2 under DC Plasma Electrolytic Oxidation. Metals 2018, 8, 112. [Google Scholar] [CrossRef]
- Aliasghari, S.; Němcová, A.; Skeldon, P.; Thompson, G.E. Influence of coating morphology on adhesive bonding of titanium pre-treated by plasma electrolytic oxidation. Surf. Coat. Technol. 2016, 289, 101–109. [Google Scholar] [CrossRef]
- Gnedenkov, S.V.; Sharkeev, Y.P.; Sinebryukhov, S.L.; Khrisanfova, O.A.; Legostaeva, E.V.; Zavidnaya, A.G.; Puz’, A.V.; Khlusov, I.A.; Opra, D.P. Functional coatings formed on the titanium and magnesium alloys as implant materials by plasma electrolytic oxidation technology: Fundamental principles and synthesis conditions. Corros. Rev. 2016, 34, 65–83. [Google Scholar] [CrossRef]
- Hariprasad, S.; Ashfaq, M.; Arunnellaiappan, T.; Harilal, M.; Rameshbabu, N. Role of electrolyte additives on in-vitro corrosion behavior of DC plasma electrolytic oxidization coatings formed on CP-Ti. Surf. Coat. Technol. 2016, 292, 20–29. [Google Scholar]
- Huang, Q.; Elkhooly, T.A.; Liu, X.; Zhang, R.; Yang, X.; Shen, Z.; Feng, Q. Effects of hierarchical micro/nano-topographies on the morphology, proliferation and differentiation of osteoblast-like cells. Colloid Surf. B Biointerfaces 2016, 145, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Kung, K.C.; Yuan, K.; Lee, T.M.; Lui, T.S. Effect of heat treatment on microstructures and mechanical behavior of porous SrCaP coatings on titanium. J. Alloy. Compd. 2012, 515, 68–73. [Google Scholar] [CrossRef]
- Zhou, R.; Wei, D.; Cheng, S.; Zhou, Y.; Jia, D.; Wang, Y.; Li, B. The effect of titanium bead diameter of porous titanium on the formation of micro-arc oxidized TiO2-based coatings containing Si and Ca. Ceram. Int. 2013, 39, 5725–5732. [Google Scholar] [CrossRef]
- Venkateswarlu, K.; Rameshbabu, N.; Sreekanth, D.; Sandhyarani, M.; Bose, A.; Muthupandi, V.; Subramanian, S. Role of electrolyte chemistry on electronic and in vitro electrochemical properties of micro-arc oxidized titania films on Cp Ti. Electrochim. Acta 2013, 105, 468–480. [Google Scholar]
- Çelik, İ.; Alsaran, A.; Purcek, G. Effect of different surface oxidation treatments on structural, mechanical and tribological properties of ultrafine-grained titanium. Surf. Coat. Technol. 2014, 258, 842–848. [Google Scholar] [CrossRef]
- Komiya, S.; Sakamoto, K.; Ohtsu, N. Structural changes of anodic layer on titanium in sulfate solution as a function of anodization duration in constant current mode. Appl. Surf. Sci. 2014, 296, 163–168. [Google Scholar] [CrossRef]
- Liu, W.; Cheng, M.; Wahafu, T.; Zhao, Y.; Qin, H.; Wang, J.; Zhang, X.; Wang, L. The in vitro and in vivo performance of a strontium-containing coating on the low-modulus Ti35Nb2Ta3Zr alloy formed by micro-arc oxidation. J. Mater. Sci. Mater. M 2015, 26, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.F.; Qiao, L.P.; Qu, B.; Zhang, S.F.; Chang, W.H.; Xiang, J.H. Biocompatibility of micro-arc oxidation coatings developed on Ti6Al4V alloy in a solution containing organic phosphate. Mater. Lett. 2015, 153, 77–80. [Google Scholar] [CrossRef]
- Sharifi, H.; Aliofkhazraei, M.; Darband, G.B.; Rouhaghdam, A.S. Tribological properties of PEO nanocomposite coatings on titanium formed in electrolyte containing ketoconazole. Tribol. Int. 2016, 102, 463–471. [Google Scholar] [CrossRef]
- Koblova, E.A.; Ustinov, A.Y.; Rudnev, V.S.; Lukiyanchuk, I.V.; Chernykh, I.V. An X-ray photoelectron spectroscopy study of Ni, Cu-containing coatings formed by plasma electrolytic oxidation on aluminum and titanium. J. Struct. Chem. 2017, 58, 1129–1136. [Google Scholar] [CrossRef]
- Wang, L.; Erasquin, U.J.; Zhao, M.; Ren, L.; Zhang, M.Y.; Cheng, G.J.; Wang, Y.; Cai, C. Stability, Antimicrobial Activity, and Cytotoxicity of Poly(amidoamine) Dendrimers on Titanium Substrates. ACS Appl. Mater. Interfaces 2011, 3, 2885–2894. [Google Scholar] [CrossRef] [PubMed]
- Rokosz, K.; Hryniewicz, T.; Matysek, D.; Raaen, S.; Valíček, J.; Dudek, Ł.; Harničarova, M. SEM, EDS and XPS analysis of the coatings obtained on titanium after plasma electrolytic oxidation in electrolytes containing copper nitrate. Materials 2016, 9, 318. [Google Scholar] [CrossRef] [PubMed]
- Rokosz, K.; Hryniewicz, T.; Gaiaschi, S.; Chapon, P.; Raaen, S.; Pietrzak, K.; Malorny, W. Characterisation of calcium- and phosphorus-enriched porous coatings on CP Titanium Grade 2 fabricated by plasma electrolytic oxidation. Metals 2017, 7, 354. [Google Scholar] [CrossRef]
- Nelis, T.; Payling, R. Practical Guide to Glow Discharge Optical Emission Spectroscopy; RSC Analytical Spectroscopy Monographs; Barnett, N.W., Ed.; Royal Society of Chemistry: Cambridge, UK, 2002. [Google Scholar]
- Casa Software Ltd. CasaXPS: Processing Software for XPS, AES, SIMS and More. 2009. Available online: http://www.casaxps.com (accessed on 24 May 2018).
- Naumkin, A.V.; Kraut-Vass, A.; Gaarenstroom, S.W.; Powell, C.J. NIST X-ray Photoelectron Spectroscopy Database: NIST Standard Reference Database 20, Version 4.1. 2012. Available online: https://srdata.nist.gov/xps/ (accessed on 24 May 2018).
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Chastain, J., Ed.; Perkin-Elmer Corporation: Eden Prairie, MN, USA, 1992. [Google Scholar]






| Electrolytes | Ref. |
|---|---|
| sodium silicate (Na2SiO3) with trisodium phosphate (Na3PO4), and sodium aluminate (NaAlO2) | [5] |
| calcium hypophosphite Ca(H2PO2)2 with phosphoric acid H3PO4 | [9] |
| sodium phosphate monobasic monohydrate (NaH2PO4·H2O) with calcium acetate hydrate (Ca(CH3COO)2·H2O) and strontium hydroxide 8-hydrate (Sr(OH)2·8H2O) | [18] |
| calcium acetate Ca(CH3COO)2 2H2O, and Na2SiO3, ethylenediaminetetraacetic acid (EDTA), and sodium hydroxide NaOH | [19] |
| tri-sodium orthophosphate (Na3PO4·12H2O) with potassium hydroxide (KOH) and di-sodium tetra borate (Na2B4O7·10H2O), and trisodium citrate dihydrate (C6H5Na3O7·2H2O), and sodium meta silicate nonahydrate (Na2SiO3·9H2O) | [20] |
| disodium phosphate (Na2HPO4) | [21] |
| ammonium sulfate solution ((NH4)2SO4) | [22] |
| sodium dihydrogen phosphate (NaH2PO4) with calcium acetate (Ca(CH3COO)2) and strontium(II) acetate (Sr(CH3COO)2) | [23] |
| sodium hydroxide (NaOH) with sodium phytate (Na12Phy) | [24] |
| sodium phosphate heptahydrate (Na2HPO4·7H2O), with α-Al2O3 and ketoconazole | [25] |
| trisodium phosphate (Na3PO4) with hydrated sodium borate (Na2B4O7), and sodium tungstate (Na2WO4) | [26] |
| ethylenediaminetetraacetic acid (EDTA) with calcium (Ca(CH3COO)2), Ca(H2PO4)2, sodium hydroxide (NaOH) | [27] |
| 3D Roughness Parameter | Voltage | Mean | Stand. Dev. | First Quartile | Third Quartile |
|---|---|---|---|---|---|
| Sa | 500 VDC | 1.74 | 0.29 | 1.44 | 2.02 |
| 575 VDC | 2.08 | 0.15 | 1.92 | 2.22 | |
| 650 VDC | 3.50 | 0.06 | 3.45 | 3.57 | |
| Sz | 500 VDC | 31.61 | 8.39 | 24.24 | 40.75 |
| 575 VDC | 25.33 | 2.72 | 22.20 | 27.15 | |
| 650 VDC | 47.25 | 1.03 | 46.18 | 48.25 |
| Ratios | Voltage | Mean | Stand. Dev. | First quartile | Third Quartile |
|---|---|---|---|---|---|
| Ca/P | 500 VDC | 0.070 | 0.009 | 0.062 | 0.079 |
| 575 VDC | 0.054 | 0.002 | 0.053 | 0.056 | |
| 650 VDC | 0.089 | 0.011 | 0.077 | 0.098 | |
| Mg/P | 500 VDC | 0.037 | 0.002 | 0.035 | 0.038 |
| 575 VDC | 0.044 | 0.002 | 0.042 | 0.046 | |
| 650 VDC | 0.045 | 0.001 | 0.043 | 0.046 | |
| Zn/P | 500 VDC | 0.034 | 0.006 | 0.029 | 0.039 |
| 575 VDC | 0.045 | 0.003 | 0.042 | 0.049 | |
| 650 VDC | 0.048 | 0.005 | 0.043 | 0.052 | |
| Cu/P | 500 VDC | 0.032 | 0.002 | 0.030 | 0.033 |
| 575 VDC | 0.037 | 0.003 | 0.033 | 0.040 | |
| 650 VDC | 0.043 | 0.011 | 0.036 | 0.052 | |
| M/P | 500 VDC | 0.172 | 0.012 | 0.162 | 0.184 |
| 575 VDC | 0.180 | 0.005 | 0.178 | 0.184 | |
| 650 VDC | 0.224 | 0.014 | 0.215 | 0.236 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rokosz, K.; Hryniewicz, T.; Kacalak, W.; Tandecka, K.; Raaen, S.; Gaiaschi, S.; Chapon, P.; Malorny, W.; Matýsek, D.; Dudek, Ł.; et al. Characterization of Porous Phosphate Coatings Enriched with Calcium, Magnesium, Zinc and Copper Created on CP Titanium Grade 2 by Plasma Electrolytic Oxidation. Metals 2018, 8, 411. https://doi.org/10.3390/met8060411
Rokosz K, Hryniewicz T, Kacalak W, Tandecka K, Raaen S, Gaiaschi S, Chapon P, Malorny W, Matýsek D, Dudek Ł, et al. Characterization of Porous Phosphate Coatings Enriched with Calcium, Magnesium, Zinc and Copper Created on CP Titanium Grade 2 by Plasma Electrolytic Oxidation. Metals. 2018; 8(6):411. https://doi.org/10.3390/met8060411
Chicago/Turabian StyleRokosz, Krzysztof, Tadeusz Hryniewicz, Wojciech Kacalak, Katarzyna Tandecka, Steinar Raaen, Sofia Gaiaschi, Patrick Chapon, Winfried Malorny, Dalibor Matýsek, Łukasz Dudek, and et al. 2018. "Characterization of Porous Phosphate Coatings Enriched with Calcium, Magnesium, Zinc and Copper Created on CP Titanium Grade 2 by Plasma Electrolytic Oxidation" Metals 8, no. 6: 411. https://doi.org/10.3390/met8060411
APA StyleRokosz, K., Hryniewicz, T., Kacalak, W., Tandecka, K., Raaen, S., Gaiaschi, S., Chapon, P., Malorny, W., Matýsek, D., Dudek, Ł., & Pietrzak, K. (2018). Characterization of Porous Phosphate Coatings Enriched with Calcium, Magnesium, Zinc and Copper Created on CP Titanium Grade 2 by Plasma Electrolytic Oxidation. Metals, 8(6), 411. https://doi.org/10.3390/met8060411