The Use of Callovo-Oxfordian Argillite as a Raw Material for Portland Cement Clinker Production
Abstract
:1. Introduction
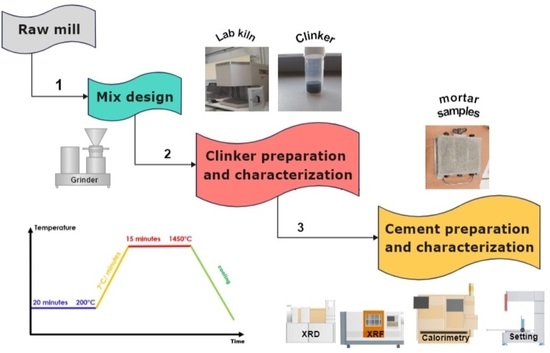
2. Materials and Methods
2.1. Materials
2.2. Clinker Synthesis at Laboratory Scale
2.3. Characterization Methods
3. Results and Discussions
3.1. COx Argillite Characterization
- -
- 100 °C corresponding to free water evaporation,
- -
- 300–500 °C corresponding to decomposition of the organic matter in addition to muscovite, kaolinite and pyrite,
- -
- 750 °C corresponding to the decarbonation of calcite.
3.2. Raw Meal Design and Characterization
3.3. Clinker Characterization
3.4. Isothermal Heat-Flow Calorimetry of COx Cement Paste
3.5. Setting Time of the COx Cement Mortar
3.6. Compressive Strength of the COx Cement Mortar
4. Conclusions
- COx argillite contains the main oxides required for a Portland clinker production: SiO2, Al2O3, CaO and Fe2O3.
- Up to 22.4% of COx argillite could be used in clinker raw meal, without any other addition to adjust, especially, the Al content.
- The COx argillite-based clinker produced contains the main phases of an ordinary Portland cement without having any secondary phase: C2S, C3S, C3A, C4AF.
- The hydration of COx cement presents a heat evolution comparable to that of Portland cement, even if the setting seems faster.
- The compressive strength of COx cement mortar at 28 days is equivalent to the one of CEM I 52.5 N commercial cement, with a compressive strength measured on normalized mortar samples, close to 56 MPa after 28 days.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Forsman, J.; Kreft-Burman, K.; Lindroos, N.; Hämäläinen, H.; Niutanen, V.; Lehtonen, K. Experiences of utilising mass stabilised low-qualit soils for infrastructure construction in the capital region of Finland-case absoils project. In Proceedings of the 28th International Baltic Road Conference, Vilnius, Lithuania, 26–28 August 2013. [Google Scholar]
- Haas, M.; Mongeard, L.; Ulrici, L.; D’Aloïa, L.; Cherrey, A.; Galler, R.; Benedikt, M. Applicability of excavated rock material: A European technical review implying opportunities for future tunnelling projects. J. Clean. Prod. 2021, 315, 128049. [Google Scholar] [CrossRef]
- Magnusson, S.; Lundberg, K.; Svedberg, B.; Knutsson, S. Sustainable management of excavated soil and rock in urban areas–A literature review. J. Clean. Prod. 2015, 93, 18–25. [Google Scholar] [CrossRef]
- Luo, W.; Liu, S.; Hu, Y.; Hu, D.; Kow, K.-W.; Pang, C.; Li, B. Sustainable reuse of excavated soil and recycled concrete aggregate in manufacturing concrete blocks. Constr. Build. Mater. 2022, 342, 127917. [Google Scholar] [CrossRef]
- Costa, F.N.; Ribeiro, D.V. Reduction in CO2 emissions during production of cement, with partial replacement of traditional raw materials with civil construction waste (CCW). J. Clean. Prod. 2020, 276, 123302. [Google Scholar] [CrossRef]
- Singh, G.B.; Subramaniam, K.V. Production and characterization of low-energy Portland composite cement from post-industrial waste. J. Clean. Prod. 2019, 239, 118024. [Google Scholar] [CrossRef]
- Özbay, E.; Erdemir, M.; Durmuş, H.I. Utilization and efficiency of ground granulated blast furnace slag on concrete properties-A review. Constr. Build. Mater. 2016, 105, 423–434. [Google Scholar] [CrossRef]
- Ghezloun, A.; Saidane, A.; Merabet, H. The COP 22 New commitments in support of the Paris Agreement. Energy Procedia 2017, 119, 10–16. [Google Scholar] [CrossRef]
- Scrivener, K.L.; John, V.M.; Gartner, E.M. Eco-Efficient Cements: Potential Economically Viable Solutions for a Low-CO2 Cement-Based Materials Industry; United Nations Environment Program: Nairobi, Kenya, 2016. [Google Scholar]
- Ahmed, H.U.; Mohammed, A.A.; Rafiq, S.; Mohammed, A.S.; Mosavi, A.; Sor, N.H.; Qaidi, S.M.A. Compressive Strength of Sustainable Geopolymer Concrete Composites: A State-of-the-Art Review. Sustainability 2021, 13, 13502. [Google Scholar] [CrossRef]
- Chajec, A. Granite powder vs. Fly ash for the sustainable production of air-cured cementitious mortars. Materials 2021, 14, 1208. [Google Scholar] [CrossRef]
- Gartner, E.; Hirao, H. A review of alternative approaches to the reduction of CO2 emissions associated with the manufacture of the binder phase in concrete. Cem. Concr. Res. 2015, 78, 126–142. [Google Scholar] [CrossRef] [Green Version]
- Aouad, G.; Laboudigue, A.; Gineys, N.; Abriak, N. Dredged sediments used as novel supply of raw material to produce Portland cement clinker. Cem. Concr. Compos. 2012, 34, 788–793. [Google Scholar] [CrossRef]
- Faure, A.; Coudray, C.; Anger, B.; Moulin, I.; Colina, H.; Izoret, L.; Théry, F.; Smith, A. Beneficial reuse of dam fine sediments as clinker raw material. Constr. Build. Mater. 2019, 218, 365–384. [Google Scholar] [CrossRef]
- DiLiberto, C.; LeComte, A.; Mechling, J.-M.; Izoret, L.; Smith, A. Valorisation of recycled concrete sands in cement raw meal for cement production. Mater. Struct. 2017, 50, 127. [Google Scholar] [CrossRef]
- Tsakiridis, P.; Papadimitriou, G.; Tsivilis, S.; Koroneos, C. Utilization of steel slag for Portland cement clinker production. J. Hazard. Mater. 2008, 152, 805–811. [Google Scholar] [CrossRef]
- Yven, B.; Sammartino, S.; Géraud, Y.; Homand, F.; Villiéras, F. Mineralogy, texture and porosity of Callovo-Oxfordian argillites of the Meuse/Haute-Marne region (eastern Paris Basin). Bull. la Soc. Geol. Fr. 2007, 178, 73–90. [Google Scholar]
- Lebon, P.; Mouroux, B. Knowledge of the three French underground laboratory sites. Eng. Geol. 1999, 52, 251–256. [Google Scholar] [CrossRef]
- Gharzouni, A.; Dupuy, C.; Sobrados, I.; Joussein, E.; Texier-Mandoki, N.; Bourbon, X.; Rossignol, S. The effect of furnace and flash heating on COx argillite for the synthesis of alkali-activated binders. J. Clean. Prod. 2017, 156, 670–678. [Google Scholar] [CrossRef]
- Tang, C.; Tang, A.; Cui, Y.; Delage, P.; Schroeder, C.; De Laure, E. Investigating the swelling pressure of compacted crushed-Callovo-Oxfordian claystone. Phys. Chem. Earth Parts A/B/C 2011, 36, 1857–1866. [Google Scholar] [CrossRef]
- Dupuy, C.; Gharzouni, A.; Sobrados, I.; Tessier-Doyen, N.; Texier-Mandoki, N.; Bourbon, X.; Rossignol, S. Formulation of an alkali-activated grout based on Callovo-Oxfordian argillite for an application in geological radioactive waste disposal. Constr. Build. Mater. 2019, 232, 117170. [Google Scholar] [CrossRef]
- Shen, W.; Kondo, D.; Dormieux, L.; Shao, J. A closed-form three scale model for ductile rocks with a plastically compressible porous matrix. Mech. Mater. 2013, 59, 73–86. [Google Scholar] [CrossRef]
- Andra, Dossier 2005 Argile–Synthèse: Evaluation de la faisabilité du stockage géologique en formation argileuse, Meuse/Haute Marne site. 2005. Available online: https://www.andra.fr/sites/default/files/2017-12/266.pdf (accessed on 1 April 2022).
- Gaucher, E.; Robelin, C.; Matray, J.; Négrel, G.; Gros, Y.; Heitz, J.; Vinsot, A.; Rebours, H.; Cassagnabère, A.; Bouchet, A. Aandra underground research laboratory: Interpretation of the mineralogical and geochemical data acquired in the Callovian–Oxfordian formation by investigative drilling. Phys. Chem. Earth Parts A/B/C 2004, 29, 55–77. [Google Scholar] [CrossRef] [Green Version]
- Descostes, M.; Blin, V.; Bazer-Bachi, F.; Meier, P.; Grenut, B.; Radwan, J.; Schlegel, M.; Buschaert, S.; Coelho, D.; Tevissen, E. Diffusion of anionic species in Callovo-Oxfordian argillites and Oxfordian limestones (Meuse/Haute–Marne, France). Appl. Geochem. 2008, 23, 655–677. [Google Scholar] [CrossRef]
- Schneider, M.; Romer, M.; Tschudin, M.; Bolio, H. Sustainable cement production-present and future. Cem. Concr. Res. 2011, 41, 642–650. [Google Scholar] [CrossRef]
- Navia, R.; Rivela, B.; Lorber, K.; Méndez, R. Recycling contaminated soil as alternative raw material in cement facilities: Life cycle assessment. Resour. Conserv. Recycl. 2006, 48, 339–356. [Google Scholar] [CrossRef]
- AFNOR NF EN 196-1; Méthode d’essais des ciments-Partie 1: Détermination des résistances mécaniques. Afnor: Saint-Denis, France, 2006.
- N. E. 196-6; Méthode d’essais des ciments-Détermination de la finesse-Partie 6: Détermination de la finesse. Afnor: Saint-Denis, France, 2018.
- Bhatty, J.I. Effect of Minor Elements on Clinker and Cement Performance; Portland Cement Association: Skokie, IL, USA, 2006. [Google Scholar]
- Kleib, J.; Aouad, G.; Khalil, N.; Zakhour, M. Incorporation of zinc in calcium sulfoaluminate cement clinker. Adv. Cem. Res. 2021, 33, 311–317. [Google Scholar] [CrossRef]
- Chatterjee, A.K. Chemistry and engineering of the clinkerization process—Incremental advances and lack of breakthroughs. Cem. Concr. Res. 2011, 41, 624–641. [Google Scholar] [CrossRef]
- Amar, M.; Benzerzour, M.; Kleib, J.; Abriak, N.E. From dredged sediment to supplementary cementitious material: Characterization, treatment, and reuse. Int. J. Sediment Res. 2021, 36, 92–109. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Cement Chemsitry, 2nd ed.; Thomas Telford Publishing: London, UK, 1997. [Google Scholar]
- NF EN 197-1; Ciment Partie 1: Composition, spécifications et critères de conformité des ciments courants. Afnor: Saint-Denis, France, 2012.
- Juilland, P. Early Hydration of Cementitious Systems. Ph.D. Thesis, Ecole Polytechnique Fédérale de Lausanne, Écublens, Switzerland, 2009. [Google Scholar]
- Zhan, B.J.; Xuan, D.X.; Poon, C.S. The effect of nanoalumina on early hydration and mechanical properties of cement pastes. Constr. Build. Mater. 2019, 202, 169–176. [Google Scholar] [CrossRef]
- Jansen, D.; Goetz-Neunhoeffer, F.; Stabler, C.; Neubauer, J. A remastered external standard method applied to the quantification of early OPC hydration. Cem. Concr. Res. 2011, 41, 602–608. [Google Scholar] [CrossRef]
- Her, S.; Park, T.; Zalnezhad, E.; Bae, S. Synthesis and characterization of cement clinker using recycled pulverized oyster and scallop shell as limestone substitutes. J. Clean. Prod. 2021, 278, 123987. [Google Scholar] [CrossRef]









| Material | Density (g/cm3) | Blaine Specific Surface (cm2/g) | BET Specific Surface (cm2/g) | LOI 950 °C (wt%) | D10 (µm) |
|---|---|---|---|---|---|
| OPC | 3.15 | 3720 | 9800 | 1.91 | 1.31 |
| Method | Clinker | C3S (wt%) | C2S (wt%) | C3A (wt%) | C4AF (wt%) | CaO Free (wt%) |
|---|---|---|---|---|---|---|
| Schlafer–Bukolowki method—Bogue formula | OPC | 61.63 | 15.76 | 9.14 | 9.18 | 1.60 |
| Rietveld method | OPC | 64.87 | 12.47 | 8.83 | 9.04 | 1.19 |
| Composition (%) | CaO | SiO2 | Al2O3 | Fe2O3 | SO3 | Na2O | K2O | MgO | ZnO | P2O5 | LOI (950 °C) | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OPC | 63.75 | 19.95 | 5.31 | 2.98 | 3.03 | 0.55 | 0.93 | 0.86 | - | - | 1.9 | 99.99 |
| Phases | C3S | C2S | C4AF | C3A | CaO |
|---|---|---|---|---|---|
| Percentages | 64 | 18 | 10 | 7 | 1 |
| Oxide | CaO | SiO2 | Al2O3 | Fe2O3 |
|---|---|---|---|---|
| Percentages | 68.86 | 23.12 | 4.74 | 3.29 |
| Oxide | LOI 950 °C | CaO | Al2O3 | Fe2O3 | SiO2 | MgO | P2O5 | SO3 | K2O | TiO2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Percentage | 16.2 | 12.0 | 12.9 | 4.7 | 46.8 | 2.3 | 0.2 | 0.2 | 3.4 | 0.7 |
| Component | CaCO3 | SiO2 | Al2O3 | Fe2O3 | COx Argillite |
|---|---|---|---|---|---|
| COx Clinker | 72.97 | 3.79 | 0.00 | 1.00 | 22.24 |
| LSF | SM | AM | |
|---|---|---|---|
| Experimental values | 0.97 | 2.95 | 1.44 |
| Theoretical values | 0.95 | 2.88 | 1.44 |
| Crystalline Phases | C3S | C2S | C4AF | C3A | C |
|---|---|---|---|---|---|
| COx clinker | 61.5 | 21.2 | 10.9 | 2.9 | 2.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kleib, J.; Amar, M.; Aouad, G.; Bourbon, X.; Benzerzour, M.; Abriak, N.-E. The Use of Callovo-Oxfordian Argillite as a Raw Material for Portland Cement Clinker Production. Buildings 2022, 12, 1421. https://doi.org/10.3390/buildings12091421
Kleib J, Amar M, Aouad G, Bourbon X, Benzerzour M, Abriak N-E. The Use of Callovo-Oxfordian Argillite as a Raw Material for Portland Cement Clinker Production. Buildings. 2022; 12(9):1421. https://doi.org/10.3390/buildings12091421
Chicago/Turabian StyleKleib, Joelle, Mouhamadou Amar, Georges Aouad, Xavier Bourbon, Mahfoud Benzerzour, and Nor-Edine Abriak. 2022. "The Use of Callovo-Oxfordian Argillite as a Raw Material for Portland Cement Clinker Production" Buildings 12, no. 9: 1421. https://doi.org/10.3390/buildings12091421
APA StyleKleib, J., Amar, M., Aouad, G., Bourbon, X., Benzerzour, M., & Abriak, N. -E. (2022). The Use of Callovo-Oxfordian Argillite as a Raw Material for Portland Cement Clinker Production. Buildings, 12(9), 1421. https://doi.org/10.3390/buildings12091421