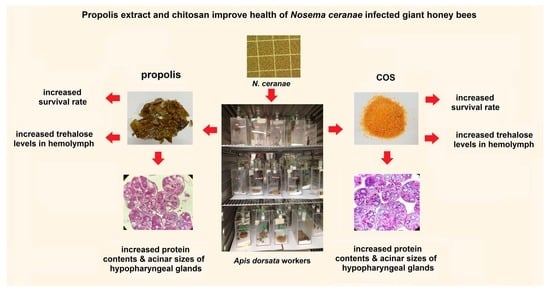
Propolis Extract and Chitosan Improve Health of Nosema ceranae Infected Giant Honey Bees, Apis dorsata Fabricius, 1793
Abstract
:1. Introduction
2. Results
2.1. Hemolymph Trehalose Levels
2.2. Hypopharyngeal Gland Protein Content
2.3. Acini Diameters of Hypopharyngeal Glands
2.4. Honey Bee Survival Rates
3. Discussion
4. Materials and Methods
4.1. Propolis Extraction
4.2. Chito-Oligosaccharide Solution Preparation
4.3. Spore Preparation
4.4. Propolis Extract and COS Treatment Experiments
4.5. Hemolymph Trehalose Measurements
4.6. Hypopharyngeal Gland Protein Content Measurements
4.7. Measurements of Acinar Sizes of the Hypopharyngeal Glands and Histological Structure
4.8. Survival Analysis
4.9. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Breeze, T.D.; Vaissière, B.E.; Bommarco, R.; Petanidou, T.; Seraphides, N.; Kozák, L.; Scheper, J.; Biesmeijer, J.C.; Kleijn, D.; Gyldenkaerne, S.; et al. Agricultural Policies Exacerbate Honeybee Pollination Service Supply-Demand Mismatches Across Europe. PLoS ONE 2014, 9, e82996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genersch, E. Honey bee pathology: Current threats to honey bees and beekeeping. Appl. Microbiol. Biotechnol. 2010, 87, 87–97. [Google Scholar] [CrossRef]
- Klein, A.-M.; Vaissière, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B Biol. Sci. 2007, 274, 303–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kremen, C.; Williams, N.M.; Aizen, M.A.; Gemmill-Herren, B.; Lebuhn, G.; Minckley, R.; Packer, L.; Potts, S.G.; Roulston, T.; Steffan-Dewenter, I.; et al. Pollination and other ecosystem services produced by mobile organisms: A conceptual framework for the effects of land-use change. Ecol. Lett. 2007, 10, 299–314. [Google Scholar] [CrossRef]
- Potts, S.G.; Petanidou, T.; Roberts, S.; O’Toole, C.; Hulbert, A.; Willmer, P. Plant-pollinator biodiversity and pollination services in a complex Mediterranean landscape. Biol. Conserv. 2006, 129, 519–529. [Google Scholar] [CrossRef]
- Suwannapong, G. Honeybees of Thailand; Nova Science Publishers, Inc.: New York, NY, USA, 2019; pp. 1–378. [Google Scholar]
- Goulson, D.; Nicholls, E.; Botías, C.; Rotheray, E.L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 2015, 347, 1255957. [Google Scholar] [CrossRef] [PubMed]
- Kulhanek, K.; Steinhauer, N.; Rennich, K.; Caron, D.M.; Sagili, R.R.; Pettis, J.S.; Ellis, J.D.; Wilson, M.E.; Wilkes, J.T.; Tarpy, D.R.; et al. A national survey of managed honey bee 2015–2016 annual colony losses in the USA. J. Apic. Res. 2017, 56, 328–340. [Google Scholar] [CrossRef] [Green Version]
- López-Uribe, M.M.; Ricigliano, V.A.; Simone-Finstrom, M. Defining Pollinator Health: A Holistic Approach Based on Ecological, Genetic, and Physiological Factors. Annu. Rev. Anim. Biosci. 2020, 8, 269–294. [Google Scholar] [CrossRef]
- McMahon, D.P.; Fürst, M.; Caspar, J.; Theodorou, P.; Brown, M.J.F.; Paxton, R. A sting in the spit: Widespread cross-infection of multiple RNA viruses across wild and managed bees. J. Anim. Ecol. 2015, 84, 615–624. [Google Scholar] [CrossRef]
- Paxton, R. Does infection by Nosema ceranae cause “Colony Collapse Disorder” in honey bees (Apis mellifera)? J. Apic. Res. 2010, 49, 80–84. [Google Scholar] [CrossRef]
- Higes, M.; Hernández, R.M.; Botías, C.; Bailón, E.G.; González-Porto, A.V.; Barrios, L.; Nozal, M.J.; Bernal, J.L.; Jiménez, J.J.; Palencia, P.G.; et al. How natural infection by Nosema ceranae causes honeybee colony collapse. Environ. Microbiol. 2008, 10, 2659–2669. [Google Scholar] [CrossRef]
- Klee, J.; Besana, A.M.; Genersch, E.; Gisder, S.; Nanetti, A.; Tam, D.Q.; Chinh, T.X.; Puerta, F.; Ruz, J.M.; Kryger, P.; et al. Widespread dispersal of the microsporidian Nosema ceranae, an emergent pathogen of the western honey bee, Apis mellifera. J. Invertebr. Pathol. 2007, 96, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Martín-Hernández, R.; Bartolomé, C.; Chejanovsky, N.; Le Conte, Y.; Dalmon, A.; Dussaubat, C.; García-Palencia, P.; Meana, A.; Pinto, M.A.; Soroker, V.; et al. Nosema ceranaein in Apis mellifera: A 12 years postdetectionperspective. Environ. Microbiol. 2018, 20, 1302–1329. [Google Scholar] [CrossRef] [Green Version]
- Chemurot, M.; De Smet, L.; Brunain, M.; De Rycke, R.; de Graaf, D.C. Nosema neumanni n. sp. (Microsporidia, Nosematidae), a new microsporidian parasite of honeybees, Apis mellifera in Uganda. Eur. J. Protistol. 2017, 61, 13–19. [Google Scholar] [CrossRef]
- Zander, E. Tierische Parasiten als Krankheitserreger bei der Biene. Munch. Bienenztg. 1909, 21, 196–204. [Google Scholar]
- Antúnez, K.; Hernández, R.M.; Prieto, L.; Meana, A.; Zunino, P.; Higes, M. Immune suppression in the honey bee (Apis mellifera) following infection by Nosema ceranae (Microsporidia). Environ. Microbiol. 2009, 11, 2284–2290. [Google Scholar] [CrossRef]
- Broadrup, R.L.; Mayack, C.; Schick, S.J.; Eppley, E.J.; White, H.K.; Macherone, A. Honey bee (Apis mellifera) exposomes and dysregulated metabolic pathways associated with Nosema ceranae infection. PLoS ONE 2019, 14, e0213249. [Google Scholar] [CrossRef] [Green Version]
- Goblirsch, M.; Huang, Z.Y.; Spivak, M. Physiological and Behavioral Changes in Honey Bees (Apis mellifera) Induced by Nosema ceranae Infection. PLoS ONE 2013, 8, e58165. [Google Scholar] [CrossRef]
- Lecocq, A.; Jensen, A.B.; Kryger, P.; Nieh, J.C. Parasite infection accelerates age polyethism in young honey bees. Sci. Rep. 2016, 6, 22042. [Google Scholar] [CrossRef] [PubMed]
- Vidau, C.; Panek, J.; Texier, C.; Biron, D.G.; Belzunces, L.P.; Le Gall, M.; Broussard, C.; Delbac, F.; El Alaoui, H. Differential proteomic analysis of midguts from Nosema ceranae-infected honeybees reveals manipulation of key host functions. J. Invertebr. Pathol. 2014, 121, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Corby-Harris, V.; Deeter, M.E.; Snyder, L.; Meador, C.; Welchert, A.C.; Hoffman, A.; Obernesser, B.T. Octopamine mobilizes lipids from honey bee (Apis mellifera) hypopharyngeal glands. J. Exp. Biol. 2020, 223, 216135. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, Y.; Cook, S.C. Chronic Nosema ceranae infection inflicts comprehensive and persistent immunosuppression and accelerated lipid loss in host Apis mellifera honey bees. Int. J. Parasitol. 2018, 48, 433–444. [Google Scholar] [CrossRef]
- Mayack, C.; Naug, D. Parasitic infection leads to decline in hemolymph sugar levels in honeybee foragers. J. Insect Physiol. 2010, 56, 1572–1575. [Google Scholar] [CrossRef]
- Dussaubat, C.; Brunet, J.-L.; Higes, M.; Colbourne, J.K.; Lopez, J.; Choi, J.-H.; Hernández, R.M.; Botías, C.; Cousin, M.; McDonnell, C.; et al. Gut Pathology and Responses to the Microsporidium Nosema ceranae in the Honey Bee Apis mellifera. PLoS ONE 2012, 7, e37017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Palencia, P.; Hernández, R.M.; González-Porto, A.-V.; Marin, P.; Meana, A.; Higes, M. Natural infection by Nosema ceranaecauses similar lesions as in experimentally infected caged-worker honey bees (Apis mellifera). J. Apic. Res. 2010, 49, 278–283. [Google Scholar] [CrossRef]
- Mayack, C.; Natsopoulou, M.E.; McMahon, D. Nosema ceranaealters a highly conserved hormonal stress pathway in honeybees. Insect Mol. Biol. 2015, 24, 662–670. [Google Scholar] [CrossRef]
- Mayack, C.; Naug, D. Energetic stress in the honeybee Apis mellifera from Nosema ceranae infection. J. Invertebr. Pathol. 2009, 100, 185–188. [Google Scholar] [CrossRef]
- Mayack, C.; Naug, D. Individual energetic state can prevail over social regulation of foraging in honeybees. Behav. Ecol. Sociobiol. 2013, 67, 929–936. [Google Scholar] [CrossRef]
- Suwannapong, G.; Maksong, S.; Phainchajoen, M.; Benbow, M.; Mayack, C. Survival and health improvement of Nosema infected Apis florea (Hymenoptera: Apidae) bees after treatment with propolis extract. J. Asia-Pacific Èntomol. 2018, 21, 437–444. [Google Scholar] [CrossRef]
- Shimanuki, H.; Knox, D.A.; Furgala, B.; Caron, D.M.; Williams, J.L. Diseases and pests of honey bee. In The Hive and the Honey Bee; Graham, J.M., Ed.; Dadant and Sons: Hamilton, IL, USA, 1992; pp. 1083–1152. [Google Scholar]
- Giacobino, A.; Rivero, R.; Molineri, A.I.; Cagnolo, N.B.; Merke, J.; Orellano, E.; Salto, C.; Signorini, M. Fumagillin control of Nosema ceranae (Microsporidia:Nosematidae) infection in honey bee (Hymenoptera:Apidae) colonies in Argentina. Vet. Ital. 2016, 52, 145–151. [Google Scholar] [PubMed]
- Mendoza, Y.; Diaz-Cetti, S.; Ramallo, G.; Santos, E.; Porrini, M.; Invernizzi, C. Nosema ceranae Winter Control: Study of the Effectiveness of Different Fumagillin Treatments and Consequences on the Strength of Honey Bee (Hymenoptera: Apidae) Colonies. J. Econ. Èntomol. 2016, 110, 1–5. [Google Scholar] [CrossRef]
- Maistrello, L.; Lodesani, M.; Costa, C.; Leonardi, F.; Marani, G.; Caldon, M.; Mutinelli, F.; Granato, A. Screening of natural compounds for the control of nosema disease in honeybees (Apis mellifera). Apidologie 2008, 39, 436–445. [Google Scholar] [CrossRef] [Green Version]
- Gajger, I.T.; Ribaric, J.; Matak, M.; Svecnjak, L.; Kozaric, Z.; Nejedli, S.; Skerl, I.S. Zeolite clinoptilolite as a dietary supplement and remedy for honeybee (Apis mellifera L.) colonies. Veterinární Med. 2017, 60, 696–705. [Google Scholar] [CrossRef] [Green Version]
- Tlak-Gajger, I.; Tomljanovic, Z.; Stanisavljevic, L. An environmentally friendly approach to the control of Varroa destructor mite and Nosema ceranae disease in Carniolan honeybee (Apis mellifera Carnica) colonies. Arch. Biol. Sci. 2013, 65, 1585–1592. [Google Scholar] [CrossRef] [Green Version]
- Higes, M.; Gómez-Moracho, T.; Rodríguez-García, C.; Botías, C.; Hernández, R.M. Preliminary effect of an experimental treatment with “Nozevit®”, (a phyto-pharmacological preparation) for Nosema ceranae control. J. Apic. Res. 2014, 53, 472–474. [Google Scholar] [CrossRef]
- Porrini, M.P.; Fernández, N.J.; Garrido, P.M.; Gende, L.B.; Medici, S.K.; Eguaras, M.J. In vivo evaluation of antiparasitic activity of plant extracts on Nosema ceranae (Microsporidia). Apidologie 2011, 42, 700–707. [Google Scholar] [CrossRef] [Green Version]
- Cilia, G.; Garrido, C.; Bonetto, M.; Tesoriero, D.; Nanetti, A. Effect of Api-Bioxal® and ApiHerb® Treatments against Nosema ceranae Infection in Apis mellifera Investigated by Two qPCR Methods. Veter-Sci. 2020, 7, 125. [Google Scholar] [CrossRef] [PubMed]
- Borges, D.; Guzman-Novoa, E.; Goodwin, P. Effects of Prebiotics and Probiotics on Honey Bees (Apis mellifera) Infected with the Microsporidian Parasite Nosema ceranae. Microorganisms 2021, 9, 481. [Google Scholar] [CrossRef]
- El Khoury, S.; Rousseau, A.; Lecoeur, A.; Cheaib, B.; Bouslama, S.; Mercier, P.-L.; Demey, V.; Castex, M.; Giovenazzo, P.; Derome, N. Deleterious Interaction Between Honeybees (Apis mellifera) and its Microsporidian Intracellular Parasite Nosema ceranae Was Mitigated by Administrating Either Endogenous or Allochthonous Gut Microbiota Strains. Front. Ecol. Evol. 2018, 6, 58. [Google Scholar] [CrossRef] [Green Version]
- Valizadeh, P.; Guzman-Novoa, E.; Goodwin, P.H. Effect of Immune Inducers on Nosema ceranae Multiplication and Their Impact on Honey Bee (Apis mellifera L.) Survivorship and Behaviors. Insects 2020, 11, 572. [Google Scholar] [CrossRef]
- Nanetti, A.; Ugolini, L.; Cilia, G.; Pagnotta, E.; Malaguti, L.; Cardaio, I.; Matteo, R.; Lazzeri, L. Seed Meals from Brassica nigra and Eruca sativa Control Artificial Nosema ceranae Infections in Apis mellifera. Microorganisms 2021, 9, 949. [Google Scholar] [CrossRef]
- Borges, D.; Guzman-Novoa, E.; Goodwin, P.H. Control of the microsporidian parasite Nosema ceranae in honey bees (Apis mellifera) using nutraceutical and immuno-stimulatory compounds. PLoS ONE 2020, 15, e0227484. [Google Scholar] [CrossRef]
- Mura, A.; Pusceddu, M.; Theodorou, P.; Angioni, A.; Floris, I.; Paxton, R.J.; Satta, A. Propolis Consumption Reduces Nosema ceranae Infection of European Honey Bees (Apis mellifera). Insects 2020, 11, 124. [Google Scholar] [CrossRef] [Green Version]
- Naree, S.; Ellis, J.D.; Benbow, M.E.; Suwannapong, G. The use of propolis for preventing and treating Nosema ceranae infection in western honey bee (Apis mellifera Linnaeus, 1787) workers. J. Apic. Res. 2021, 1–11. [Google Scholar] [CrossRef]
- Yemor, T.; Phiancharoen, M.; Benbow, M.E.; Suwannapong, G. Effects of stingless bee propolis on Nosema ceranae infected Asian honey bees, Apis cerana. J. Apic. Res. 2015, 54, 468–473. [Google Scholar] [CrossRef]
- Arismendi, N.; Vargas, M.; López, M.D.; Barría, Y.; Zapata, N. Promising antimicrobial activity against the honey bee parasite Nosema ceranae by methanolic extracts from Chilean native plants and propolis. J. Apic. Res. 2018, 57, 522–535. [Google Scholar] [CrossRef]
- Saltykova, E.S.; Karimova, A.A.; Gataullin, A.R.; Gaifullina, L.; Matniyazov, R.T.; Frolova, M.A.; Albulov, A.I.; Nikolenko, A.G. The effect of high-molecular weight chitosans on the antioxidant and immune systems of the honeybee. Appl. Biochem. Microbiol. 2016, 52, 553–557. [Google Scholar] [CrossRef]
- Thongsong, B.; Suthongsa, S.; Pichyangkura, R.; Kalandakanond-Thongsong, S. Effects of chito-oligosaccharide supplementation with low or medium molecular weight and high degree of deacetylation on growth performance, nutrient digestibility and small intestinal morphology in weaned pigs. Livest. Sci. 2018, 209, 60–66. [Google Scholar] [CrossRef]
- Yousef, M.; Pichyangkura, R.; Soodvilai, S.; Chatsudthipong, V.; Muanprasat, C. Chitosan oligosaccharide as potential therapy of inflammatory bowel disease: Therapeutic efficacy and possible mechanisms of action. Pharmacol. Res. 2012, 66, 66–79. [Google Scholar] [CrossRef]
- Azagra-Boronat, I.; Rodríguez-Lagunas, M.J.; Castell, M.; Pérez-Cano, F.J. Chapter 14—Prebiotics for gastrointestinal infections and acute diarrhea. In Dietary Interventions in Gastrointestinal Diseases; Watson, R., Preedy, V., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 179–191. [Google Scholar]
- Guan, G.; Azad, A.K.; Lin, Y.; Kim, S.W.; Tian, Y.; Liu, G.; Wang, H. Biological Effects and Applications of Chitosan and Chito-Oligosaccharides. Front. Physiol. 2019, 10, 516. [Google Scholar] [CrossRef] [Green Version]
- Kunanusornchai, W.; Witoonpanich, B.; Tawonsawatruk, T.; Pichyangkura, R.; Chatsudthipong, V.; Muanprasat, C. Chitosan oligosaccharide suppresses synovial inflammation via AMPK activation: An in vitro and in vivo study. Pharmacol. Res. 2016, 113, 458–467. [Google Scholar] [CrossRef]
- Zhang, C.; Jiao, S.; Wang, Z.A.; Du, Y. Exploring Effects of Chitosan Oligosaccharides on Mice Gut Microbiota in in vitro Fermentation and Animal Model. Front. Microbiol. 2018, 9, 2388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurze, C.; Mayack, C.; Hirche, F.; Stangl, G.I.; Le Conte, Y.; Kryger, P.; Moritz, R.F.A. Nosema spp. infections cause no energetic stress in tolerant honeybees. Parasitol. Res. 2016, 115, 2381–2388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drescher, N.; Klein, A.-M.; Neumann, P.; Yañez, O.; Leonhardt, S.D. Inside Honeybee Hives: Impact of Natural Propolis on the Ectoparasitic Mite Varroa destructor and Viruses. Insects 2017, 8, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saltykova, E.S.; Gaifullina, L.R.; Kaskinova, M.; Gataullin, A.R.; Matniyazov, R.T.; Poskryakov, A.V.; Nikolenko, A.G. Effect of Chitosan on Development of Nosema apis Microsporidia in Honey Bees. Microbiology 2018, 87, 738–743. [Google Scholar] [CrossRef]
- Paris, L.; El Alaoui, H.; Delbac, F.; Diogon, M. Effects of the gut parasite Nosema ceranae on honey bee physiology and behavior. Curr. Opin. Insect Sci. 2018, 26, 149–154. [Google Scholar] [CrossRef]
- Higes, M.; García-Palencia, P.; Hernández, R.M.; Meana, A. Experimental infection of Apis mellifera honeybees with Nosema ceranae (Microsporidia). J. Invertebr. Pathol. 2007, 94, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Suwannapong, G.; Maksong, S.; Seanbualuang, P.; Benbow, M.E. Experimental infection of red dwarf honeybee, Apis florea, with Nosema ceranae. J. Asia-Pacific Èntomol. 2010, 13, 361–364. [Google Scholar] [CrossRef]
- Suwannapong, G.; Yemor, T.; Boonpakdee, C.; Benbow, M.E. Nosema ceranae, a new parasite in Thai honeybees. J. Invertebr. Pathol. 2011, 106, 236–241. [Google Scholar] [CrossRef]
- Suwannapong, G.; Benbow, M.E.; Nieh, J.C. Biology of Thai honeybees: Natural history and threats. In Bees: Biology, Threats and Colonies; Florio, R.M., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2011; pp. 1–98. [Google Scholar]
- Suwannapong, G.; Maksong, S.; Yemor, T.; Junsuri, N.; Benbow, M.E. Three species of native Thai honey bees exploit overlapping pollen resources: Identification of bee flora from pollen loads and midguts from Apis cerana, A. dorsata and A. florea. J. Apic. Res. 2013, 52, 196–201. [Google Scholar] [CrossRef]
- Aliferis, K.A.; Copley, T.; Jabaji, S. Gas chromatography–mass spectrometry metabolite profiling of worker honey bee (Apis mellifera L.) hemolymph for the study of Nosema ceranae infection. J. Insect Physiol. 2012, 58, 1349–1359. [Google Scholar] [CrossRef]
- Jack, C.J.; Uppala, S.S.; Lucas, H.M.; Sagili, R.R. Effects of pollen dilution on infection of Nosema ceranae in honey bees. J. Insect Physiol. 2016, 87, 12–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Evans, J.D.; Smith, I.B.; Pettis, J.S. Nosema ceranae is a long-present and wide-spread microsporidian infection of the European honey bee (Apis mellifera) in the United States. J. Invertebr. Pathol. 2008, 97, 186–188. [Google Scholar] [CrossRef]
- Chen, Y.P.; Evans, J.D.; Murphy, C.; Gutell, R.; Zuker, M.; Gundensen-Rindal, D.; Pettis, J.S. Morphological, Molecular, and Phylogenetic Characterization of Nosema ceranae, a Microsporidian Parasite Isolated from the European Honey Bee, Apis mellifera. J. Eukaryot. Microbiol. 2009, 56, 142–147. [Google Scholar] [CrossRef] [Green Version]
- Kurze, C.; Dosselli, R.; Grassl, J.; Le Conte, Y.; Kryger, P.; Baer, B.; Moritz, R.F. Differential proteomics reveals novel insights into Nosema–honey bee interactions. Insect Biochem. Mol. Biol. 2016, 79, 42–49. [Google Scholar] [CrossRef]
- Malone, L.; Giacon, H.A.; Newton, M.R. Comparison of the responses of some New Zealand and Australian honey bees (Apis mellifera L) to Nosema apis Z. Apidologie 1995, 26, 495–502. [Google Scholar] [CrossRef] [Green Version]
- Cantwell, G.E. Standard method for counting Nosema spores. Am. Bee J. 1970, 110, 222–223. [Google Scholar]
- Fries, I.; Chauzat, M.-P.; Chen, Y.-P.; Doublet, V.; Genersch, E.; Gisder, S.; Higes, M.; McMahon, D.P.; Martín-Hernández, R.; Natsopoulou, M.; et al. Standard methods for Nosema research. J. Apic. Res. 2013, 52, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Higes, M.; Martín-Hernández, R.; Garrido-Bailón, E.; García-Palencia, P.; Meana, A. Detection of infective Nosema ceranae (Microsporidia) spores in corbicular pollen of forager honeybees. J. Invertebr. Pathol. 2008, 97, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-Dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Suwannapong, G.; Chaiwongwattanakul, S.; Benbow, M.E. Histochemical Comparison of the Hypopharyngeal Gland in Apis cerana Fabricius, 1793 Workers and Apis mellifera Linnaeus, 1758 Workers. Psyche A J. Èntomol. 2010, 2010, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Suwannapong, G.; Seanbualuang, P.; Wongsiri, S. A histochemical study of the hypopharangeal glands of the dwarf honey bees Apis andreniformis and Apis florea. J. Apic. Res. 2007, 46, 260–263. [Google Scholar] [CrossRef]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naree, S.; Ponkit, R.; Chotiaroonrat, E.; Mayack, C.L.; Suwannapong, G. Propolis Extract and Chitosan Improve Health of Nosema ceranae Infected Giant Honey Bees, Apis dorsata Fabricius, 1793. Pathogens 2021, 10, 785. https://doi.org/10.3390/pathogens10070785
Naree S, Ponkit R, Chotiaroonrat E, Mayack CL, Suwannapong G. Propolis Extract and Chitosan Improve Health of Nosema ceranae Infected Giant Honey Bees, Apis dorsata Fabricius, 1793. Pathogens. 2021; 10(7):785. https://doi.org/10.3390/pathogens10070785
Chicago/Turabian StyleNaree, Sanchai, Rujira Ponkit, Evada Chotiaroonrat, Christopher L. Mayack, and Guntima Suwannapong. 2021. "Propolis Extract and Chitosan Improve Health of Nosema ceranae Infected Giant Honey Bees, Apis dorsata Fabricius, 1793" Pathogens 10, no. 7: 785. https://doi.org/10.3390/pathogens10070785
APA StyleNaree, S., Ponkit, R., Chotiaroonrat, E., Mayack, C. L., & Suwannapong, G. (2021). Propolis Extract and Chitosan Improve Health of Nosema ceranae Infected Giant Honey Bees, Apis dorsata Fabricius, 1793. Pathogens, 10(7), 785. https://doi.org/10.3390/pathogens10070785