Metabolic Alteration of Trypanosoma cruzi during Differentiation of Epimastigote to Trypomastigote Forms
Abstract
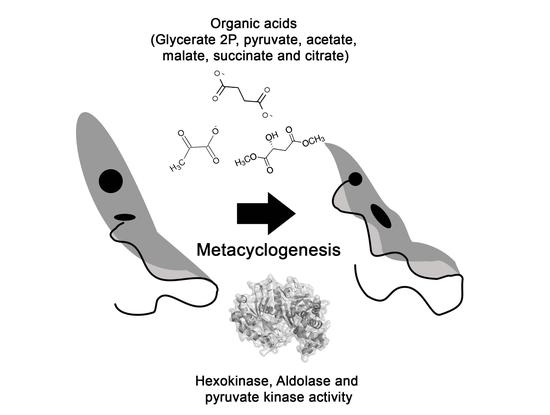
:1. Introduction
2. Results
2.1. Organic Acids
2.2. Enzymatic Activities
3. Discussion
4. Materials and Methods
4.1. Parasites and Growth Condition
4.2. Extraction of Organic Acids
4.3. HPLC Analysis
4.4. Enzymatic Assay and Protein Estimation
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tyler, K.M.; Engman, D.M. The life cycle of Trypanosoma cruzi revisited. Int. J. Parasitol. 2001, 31, 472–481. [Google Scholar] [CrossRef]
- Contreras, V.T.; Morel, C.M.; Goldenberg, S. Stage-specific gene expression precedes morphological changes during Trypanosoma cruzi metacyclogenesis. Mol. Biochem. Parasitol. 1985, 14, 83–96. [Google Scholar] [CrossRef] [Green Version]
- Krassner, S.M.; Granger, B.; Phermsangngnam, P.; Le, T.; Linden, V. Further studies on substrates inducing metacyclogenesis in Trypanosoma cruzi. J. Protozool. 1990, 37, 128–132. [Google Scholar] [CrossRef]
- Parodi-Talice, A.; Monteiro-Goes, V.; Arrambide, N.; Avila, A.R.; Duran, R.; Correa, A.; Dallagiovanna, B.; Cayota, A.; Krieger, M.; Goldenberg, S.; et al. Proteomic analysis of metacyclic trypomastigotes undergoing Trypanosoma cruzi metacyclogenesis. J. Mass Spectrom. 2007, 42, 1422–1432. [Google Scholar] [CrossRef] [PubMed]
- Contreras, V.T.; Salles, J.M.; Thomas, N.; Morel, C.M.; Goldenberg, S. In vitro differentiation of Trypanosoma cruzi under chemically defined conditions. Mol. Biochem. Parasitol. 1985, 16, 315–327. [Google Scholar] [CrossRef] [Green Version]
- Isola, E.L.; Lammel, E.M.; Redruello, M. Trypanosoma cruzi: Conditions required to improve metacyclic differentiation in axenic culture. Rev. Argent. Microbiol. 1989, 21, 9–14. [Google Scholar] [PubMed]
- Schaub, G.A. Trypanosoma cruzi: Quantitative studies of development of two strains in small intestine and rectum of the vector Triatoma infestans. Exp. Parasitol. 1989, 68, 260–273. [Google Scholar] [CrossRef]
- De Lima, A.R.; Navarro, M.C.; Arteaga, R.Y.; Contreras, V.T. Cultivation of Trypanosoma cruzi epimastigotes in low glucose axenic media shifts its competence to differentiate at metacyclic trypomastigotes. Exp. Parasitol. 2008, 119, 336–342. [Google Scholar] [CrossRef]
- Hamedi, A.; Botelho, L.; Britto, C.; Fragoso, S.P.; Umaki, A.C.; Goldenberg, S.; Bottu, G.; Salmon, D. In vitro metacyclogenesis of Trypanosoma cruzi induced by starvation correlates with a transient adenylyl cyclase stimulation as well as with a constitutive upregulation of adenylyl cyclase expression. Mol. Biochem. Parasitol. 2015, 200, 9–18. [Google Scholar] [CrossRef]
- Schaub, G.A.; Lösch, P. Trypanosoma cruzi: Origin of metacyclic trypomastigotes in the urine of the vector Triatoma infestans. Exp. Parasitol. 1988, 65, 174–186. [Google Scholar] [CrossRef]
- de Andrade, A.F.; Esteves, M.J.; Angluster, J.; Gonzales-Perdomo, M.; Goldenberg, S. Changes in cell-surface carbohydrates of Trypanosoma cruzi during metacyclogenesis under chemically defined conditions. J. Gen. Microbiol. 1991, 137, 2845–2849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaneda, Y.; Nagakura, K.; Goutsu, T. Lipid composition of three morphological stages of Trypanosoma cruzi. Comp. Biochem. Physiol. B 1986, 83, 533–536. [Google Scholar] [CrossRef]
- Booth, L.A.; Smith, T.K. Lipid metabolism in Trypanosoma cruzi: A review. Mol. Biochem. Parasitol. 2020, 240, 111324. [Google Scholar] [CrossRef]
- Esteves, M.G.; Gonzales-Perdomo, M.; Alviano, C.S.; Angluster, J.; Goldenberg, S. Changes in fatty acid composition associated with differentiation of Trypanosoma cruzi. FEMS Microbiol. Lett. 1989, 50, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Cazzulo, J.J.; Franke de Cazzulo, B.M.; Engel, J.C.; Cannata, J.J. End products and enzyme levels of aerobic glucose fermentation in trypanosomatids. Mol. Biochem. Parasitol. 1985, 16, 329–343. [Google Scholar] [CrossRef]
- Adroher, F.J.; Osuna, A.; Lupianez, J.A. Differential energetic metabolism during Trypanosoma cruzi differentiation. II Hexokinase, phosphofructokinase, and pyruvate kinase. Mol. Cell. Biochem. 1990, 94, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Durieux, P.O.; Schutz, P.; Brun, R.; Kohler, P. Alterations in Krebs cycle enzyme activities and carbohydrate catabolism in two strains of Trypanosoma brucei during in differentiation of their bloodstream to procyclic stages. Mol. Biochem. Parasitol. 1991, 45, 19–28. [Google Scholar] [CrossRef]
- Cazzulo, J.J. Intermediate metabolism in Trypanosoma cruzi. J. Bioenerg. Biomembr. 1994, 26, 157–165. [Google Scholar] [CrossRef]
- Maugeri, D.A.; Cannata, J.J.; Cazzulo, J.J. Glucose metabolism in Trypanosoma cruzi. Essays Biochem. 2011, 51, 15–30. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Moreno, M.; Fernandez-Becerra, M.C.; Castilla-Calvente, J.J.; Osuna, A. Metabolic studies by 1H NMR of different forms of Trypanosoma cruzi as obtained by ‘in vitro’ culture. FEMS Microbiol. Lett. 1995, 33, 119–125. [Google Scholar] [CrossRef]
- Wood, E.D. Trypanosoma cruzi: Organic acid metabolism in vitro. Exp. Parasitol. 1975, 37, 60–66. [Google Scholar] [CrossRef]
- Silber, A.M.; Colli, W.; Ulrich, H.; Alves, M.J.; Pereira, C.A. Amino acid metabolic routes in Trypanosoma cruzi: Possible therapeutic targets against Chagas’ disease. Curr. Drug Targets Infect. Disord. 2005, 5, 53–64. [Google Scholar] [CrossRef] [Green Version]
- Manchola, N.C.; Rapado, L.N.; Barisón, M.J.; Silber, A.M.J. Biochemical characterization of branched-chain amino acids uptake in Trypanosoma cruzi. J. Eukaryot. Microbiol. 2016, 63, 299–308. [Google Scholar] [CrossRef]
- Cazzulo, J.J. Aerobic fermentation of glucose by trypanosomatids. FASEB J. 1992, 6, 3153–3161. [Google Scholar] [CrossRef] [PubMed]
- Opperdoes, F.R.; Michels, P.A. Enzymes of carbohydrate metabolism as potential drug targets. Int. J. Parasitol. 2001, 31, 482–490. [Google Scholar] [CrossRef]
- Cruz-Saavedra, L.; Vallejo, G.A.; Guhl, F.; Messenger, L.A.; Ramírez, J.D. Transcriptional remodeling during metacyclogenesis in Trypanosoma cruzi I. Virulence 2020, 11, 969–980. [Google Scholar] [CrossRef] [PubMed]
- Shah-Simpson, S.; Lentini, G.; Dumoulin, P.C.; Burleigh, B.A. Modulation of host central carbon metabolism and in situ glucose uptake by intracellular Trypanosoma cruzi amastigotes. PLoS Pathog. 2017, 13, e1006747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiratsubaki, I.S.; Fang, X.; Souza, R.O.O.; Palsson, B.O.; Silber, A.M.; Siqueira-Neto, J.L. Genome-scale metabolic models highlight stage-specific differences in essential metabolic pathways in Trypanosoma cruzi. PLoS Negl. Trop. Dis. 2020, 14, e0008728. [Google Scholar] [CrossRef] [PubMed]
- Parsons, M. Glycosomes: Parasites and the divergence of peroxisomal purpose. Mol. Microbiol. 2004, 53, 717–724. [Google Scholar] [CrossRef]
- Michels, P.A.M.; Villafraz, O.; Pineda, E.; Alencar, M.B.; Cáceres, A.J.; Silber, A.M.; Bringaud, F. Carbohydrate metabolism in trypanosomatids: New insights revealing novel complexity, diversity, and species-unique features. Exp. Parasitol. 2021, 224, 108102. [Google Scholar] [CrossRef]
- Shah-Simpson, S.; Pereira, C.F.; Dumoulin, P.C.; Caradonna, K.L.; Burleigh, B.A. Bioenergetic profiling of Trypano soma cruzi life stages using Seahorse extracellular flux technology. Mol. Biochem. Parasitol. 2016, 208, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Avila, A.R.; Dallagiovanna, B.; Yamada-Ogatta, S.F.; Monteiro-Góes, V.; Fragoso, S.P.; Krieger, M.A.; Goldenberg, S. Stage-specific gene expression during Trypanosoma cruzi metacyclogenesis. Genet. Mol. Res. 2003, 2, 159–168. [Google Scholar]
- Paes, M.C.; Saraiva, F.M.S.; Nogueira, N.P.; Vieira, C.S.D.; Dias, F.A.; Rossini, A.; Coelho, V.L.; Pane, A.; Sang, F.; Alcocer, M. Gene expression profiling of Trypanosoma cruzi in the presence of heme points to glycosomal metabo lic adaptation of epimastigotes inside the vector. PLoS Negl. Trop. Dis. 2020, 14, e0007945. [Google Scholar] [CrossRef] [PubMed]
- Barisón, M.J.; Rapado, L.N.; Merino, E.F.; Furusho Pral, E.M.; Mantilla, B.S.; Marchese, L.; Nowicki, C.; Silber, A.M.; Cassera, M.B. Metabolomic profiling reveals a finely tuned, starvation-induced metabolic switch in Trypanosoma cruzi epimastigotes. J Biol. Chem. 2017, 292, 8964–8977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibáñez, A.B.; Bauer, S. Analytical method for the determination of organic acids in dilute acid pretreated biomass hydrolysate by liquid chromatography-time-of-flight mass spectrometry. Biotechnol. Biofuels 2014, 7, 145. [Google Scholar] [CrossRef] [Green Version]
- Masson, S.; Sciaky, M.; Desmoulin, F.; Fontanara, E.; Cozzone, P.J. Simple cation-exchange high-performance liquid chromatography optimized to measure of metabolites in the effluents from perfused rat livers using refrac tive index and ultraviolet detectors. J. Chromatogr. 1991, 563, 231–242. [Google Scholar] [CrossRef]
- Toyoka, T. Use of derivation to improve the chromatographic properties and detection selectivity of physiologically important carboxylic acids. J. Chromatogr. B 1995, 671, 91–112. [Google Scholar] [CrossRef]
- Ding, M.; Chen, P.; Luo, G. High performance liquid chromatographic analysis of organic acids in foods. Chin. J. Chromatogr. 1997, 15, 212–215. [Google Scholar]
- Cazzulo, J.J.; Franke, M.C.C.; Cazzulo, B.M.F. On the regulatory properties of the pyruvate kinase from Trypanosoma cruzi epimastigotes. FEMS Microbiol. Lett. 1989, 59, 259–263. [Google Scholar] [CrossRef]
- Schaub, G.A. An update on the knowledge of parasite-vector interactions of Chagas Disease. Res. Rep. Trop. Med. 2021, 12, 63–76. [Google Scholar] [CrossRef]
- Urbina, J.A. Intermediary metabolism of Trypanosoma cruzi. Parasitol. Today 1994, 10, 107–110. [Google Scholar] [CrossRef]
- Sant’Anna, C.; de Souza, W.; Cunha-e-Silva, N. Biogenesis of the reservosomes of Trypanosoma cruzi. Microsc. Microanal. 2004, 10, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, R.C.Q.; Steindel, M.; Soares, M.J. The reservosomes of epimastigotes forms of Trypanosoma cruzi: Occurrence during in vitro cultivation. Parasitol. Res. 1994, 80, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Sant’Anna, C.; Nakayasu, E.S.; Pereira, M.G.; Lourenço, D.; De Souza, W. Subcellular proteomics of Trypanosoma cruzi reservosomes. Proteomics 2009, 9, 1782–1794. [Google Scholar] [CrossRef] [Green Version]
- Rogerson, G.W.; Gutteridge, W.E. Catabolic metabolism in Trypanosoma cruzi. Int. J. Parasitol. 1980, 10, 31–35. [Google Scholar] [CrossRef]
- Engel, J.C.; Franke de Cazzulo, B.M.; Stoppani, A.O.; Cannata, J.J.; Cazzulo, J.J. Aerobic glucose fermentation by Trypanosoma cruzi axenic culture amastigote-like forms during growth and differentiation to epimastigotes. Mol. Biochem. Parasitol. 1987, 26, 1–10. [Google Scholar] [CrossRef]
- Wood, D.E.; Schiller, E.L. Trypanosoma cruzi: Comparative fatty acid metabolism of the epimastigotes and trypomastigotes in vitro. Exp Parasitol. 1975, 38, 202–207. [Google Scholar] [CrossRef]
- Souza, R.O.O.; Damasceno, F.S.; Marsiccobetre, S.; Biran, M.; Murata, G.; Curi, R.; Bringaud, F.; Silber, A.M. Fatty acid oxidation participates in resistance to nutrient-depleted environments in the insect stages of Trypanosoma cruzi. PLoS Pathog. 2021, 17, e1009. [Google Scholar] [CrossRef]
- Guarneri, A.A.; Schaub, G.A. Interaction of triatomines, trypanosomes, and microbiota. In Triatominae—The Biology of Chagas Disease Vectors; Guarneri, A.A., Lorenzo, M.G., Eds.; Springer Nature: New York, NY, USA, 2021; pp. 345–386. [Google Scholar]
- Pereira, M.G.; Nakayasu, E.S.; Sant’Anna, C.; De Cicco, N.N.; Atella, G.C.; de Souza, W.; Almeida, I.C.; Cunha-e-Silva, N. Trypanosoma cruzi epimastigotes are able to store and mobilize high amounts of cholesterol in reservosome lipid inclusions. PLoS ONE 2011, 6, e22359. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Saavedra, L.; Vallejo, G.A.; Guhl, F.; Ramírez, J.D. Transcriptomic changes across the life cycle of Trypanosoma cruzi II. Peer J. 2020, 8, e8947. [Google Scholar] [CrossRef]
- Denicola-Seoane, A.; Rubbo, H.; Prodanov, E.; Turrens, J.F. Succinate-depende metabolism in Trypanosoma cruzi epimastigotes. Mol. Biochem. Parasitol. 1992, 54, 43–50. [Google Scholar] [CrossRef]
- Contreras, V.T.; De Lima, A.R.; Zorrilla, G. Trypanosoma cruzi: Maintenance in culture modify gene and antigenic expression of metacyclic trypomastigotes. Mem. Inst. Oswaldo Cruz 1998, 93, 753–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De-Simone, S.G.; Bourguignon, S.C.; Gonçalves, P.S.; Lechuga, G.C.; Provance, D.W., Jr. Metabolic Alteration of Trypanosoma cruzi during Differentiation of Epimastigote to Trypomastigote Forms. Pathogens 2022, 11, 268. https://doi.org/10.3390/pathogens11020268
De-Simone SG, Bourguignon SC, Gonçalves PS, Lechuga GC, Provance DW Jr. Metabolic Alteration of Trypanosoma cruzi during Differentiation of Epimastigote to Trypomastigote Forms. Pathogens. 2022; 11(2):268. https://doi.org/10.3390/pathogens11020268
Chicago/Turabian StyleDe-Simone, Salvatore G., Saulo C. Bourguignon, Priscila S. Gonçalves, Guilherme C. Lechuga, and David W. Provance, Jr. 2022. "Metabolic Alteration of Trypanosoma cruzi during Differentiation of Epimastigote to Trypomastigote Forms" Pathogens 11, no. 2: 268. https://doi.org/10.3390/pathogens11020268
APA StyleDe-Simone, S. G., Bourguignon, S. C., Gonçalves, P. S., Lechuga, G. C., & Provance, D. W., Jr. (2022). Metabolic Alteration of Trypanosoma cruzi during Differentiation of Epimastigote to Trypomastigote Forms. Pathogens, 11(2), 268. https://doi.org/10.3390/pathogens11020268