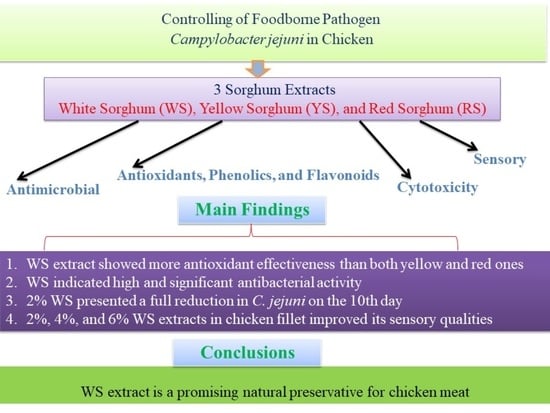
Estimating the Prevalence of Foodborne Pathogen Campylobacter jejuni in Chicken and Its Control via Sorghum Extracts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Growth Conditions of Bacterial Strains
2.2. Collection of Chicken Samples and Detection of C. jejuni
2.3. Sorghum Materials and Extraction
2.4. Antibacterial Activity
2.4.1. Antibacterial Potential of Sorghum Extract
2.4.2. Assessment MICs of Sorghum Extract against C. jejuni
2.5. Phytochemical Analysis of White Sorghum Extract
2.5.1. Total Phenolic Compounds (TPCs) of White Sorghum Extract
2.5.2. Total Flavonoid Compounds (TFCs) of White Sorghum Extract
2.5.3. Diphenyl-1-Picrylhydrazyl (DPPH) Radical Scavenging Assay
2.6. Safety and Cytotoxicity Assay of White Sorghum Extract
2.7. Experimental Application and Evaluation of the Antimicrobial Power of White Sorghum Extract against C. jejuni Experimentally Inoculated into Chicken Fillet
2.7.1. Microbes
2.7.2. Refrigerated Storage Study of Chicken Breast Fillets
2.7.3. Sensory Evaluation of the Acceptability of Chicken Fillet Fortified with White Sorghum Extract
2.8. Statistical Analysis
3. Results and Discussion
3.1. Occurrence of C. jejuni in Chicken Meat
3.2. Antibacterial Activity of Lyophilized Sorghum Extracts
3.3. Minimal Inhibitory Concentration (MIC) of Sorghum Plant Extract
3.4. Total Phenolic Compounds (TPCs) and Total Flavonoid Compounds (TFCs) of Lyophilized Sorghum Extract
3.5. Antioxidant Potential and DPPH Radical Scavenging Ability
3.6. Safety Assay and Cytotoxicity of White Sorghum Extract
3.7. Preparation of Chicken Fillets and Their Acceptability after the Fortification with Lyophilized White Sorghum Extract
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meurer, L.; Payne, W.; Guffey, J.S. Visible light as an inhibitor of Campylobacter jejuni. Int. J. Antimicrob. Agent. 2020, 55, 105818. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-C.; Oh, E.; Kim, J.; Jeon, B. Regulation of oxidative stress resistance in Campylobacter jejuni, a microaerophilic foodborne pathogen. Front. Microbiol. 2015, 6, 751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allos, B.M.; Blaser, M.J. Campylobacter jejuni and the expanding spectrum of related infections. Clin. Infect. Dis. 1995, 20, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Acheson, D.; Allos, B.M. Campylobacter jejuni infections: Update on emerging issues and trends. Clin. Infect. Dis. 2001, 32, 1201–1206. [Google Scholar] [CrossRef]
- Ruiz-Palacios, G.M. The Health Burden of Campylobacter Infection and the Impact of Antimicrobial Resistance: Playing Chicken; The University of Chicago Press: Chicago, IL, USA, 2007; Volume 44, pp. 701–703. [Google Scholar]
- Hermans, D.; Pasmans, F.; Heyndrickx, M.; Van Immerseel, F.; Martel, A.; Van Deun, K.; Haesebrouck, F. A tolerogenic mucosal immune response leads to persistent Campylobacter jejuni colonization in the chicken gut. Cri. Rev. Microbiol. 2012, 38, 17–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newell, D.; Elvers, K.; Dopfer, D.; Hansson, I.; Jones, P.; James, S.; Gittins, J.; Stern, N.; Davies, R.; Connerton, I. Biosecurity-based interventions and strategies to reduce Campylobacter spp. on poultry farms. Appl. Environ. Microbiol. 2011, 77, 8605–8614. [Google Scholar] [CrossRef] [Green Version]
- Sibanda, N.; McKenna, A.; Richmond, A.; Ricke, S.C.; Callaway, T.; Stratakos, A.C.; Gundogdu, O.; Corcionivoschi, N. A review of the effect of management practices on Campylobacter prevalence in poultry farms. Front. Microbiol. 2018, 9, 2002. [Google Scholar] [CrossRef]
- Abbas, S.G.E.; Karmi, M.; Mubarak, A.G.; Youseef, A.G. Prevalence and virulence genes profile of zoonotic Campylobacter species in chickens and human in Aswan governorate. SVU-Int. J. Vet. Sci. 2022, 5, 15–32. [Google Scholar] [CrossRef]
- Abdallah, M.; Abaza, M.A.; Fathy, R.R.; Youseef, A.G.; Sobhy, M.; Abd Elhamid, H.S.; Ahmed, W. Detection of Some Virulence and Antibiotic Resistance Genes in Campylobacter jejuni isolated from Poultry and Human. Egypt. J. Hosp. Med. 2022, 89, 6373–6381. [Google Scholar] [CrossRef]
- Burnham, P.M.; Hendrixson, D.R. Campylobacter jejuni: Collective components promoting a successful enteric lifestyle. Nature Rev. Microbiol. 2018, 16, 551–565. [Google Scholar] [CrossRef]
- Fonseca, B.B.; Fernandez, H.; Rossi, D.A. Campylobacter Spp. and Related Organisms in Poultry; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Al Hakeem, W.G.; Fathima, S.; Shanmugasundaram, R.; Selvaraj, R.K. Campylobacter jejuni in Poultry: Pathogenesis and Control Strategies. Microorganisms 2022, 10, 2134. [Google Scholar] [CrossRef] [PubMed]
- Siringan, P.; Connerton, P.L.; Payne, R.J.; Connerton, I.F. Bacteriophage-mediated dispersal of Campylobacter jejuni biofilms. Appl. Environ. Microbiol. 2011, 77, 3320–3326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehany, T.; Siddiqui, S.A.; Olawoye, B.; Olabisi Popoola, O.; Hassoun, A.; Manzoor, M.F.; Punia Bangar, S. Recent innovations and emerging technological advances used to improve quality and process of plant-based milk analogs. Crit. Rev. Food Sci. Nutr. 2023, 2023, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Gashaw, G.; Fassil, A.; Redi, F. Evaluation of the antibacterial activity of Pleurotus spp. cultivated on different agricultural wastes in Chiro, Ethiopia. Int. J. Microbiol. 2020, 2020, 9312489. [Google Scholar] [CrossRef]
- Mehany, T.; Rashad, Y.M.; Olawoye, B.; Cacciotti, I.; Johnson, E.O.; Popoola, O.O.; Han, Z.; Fekry, W.M. Pigmented Sorghum: Functional Properties and Bioactive Diversity. In Pigmented Cereals and Millets: Bioactive Profile and Food Applications; Royal Society of Chemistry: London, UK, 2023; pp. 109–143. [Google Scholar]
- Chen, W.; Zhang, T.; Ma, Q.; Zhu, Y.; Shen, R. Structure Characterization and Potential Probiotic Effects of Sorghum and Oat Resistant Dextrins. Foods 2022, 11, 1877. [Google Scholar] [CrossRef]
- Navarro, M.; Stanley, R.; Cusack, A.; Sultanbawa, Y. Combinations of plant-derived compounds against Campylobacter in vitro. J. Appl. Poultry Res. 2015, 24, 352–363. [Google Scholar] [CrossRef]
- Tribble, D.R.; Baqar, S.; Carmolli, M.P.; Porter, C.; Pierce, K.K.; Sadigh, K.; Guerry, P.; Larsson, C.J.; Rockabrand, D.; Ventone, C.H. Campylobacter jejuni strain CG8421: A refined model for the study of Campylobacteriosis and evaluation of Campylobacter vaccines in human subjects. Clin. Infect. Dis. 2009, 49, 1512–1519. [Google Scholar] [CrossRef] [Green Version]
- El-Khawas, K.M.; Hendy, B.A.S. Assessment and improvement of hygienic status of chicken fillet from slaughterhouses using organic acids from natural sources. Assiut Vet. Med. J. 2015, 61, 8–17. [Google Scholar]
- Hamad, G.; Amer, A.; Kirrella, G.; Mehany, T.; Elfayoumy, R.A.; Elsabagh, R.; Elghazaly, E.M.; Esatbeyoglu, T.; Taha, A.; Zeitoun, A. Evaluation of the Prevalence of Staphylococcus aureus in Chicken Fillets and Its Bio-Control Using Different Seaweed Extracts. Foods 2023, 12, 20. [Google Scholar] [CrossRef]
- Hamad, G.; Ombarak, R.A.; Eskander, M.; Mehany, T.; Anees, F.R.; Elfayoumy, R.A.; Omar, S.A.; Lorenzo, J.M.; Abou-Alella, S.A.E. Detection and inhibition of Clostridium botulinum in some Egyptian fish products by probiotics cell-free supernatants as bio-preservation agents. LWT 2022, 163, 113603. [Google Scholar] [CrossRef]
- Klančnik, A.; Piskernik, S.; Jeršek, B.; Možina, S.S. Evaluation of diffusion and dilution methods to determine the antibacterial activity of plant extracts. J. Microbiol. Methods 2010, 81, 121–126. [Google Scholar] [CrossRef]
- Solís-Soto, L.; Prabhakarankutty, L.K.; García, S.; Ortíz-Reyes, Y.; Heredia, N. Controlling Campylobacter jejuni in vitro and in chicken using combinations of citrus-based and trisodium phosphate formulations. J. Food Saf. 2021, 41, e12938. [Google Scholar] [CrossRef]
- Eldin, R.M.B.; Talaat, D.; Elbaba, A.H.; Ibrahim, M.S. Antibacterial activity y of some plant extracts on different bacteria in chicken fillet. Eur. J. Pharm. Med. Res. 2020, 7, 84–95. [Google Scholar]
- Hamad, G.M.; Mohdaly, A.A.A.; El-Nogoumy, B.A.; Ramadan, M.F.; Hassan, S.A.; Zeitoun, A.M. Detoxification of aflatoxin B1 and ochratoxin A using Salvia farinacea and Azadirachta indica water extract and application in meat products. Appl. Biochem. Biotechnol. 2021, 193, 3098–3120. [Google Scholar] [CrossRef]
- Catarino, M.D.; Silva, A.M.; Saraiva, S.C.; Sobral, A.J.; Cardoso, S.M. Characterization of phenolic constituents and evaluation of antioxidant properties of leaves and stems of Eriocephalus africanus. Arab. J. Chem. 2018, 11, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Popiołkiewicz, J.; Polkowski, K.; Skierski, J.S.; Mazurek, A.P. In vitro toxicity evaluation in the development of new anticancer drugs—Genistein glycosides. Cancer Lett. 2005, 229, 67–75. [Google Scholar] [CrossRef]
- Salem, A.; Abou El Roos, N.; Nassar, Y. Antimicrobial Effects of some Essential Oils on the Foodborne Pathogen Campylobacter jejuni. Benha Vet. Med. J. 2019, 36, 65–70. [Google Scholar] [CrossRef]
- Morsy, M.K.; Elsabagh, R.; Trinetta, V. Evaluation of novel synergistic antimicrobial activity of nisin, lysozyme, EDTA nanoparticles, and/or ZnO nanoparticles to control foodborne pathogens on minced beef. Food Control. 2018, 92, 249–254. [Google Scholar] [CrossRef]
- Grant, F.S. Fish and Fishery Products Hazards and Controls Guidance, 4th ed.; United States Department of Health and Human Services: Washington, DC, USA, 2011. [Google Scholar]
- Hamad, G.M.; Abdelmotilib, N.M.; Darwish, A.M.; Zeitoun, A.M. Commercial probiotic cell-free supernatants for inhibition of Clostridium perfringens poultry meat infection in Egypt. Anaerobe 2020, 62, 102181. [Google Scholar] [CrossRef]
- Kaakoush, N.O.; Castaño-Rodríguez, N.; Mitchell, H.M.; Man, S.M. Global epidemiology of Campylobacter infection. Clin. Microbiol. Rev. 2015, 28, 687–720. [Google Scholar] [CrossRef] [Green Version]
- Walker, L.J.; Wallace, R.L.; Smith, J.J.; Graham, T.; Saputra, T.; Symes, S.; Stylianopoulos, A.; Polkinghorne, B.G.; Kirk, M.D.; Glass, K. Prevalence of Campylobacter coli and Campylobacter jejuni in retail chicken, beef, lamb, and pork products in three Australian states. J. Food Prot. 2019, 82, 2126–2134. [Google Scholar] [CrossRef] [PubMed]
- Piskernik, S.; Klančnik, A.; Riedel, C.T.; Brøndsted, L.; Možina, S.S. Reduction of Campylobacter jejuni by natural antimicrobials in chicken meat-related conditions. Food Control 2011, 22, 718–724. [Google Scholar] [CrossRef]
- Chen, H.; Xu, Y.; Chen, H.; Liu, H.; Yu, Q.; Han, L. Isolation and identification of polyphenols from fresh sweet sorghum stems and their antibacterial mechanism against foodborne pathogens. Front. Bioeng. Biotechnol. 2022, 9, 770726. [Google Scholar] [CrossRef]
- Garzón, A.G.; Veras, F.F.; Brandelli, A.; Drago, S.R. Purification, identification and in silico studies of antioxidant, antidiabetogenic and antibacterial peptides obtained from sorghum spent grain hydrolysate. LWT 2022, 153, 112414. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Paiva, L.; Lima, E.; Neto, A.I.; Baptista, J. Seasonal variability of the biochemical composition and antioxidant properties of Fucus spiralis at two Azorean Islands. Mar. Drugs 2018, 16, 248. [Google Scholar] [CrossRef] [Green Version]
- Nazarudin, M.; Yasin, I.; Mazli, N.; Saadi, A.; Azizee, M.; Nooraini, M.; Saad, N.; Ferdous, U.; Fakhrulddin, I. Preliminary screening of antioxidant and cytotoxic potential of green seaweed, Halimeda opuntia (Linnaeus) Lamouroux. Saudi J. Biol. Sci. 2022, 29, 2698–2705. [Google Scholar] [CrossRef]
- Pontieri, P.; Pepe, G.; Campiglia, P.; Merciai, F.; Basilicata, M.G.; Smolensky, D.; Calcagnile, M.; Troisi, J.; Romano, R.; Del Giudice, F. Comparison of Content in Phenolic Compounds and Antioxidant Capacity in Grains of White, Red, and Black Sorghum Varieties Grown in the Mediterranean Area. ACS Food Sci. Technol. 2021, 1, 1109–1119. [Google Scholar] [CrossRef]
- De Morais Cardoso, L.; Pinheiro, S.S.; Martino, H.S.D.; Pinheiro-Sant’Ana, H.M. Sorghum (Sorghum bicolor L.): Nutrients, bioactive compounds, and potential impact on human health. Crit. Rev. Food Sci. Nutr. 2017, 57, 372–390. [Google Scholar] [CrossRef]
- Stefoska-Needham, A.; Beck, E.J.; Johnson, S.K.; Tapsell, L.C. Sorghum: An underutilized cereal whole grain with the potential to assist in the prevention of chronic disease. Food Rev. Int. 2015, 31, 401–437. [Google Scholar] [CrossRef] [Green Version]
- Daghir, N.; Diab-El-Harake, M.; Kharroubi, S. Poultry production and its effects on food security in the Middle Eastern and North African region. J. Appl. Poult. Res. 2021, 30, 100110. [Google Scholar] [CrossRef]
- Mohamed, H.I.; Fawzi, E.M.; Basit, A.; Lone, R.; Sofy, M.R. Sorghum: Nutritional Factors, Bioactive Compounds, Pharmaceutical and Application in Food Systems: A Review. Phyton 2022, 91, 1303. [Google Scholar] [CrossRef]
- Huang, J.C.; Zayas, J.F.; Bowers, J.A. Functional properties of sorghum flour as an extender in ground beef patties 1. J. Food Qual. 1999, 22, 51–61. [Google Scholar] [CrossRef]




| Samples | Positive Samples | ||
|---|---|---|---|
| Types of Chicken Fillet | No. of Samples | No. | % |
| Breast | 25 | 20 | 80 |
| Thigh | 25 | 22 | 88 |
| Liver | 25 | 15 | 60 |
| Gizzard | 25 | 18 | 72 |
| Total | 100 | 75 | 75 |
| Sorghum Extracts/Antibiotics | Concentration | Inhibition Zone Diameter (mm) |
|---|---|---|
| White sorghum extracts | 100 mg/mL | 39.1 ± 0.2 a |
| Yellow sorghum extracts | 100 mg/mL | 30.1 ± 0.2 c |
| Red sorghum extracts | 100 mg/mL | ND |
| Gentamicin (GEN) | 30 mg/mL | 35.3 ± 0.1 b |
| Erythromycin (ERY) | 100 mg/mL | 29.1 ± 0.1 cd |
| Amoxicillin (AMX) | 30 mg/mL | 26.4 ± 0.1 e |
| Strains/Extract | White Sorghum Extracts (mg/mL) | |
| Conc. (%) | Inhibition zone (mm) | |
| 100 | 38.7 ± 0.6 a | |
| 50 | 24.8 ± 0.8 b | |
| 25 | 16.1 ± 0.7 c | |
| 12.5 | 11.8 ± 0.8 d | |
| 6.25 | 7.8 ± 0.3 e | |
| 3.12 | ND | |
| Extracts | Total Phenolic Content (mg GAE/g) | Total Flavonoid Content (mg CE/g) |
|---|---|---|
| White sorghum extract | 64.2 ± 0.8 a | 33.9 ± 0.4 a |
| Yellow sorghum extract | 31.6 ± 1.4 b | 21.9 ± 0.4 b |
| Red sorghum extract | 15.5 ± 0.5 c | 7.20 ± 0.3 c |
| Conc. (μg/mL) | Ascorbic Acid | White Sorghum Extract | Yellow Sorghum Extract | Red Sorghum Extract | ||||
|---|---|---|---|---|---|---|---|---|
| Inhibition (%) | IC50 (μg/mL) | Inhibition (%) | IC50 (μg/mL) | Inhibition (%) | IC50 (μg/mL) | Inhibition (%) | IC50 (μg/mL) | |
| 10 | 33.2 (0.0) a | 20.1 | 25.6 (0.0) b | 34.6 | 11.3 (0.0) c | 51.5 | 5.2 (0.0) d | 65.8 |
| 20 | 49.8 (0.0) a | 34.6 (0.0) b | 19.2 (0.0) c | 12.5 (0.0) d | ||||
| 30 | 78.4 (0.0) a | 43.3 (0.0) b | 27.3(0.0) c | 18.6(0.0) d | ||||
| 40 | 84.5 (0.0) a | 51.4 (0.0) b | 36.3 (0.0) c | 28.2 (0.0) d | ||||
| 50 | 90.7 (0.0) a | 63.6 (0.0) b | 48.5 (0.0) c | 35.3 (0.0) d | ||||
| 60 | 93.3 (0.0) a | 75.2 (0.0) b | 56.9 (0.0) c | 45.6 (0.0) d | ||||
| 70 | 95.3 (0.0) a | 92.5 (0.0) b | 71.2 (0.0) c | 55.5 (0.0) d | ||||
| 80 | 97.4 (0.0) a | 95.2 (0.0) b | 86.4 (0.0) c | 69.2 (0.0) d | ||||
| 90 | 98.4 (0.0) a | 97.3 (0.0) ab | 95.7 (0.0) c | 83.4 (0.0) d | ||||
| 100 | 99.2 (0.0) a | 98.8 (0.0) ab | 97.6 (0.0) c | 89.2 (0.0) d | ||||
| Concentration (µg/mL) | Inhibition % | Viability % |
|---|---|---|
| 10,000 | 100 | 0 |
| 5000 | 100 | 0 |
| 2500 | 97 | 3 |
| 1250 | 88 | 12 |
| 625 | 69 | 31 |
| 312 | 53 | 47 |
| 156 | 43 | 57 |
| 78 | 32 | 68 |
| 39 | 28 | 72 |
| 19.5 | 16 | 84 |
| IC50 = 482.4 |
| Storage (Days) | Negative Control (1) | Negative Control (2) | Positive Control | Treatment (2%) | Treatment (4%) | Treatment (6%) |
|---|---|---|---|---|---|---|
| 0 | 0.00 | 0.00 | 1 × 107 | 1 × 107 (0.0) | 1 × 107 (0.1) | 1 × 107 (0.1) |
| 2nd | 0.00 | 0.00 | 1 × 107 | 1.81 × 106 (0.0) | 6.21 × 105 (0.0) | 4.1 × 104 (0.1) |
| 4th | 0.00 | 0.00 | 1 × 107 | 7.21 × 105 (0.0) | 2.51 × 104 (0.0) | 2.1 × 102 (0.0) |
| 6th | 0.00 | 0.00 | 1 × 107 | 3.21 × 104 (0.0) | 1.4 × 102 (0.0) | 0.00 (0.0) |
| 8th | 0.00 | 0.00 | 1 × 107 | 1.8 × 106 (0.0) | 0.00 (0.0) | 0.00 (0.0) |
| 10th | 0.00 | 0.00 | 1 × 107 | 0.00 (0.0) | 0.00 (0.0) | 0.00 (0.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamad, G.M.; Gerges, M.; Mehany, T.; Hussein, S.M.; Eskander, M.; Tawfik, R.G.; El-Halmouch, Y.; Mansour, A.M.; Hafez, E.E.; Esatbeyoglu, T.; et al. Estimating the Prevalence of Foodborne Pathogen Campylobacter jejuni in Chicken and Its Control via Sorghum Extracts. Pathogens 2023, 12, 958. https://doi.org/10.3390/pathogens12070958
Hamad GM, Gerges M, Mehany T, Hussein SM, Eskander M, Tawfik RG, El-Halmouch Y, Mansour AM, Hafez EE, Esatbeyoglu T, et al. Estimating the Prevalence of Foodborne Pathogen Campylobacter jejuni in Chicken and Its Control via Sorghum Extracts. Pathogens. 2023; 12(7):958. https://doi.org/10.3390/pathogens12070958
Chicago/Turabian StyleHamad, Gamal M., Mariam Gerges, Taha Mehany, Saleh M. Hussein, Michael Eskander, Rasha G. Tawfik, Yasser El-Halmouch, Alaa M. Mansour, Elsayed E. Hafez, Tuba Esatbeyoglu, and et al. 2023. "Estimating the Prevalence of Foodborne Pathogen Campylobacter jejuni in Chicken and Its Control via Sorghum Extracts" Pathogens 12, no. 7: 958. https://doi.org/10.3390/pathogens12070958
APA StyleHamad, G. M., Gerges, M., Mehany, T., Hussein, S. M., Eskander, M., Tawfik, R. G., El-Halmouch, Y., Mansour, A. M., Hafez, E. E., Esatbeyoglu, T., & Elghazaly, E. M. (2023). Estimating the Prevalence of Foodborne Pathogen Campylobacter jejuni in Chicken and Its Control via Sorghum Extracts. Pathogens, 12(7), 958. https://doi.org/10.3390/pathogens12070958