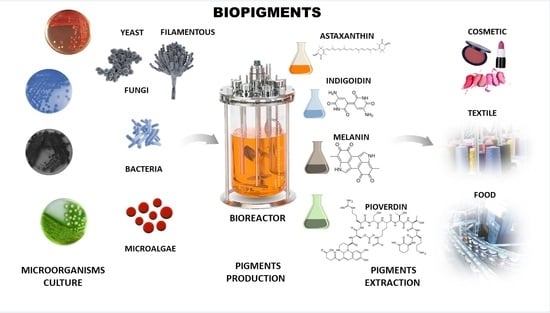
Microbial Pigments: Major Groups and Industrial Applications
Abstract
:1. Introduction
2. Pigments and Dyes
3. Synthetic and Natural Pigments
4. Microbial Pigments: Major Chemical Groups and Functions
4.1. Isoprenoids Pigments
4.2. Flavins Pigments
4.3. Tetrapyrrole-Containing Pigments
4.4. Alkaloid Pigments
4.4.1. Prodigiosines and Tamjamines
4.4.2. Betalains
4.4.3. Other Alkaloid Pigments
4.5. Polyketide Pigments
4.5.1. Quinones
4.5.2. Azaphilones
4.6. Phenol-Containing-Pigments
4.7. Melanins
5. Challenges of the Industrial Application
5.1. Converging Frontiers: Exploring the Synergy of Multiomic Integration, Synthetic Biology, Artificial Intelligence, and Metabolic Engineering
5.2. Fermentation Process
5.3. Pigment Extraction
5.4. Micro and Nanoencapsulation
6. Major Biotechnological Applications
6.1. Pharmaceutical and Medicine
6.2. Cosmetics
6.3. Food Industry
6.4. Textile Industry
7. Pigment Market
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yadav, S.; Tiwari, K.S.; Gupta, C.; Tiwari, M.K.; Khan, A.; Sonkar, S.P. A Brief Review on Natural Dyes, Pigments: Recent Advances and Future Perspectives. Results Chem. 2023, 5, 100733. [Google Scholar] [CrossRef]
- Kumar, A.; Vishwakarma, H.S.; Singh, S.; Kumar, M. Microbial pigments: Production and their applications in various industries. Int. J. Pharm. Chem. Sci. 2015, 5, 203–212. [Google Scholar]
- Sen, T.; Barrow, C.J.; Deshmukh, S.K. Microbial Pigments in the Food Industry—Challenges and the Way Forward. Front. Nutr. 2019, 6, 7. [Google Scholar] [CrossRef]
- Agarwal, H.; Bajpai, S.; Mishra, A.; Kohli, I.; Varma, A.; Fouillaud, M.; Dufossé, L.; Joshi, N.C. Bacterial Pigments and Their Multifaceted Roles in Contemporary Biotechnology and Pharmacological Applications. Microorganisms 2023, 11, 614. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Li, Y.; Lawson, D.; Xie, D.-Y. Metabolic Engineering of Anthocyanins in Dark Tobacco Varieties. Physiol. Plant. 2017, 159, 2–12. [Google Scholar] [CrossRef]
- Cuthill, I.C.; Allen, W.L.; Arbuckle, K.; Caspers, B.; Chaplin, G.; Hauber, M.E.; Hill, G.E.; Jablonski, N.G.; Jiggins, C.D.; Kelber, A.; et al. The Biology of Color. Science 2017, 357, eaan0221. [Google Scholar] [CrossRef]
- Walter, M.H.; Strack, D. Carotenoids and Their Cleavage Products: Biosynthesis and Functions. Nat. Prod. Rep. 2011, 28, 663. [Google Scholar] [CrossRef]
- Gahlawat, G.; Choudhury, A.R. A Review on the Biosynthesis of Metal and Metal Salt Nanoparticles by Microbes. RSC Adv. 2019, 9, 12944–12967. [Google Scholar] [CrossRef]
- Palacio-Barrera, A.M.; Areiza, D.; Zapata, P.; Atehortúa, L.; Correa, C.; Peñuela-Vásquez, M. Induction of Pigment Production through Media Composition, Abiotic and Biotic Factors in Two Filamentous Fungi. Biotechnol. Rep. 2019, 21, e00308. [Google Scholar] [CrossRef]
- Lincke, G. Molecular Stacks as a Common Characteristic in the Crystal Lattice of Organic Pigment Dyes A Contribution to the “Soluble–Insoluble” Dichotomy of Dyes and Pigments from the Technological Point of View. Dyes Pigments 2003, 59, 1–24. [Google Scholar] [CrossRef]
- Rothon, R. Pigment and Nanopigment Dispersion Technologies. In Pigment and Nanopigment Dispersion Technologies; iSmithers Rapra Publishing: Shrewsbury, UK, 2012. [Google Scholar]
- Dasgupta Mandal, D.; Majumdar, S. Bacteria as Biofactory of Pigments: Evolution beyond Therapeutics and Biotechnological Advancements. J. Biosci. Bioeng. 2023, 135, 349–358. [Google Scholar] [CrossRef]
- Arora, K.; Ameur, H.; Polo, A.; Di Cagno, R.; Rizzello, C.G.; Gobbetti, M. Thirty Years of Knowledge on Sourdough Fermentation: A Systematic Review. Trends Food Sci. Technol. 2021, 108, 71–83. [Google Scholar] [CrossRef]
- De Souza Mesquita, L.M.; Martins, M.; Pisani, L.P.; Ventura, S.P.M.; De Rosso, V.V. Insights on the Use of Alternative Solvents and Technologies to Recover Bio-based Food Pigments. Comp. Rev. Food Sci. Food Saf. 2021, 20, 787–818. [Google Scholar] [CrossRef]
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic Organic Dyes as Contaminants of the Aquatic Environment and Their Implications for Ecosystems: A Review. Sci. Total Environ. 2020, 717, 137222. [Google Scholar] [CrossRef] [PubMed]
- Kant, R. Textile Dyeing Industry an Environmental Hazard. Nat. Sci. 2012, 4, 22–26. [Google Scholar] [CrossRef]
- Oginawati, K.; Susetyo, S.H.; Sulung, G.; Chazanah, N.; Kusumah, S.W.D.; Fahimah, N. Investigation of Dermal Exposure to Heavy Metals (Cu, Zn, Ni, Al, Fe and Pb) in Traditional Batik Industry Workers. Heliyon 2022, 8, e08914. [Google Scholar] [CrossRef] [PubMed]
- Coccato, A.; Moens, L.; Vandenabeele, P. On the Stability of Mediaeval Inorganic Pigments: A Literature Review of the Effect of Climate, Material Selection, Biological Activity, Analysis and Conservation Treatments. Herit. Sci. 2017, 5, 12. [Google Scholar] [CrossRef]
- Anzum, N.; Khan, F.I.; Hossain, M.Z.; Islam, M.N.; Saha, M.L. Isolation and Identification of Pigment Producing Bacteria from the Ratargul Swamp Forest Soil. Dhaka Univ. J. Biol. Sci 2022, 31, 1–8. [Google Scholar] [CrossRef]
- Jones, O.; Selinger, B. The Chemistry of Cosmetics; Australian Academic of Science: Canberra, Australia, 2019. [Google Scholar]
- Veuthey, T. Dyes and Stains: From Molecular Structure to Histological Application. Front. Biosci. 2014, 19, 91. [Google Scholar] [CrossRef] [PubMed]
- Gondal, M.A.; Seddigi, Z.S.; Nasr, M.M.; Gondal, B. Spectroscopic Detection of Health Hazardous Contaminants in Lipstick Using Laser Induced Breakdown Spectroscopy. J. Hazard. Mater. 2010, 175, 726–732. [Google Scholar] [CrossRef]
- Elsahida, K.; Fauzi, A.M.; Sailah, I.; Siregar, I.Z. Sustainability of the Use of Natural Dyes in the Textile Industry. IOP Conf. Ser. Earth Environ. Sci. 2019, 399, 012065. [Google Scholar] [CrossRef]
- Michalek, I.M.; Benn, E.K.T.; Dos Santos, F.L.C.; Gordon, S.; Wen, C.; Liu, B. A Systematic Review of Global Legal Regulations on the Permissible Level of Heavy Metals in Cosmetics with Particular Emphasis on Skin Lightening Products. Environ. Res. 2019, 170, 187–193. [Google Scholar] [CrossRef]
- Mantri, S.; Dondapati, M.; Ramakrishna, K.; Audipudi, A.V.; Srinath, B.S. Production, Characterization, and Applications of Bacterial Pigments- a Decade of Review. Biomedicine 2022, 42, 434–440. [Google Scholar] [CrossRef]
- Lyrio, E.S.; Ferreira, G.G.; Zuqui, S.N.; Silva, A.G. Plant Resources in Biocosmetic: A New Concept on Beauty, Health, and Sustainability. ESFA 2011, 9, 47–51. [Google Scholar]
- Doulati Ardejani, F.; Badii, K.; Limaee, N.Y.; Shafaei, S.Z.; Mirhabibi, A.R. Adsorption of Direct Red 80 Dye from Aqueous Solution onto Almond Shells: Effect of pH, Initial Concentration and Shell Type. J. Hazard. Mater. 2008, 151, 730–737. [Google Scholar] [CrossRef]
- Bom, S.; Jorge, J.; Ribeiro, H.M.; Marto, J. A Step Forward on Sustainability in the Cosmetics Industry: A Review. J. Clean. Prod. 2019, 225, 270–290. [Google Scholar] [CrossRef]
- Dikshit, R.; Tallapragada, P. Comparative Study of Natural and Artificial Flavoring Agents and Dyes. In Natural and Artificial Flavoring Agents and Food Dyes; Elsevier: Amsterdam, The Netherlands, 2018; pp. 83–111. [Google Scholar]
- Bisbis, M.B.; Gruda, N.; Blanke, M. Potential Impacts of Climate Change on Vegetable Production and Product Quality—A Review. J. Clean. Prod. 2018, 170, 1602–1620. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Salinity Stress Enhances Color Parameters, Bioactive Leaf Pigments, Vitamins, Polyphenols, Flavonoids and Antioxidant Activity in Selected Amaranthus Leafy Vegetables. J. Sci. Food Agric. 2019, 99, 2275–2284. [Google Scholar] [CrossRef]
- Sonar, C.R.; Rasco, B.; Tang, J.; Sablani, S.S. Natural Color Pigments: Oxidative Stability and Degradation Kinetics during Storage in Thermally Pasteurized Vegetable Purees. J. Sci. Food Agric. 2019, 99, 5934–5945. [Google Scholar] [CrossRef]
- Yusuf, M.; Shabbir, M.; Mohammad, F. Natural Colorants: Historical, Processing and Sustainable Prospects. Nat. Prod. Bioprospecting 2017, 7, 123–145. [Google Scholar] [CrossRef]
- Shamim, G.; Ranjan, S.K.; Pandey, D.M.; Ramani, R. Biochemistry and Biosynthesis of Insect Pigments. Eur. J. Entomol. 2014, 111, 149–164. [Google Scholar] [CrossRef]
- Qin, Z.; Wang, X.; Gao, S.; Li, D.; Zhou, J. Production of Natural Pigments Using Microorganisms. J. Agric. Food Chem. 2023, 71, 9243–9254. [Google Scholar] [CrossRef] [PubMed]
- Müller-Maatsch, J.; Gras, C. The “Carmine Problem” and Potential Alternatives. In Handbook on Natural Pigments in Food and Beverages; Elsevier: Amsterdam, The Netherlands, 2016; pp. 385–428. [Google Scholar]
- Dave, S.; Das, J.; Varshney, B.; Sharma, V.P. Dyes and Pigments: Interventions and How Safe and Sustainable Are Colors of Life!!! In Trends and Contemporary Technologies for Photocatalytic Degradation of Dyes; Dave, S., Das, J., Eds.; Environmental Science and Engineering; Springer International Publishing: Cham, Switerland, 2022; pp. 1–20. [Google Scholar]
- Kumar, S.; Kumar, V.; Ambika, A.A.A.; Nag, D.; Kumar, V.; Darnal, S.; Thakur, V.; Patial, V.; Singh, D. Microbial Pigments: Learning from Himalayan Perspective to Industrial Applications. J. Ind. Microbiol. Biotechnol. 2022, 49, kuac017. [Google Scholar] [CrossRef] [PubMed]
- Rather, L.J.; Mir, S.S.; Ganie, S.A.; Shahid-ul-Islam; Li, Q. Research Progress, Challenges, and Perspectives in Microbial Pigment Production for Industrial Applications—A Review. Dyes Pigments 2023, 210, 110989. [Google Scholar] [CrossRef]
- Vermelho, A.B.; Noronha, E.F.; Filho, E.X.F.; Ferrara, M.A.; Bon, E.P.S. Diversity and Biotechnological Applications of Prokaryotic Enzymes. In The Prokaryotes; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 213–240. [Google Scholar]
- Scarano, P.; Naviglio, D.; Prigioniero, A.; Tartaglia, M.; Postiglione, A.; Sciarrillo, R.; Guarino, C. Sustainability: Obtaining Natural Dyes from Waste Matrices Using the Prickly Pear Peels of Opuntia ficus-indica (L.) Miller. Agronomy 2020, 10, 528. [Google Scholar] [CrossRef]
- Lopes, F.C.; Ligabue-Braun, R. Agro-Industrial Residues: Eco-Friendly and Inexpensive Substrates for Microbial Pigments Production. Front. Sustain. Food Syst. 2021, 5, 589414. [Google Scholar] [CrossRef]
- Lyu, X.; Lyu, Y.; Yu, H.; Chen, W.; Ye, L.; Yang, R. Biotechnological Advances for Improving Natural Pigment Production: A State-of-the-Art Review. Bioresour. Bioprocess. 2022, 9, 8. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Q.; Sun, T.; Zhu, X.; Xu, H.; Tang, J.; Zhang, X.; Ma, Y. Engineering Central Metabolic Modules of Escherichia Coli for Improving β-Carotene Production. Metab. Eng. 2013, 17, 42–50. [Google Scholar] [CrossRef]
- Tuli, H.S.; Chaudhary, P.; Beniwal, V.; Sharma, A.K. Microbial Pigments as Natural Color Sources: Current Trends and Future Perspectives. J. Food Sci. Technol. 2015, 52, 4669–4678. [Google Scholar] [CrossRef]
- Kaur, P.; Singh, S.; Ghoshal, G.; Ramamurthy, P.C.; Parihar, P.; Singh, J.; Singh, A. Valorization of Agri-Food Industry Waste for the Production of Microbial Pigments: An Eco-Friendly Approach. In Advances in Agricultural and Industrial Microbiology; Nayak, S.K., Baliyarsingh, B., Mannazzu, I., Singh, A., Mishra, B.B., Eds.; Springer Nature: Singapore, 2022; pp. 137–167. [Google Scholar]
- Panesar, R.; Kaur, S.; Panesar, P.S. Production of Microbial Pigments Utilizing Agro-Industrial Waste: A Review. Curr. Opin. Food Sci. 2015, 1, 70–76. [Google Scholar] [CrossRef]
- Sinha, S.; Choubey, S.; Kumar, A.; Bhosale, P. Identification, Characterization of Pigment Producing Bacteria from Soil and Water and Testing of Antimicrobial Activity of Bacterial Pigments. Int. J. Pharm. Sci. Rev. Res. 2017, 42, 119–124. [Google Scholar]
- Aruldass, C.A.; Dufossé, L.; Ahmad, W.A. Current Perspective of Yellowish-Orange Pigments from Microorganisms—A Review. J. Clean. Prod. 2018, 180, 168–182. [Google Scholar] [CrossRef]
- Jeong, S.W.; Yang, J.E.; Choi, Y.J. Isolation and Characterization of a Yellow Xanthophyll Pigment-Producing Marine Bacterium, Erythrobacter Sp. SDW2 Strain, in Coastal Seawater. Mar. Drugs 2022, 20, 73. [Google Scholar] [CrossRef]
- Ramesh, C.; Vinithkumar, N.V.; Kirubagaran, R.; Venil, C.K.; Dufossé, L. Multifaceted Applications of Microbial Pigments: Current Knowledge, Challenges and Future Directions for Public Health Implications. Microorganisms 2019, 7, 186. [Google Scholar] [CrossRef]
- Reis-Mansur, M.C.P.P.; Cardoso-Rurr, J.S.; Silva, J.V.M.A.; De Souza, G.R.; Cardoso, V.D.S.; Mansoldo, F.R.P.; Pinheiro, Y.; Schultz, J.; Lopez Balottin, L.B.; Da Silva, A.J.R.; et al. Carotenoids from UV-Resistant Antarctic Microbacterium sp. LEMMJ01. Sci. Rep. 2019, 9, 9554. [Google Scholar] [CrossRef] [PubMed]
- Sankari, M.; Rao, P.R.; Hemachandran, H.; Pullela, P.K.; Doss, C.G.P.; Tayubi, I.A.; Subramanian, B.; Gothandam, K.; Singh, P.; Ramamoorthy, S. Prospects and Progress in the Production of Valuable Carotenoids: Insights from Metabolic Engineering, Synthetic Biology, and Computational Approaches. J. Biotechnol. 2018, 266, 89–101. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids as Natural Functional Pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef]
- Liang, M.-H.; Zhu, J.; Jiang, J.-G. Carotenoids Biosynthesis and Cleavage Related Genes from Bacteria to Plants. Crit. Rev. Food Sci. Nutr. 2018, 58, 2314–2333. [Google Scholar] [CrossRef] [PubMed]
- Shumskaya, M.; Wurtzel, E.T. The Carotenoid Biosynthetic Pathway: Thinking in All Dimensions. Plant Sci. 2013, 208, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Mata-Gómez, L.C.; Montañez, J.C.; Méndez-Zavala, A.; Aguilar, C.N. Biotechnological Production of Carotenoids by Yeasts: An Overview. Microb. Cell Factories 2014, 13, 12. [Google Scholar] [CrossRef]
- Meddeb-Mouelhi, F.; Moisan, J.K.; Bergeron, J.; Daoust, B.; Beauregard, M. Structural Characterization of a Novel Antioxidant Pigment Produced by a Photochromogenic Microbacterium Oxydans Strain. Appl. Biochem. Biotechnol. 2016, 180, 1286–1300. [Google Scholar] [CrossRef]
- Ghilardi, C.; Sanmartin Negrete, P.; Carelli, A.A.; Borroni, V. Evaluation of Olive Mill Waste as Substrate for Carotenoid Production by Rhodotorula Mucilaginosa. Bioresour. Bioprocess. 2020, 7, 52. [Google Scholar] [CrossRef]
- Mussagy, C.U.; Gonzalez-Miquel, M.; Santos-Ebinuma, V.C.; Pereira, J.F.B. Microbial Torularhodin—A Comprehensive Review. Crit. Rev. Biotechnol. 2023, 43, 540–558. [Google Scholar] [CrossRef] [PubMed]
- De Maayer, P.; Anderson, D.; Cary, C.; Cowan, D.A. Some like It Cold: Understanding the Survival Strategies of Psychrophiles. EMBO Rep. 2014, 15, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, C.; Rizzo, C.; Lo Giudice, A.; Saggiomo, M. Physiological and Molecular Responses to Main Environmental Stressors of Microalgae and Bacteria in Polar Marine Environments. Microorganisms 2020, 8, 1957. [Google Scholar] [CrossRef] [PubMed]
- Asker, D.; Awad, T.S.; Beppu, T.; Ueda, K. Isolation, Characterization, and Diversity of Novel Radiotolerant Carotenoid-Producing Bacteria. In Microbial Carotenoids from Bacteria and Microalgae; Barredo, J.-L., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; Volume 892, pp. 21–60. [Google Scholar]
- Nupur, L.N.U.; Vats, A.; Dhanda, S.K.; Raghava, G.P.S.; Pinnaka, A.K.; Kumar, A. ProCarDB: A Database of Bacterial Carotenoids. BMC Microbiol. 2016, 16, 96. [Google Scholar] [CrossRef] [PubMed]
- Gabani, P.; Singh, O.V. Radiation-Resistant Extremophiles and Their Potential in Biotechnology and Therapeutics. Appl. Microbiol. Biotechnol. 2013, 97, 993–1004. [Google Scholar] [CrossRef]
- Yoo, A.Y.; Alnaeeli, M.; Park, J.K. Production Control and Characterization of Antibacterial Carotenoids from the Yeast Rhodotorula Mucilaginosa AY-01. Process. Biochem. 2016, 51, 463–473. [Google Scholar] [CrossRef]
- Abbas, C.A.; Sibirny, A.A. Genetic Control of Biosynthesis and Transport of Riboflavin and Flavin Nucleotides and Construction of Robust Biotechnological Producers. Microbiol. Mol. Biol. Rev. 2011, 75, 321–360. [Google Scholar] [CrossRef]
- Liu, S.; Hu, W.; Wang, Z.; Chen, T. Production of Riboflavin and Related Cofactors by Biotechnological Processes. Microb. Cell Factories 2020, 19, 31. [Google Scholar] [CrossRef]
- Bacher, A.; Eberhardt, S.; Fischer, M.; Kis, K.; Richter, G. Biosynthesis of Vitamin B2 (Riboflavin). Annu. Rev. Nutr. 2000, 20, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Philmus, B.; Decamps, L.; Berteau, O.; Begley, T.P. Biosynthetic Versatility and Coordinated Action of 5′-Deoxyadenosyl Radicals in Deazaflavin Biosynthesis. J. Am. Chem. Soc. 2015, 137, 5406–5413. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.M. Structure and General Properties of Flavins. In Flavins and Flavoproteins; Weber, S., Schleicher, E., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2014; Volume 1146, pp. 3–13. [Google Scholar]
- Behera, H.T.; Mojumdar, A.; Nivedita, S.; Ray, L. Microbial Pigments: Secondary Metabolites with Multifaceted Roles. In Microbial Polymers; Vaishnav, A., Choudhary, D.K., Eds.; Springer: Singapore, 2021; pp. 631–654. [Google Scholar]
- Busch, A.W.U.; Montgomery, B.L. Interdependence of Tetrapyrrole Metabolism, the Generation of Oxidative Stress and the Mitigative Oxidative Stress Response. Redox Biol. 2015, 4, 260–271. [Google Scholar] [CrossRef]
- Battersby, A.R. Tetrapyrroles: The Pigments of Life. Nat. Prod. Rep. 2000, 17, 507–526. [Google Scholar] [CrossRef] [PubMed]
- Ptaszek, M. Rational Design of Fluorophores for In Vivo Applications. In Progress in Molecular Biology and Translational Science; Elsevier Inc.: Amsterdam, The Netherlands, 2013; Volume 113, pp. 59–108. [Google Scholar]
- Shepherd, M.; Medlock, A.E.; Dailey, H.A. Porphyrin Metabolism. In Encyclopedia of Biological Chemistry; Elsevier: Amsterdam, The Netherlands, 2013; Volume 3, pp. 544–549. [Google Scholar]
- Kang, Z.; Wang, Y.; Wang, Q.; Qi, Q. Metabolic Engineering to Improve 5-Aminolevulinic Acid Production. Bioeng. Bugs 2011, 2, 342–345. [Google Scholar] [CrossRef]
- Hörtensteiner, S. Chlorophyll Degradation during Senescence. Annu. Rev. Plant Biol. 2006, 57, 55–77. [Google Scholar] [CrossRef]
- Chen, M.; Schliep, M.; Willows, R.D.; Cai, Z.-L.; Neilan, B.A.; Scheer, H. A Red-Shifted Chlorophyll. Science 2010, 329, 1318–1319. [Google Scholar] [CrossRef]
- Scheer, H. An Overview of Chlorophylls and Bacteriochlorophylls: Biochemistry, Biophysics, Functions and Applications. In Chlorophylls and Bacteriochlorophylls; Springer: Dordrecht, The Netherlands, 2007; pp. 1–26. [Google Scholar]
- Senge, M.O.; Smith, K.M. Biosynthesis and Structures of the Bacteriochlorophylls. In Anoxygenic Photosynthetic Bacteria; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2006; pp. 137–151. [Google Scholar]
- Li, W.; Su, H.-N.; Pu, Y.; Chen, J.; Liu, L.-N.; Liu, Q.; Qin, S. Phycobiliproteins: Molecular Structure, Production, Applications, and Prospects. Biotechnol. Adv. 2019, 37, 340–353. [Google Scholar] [CrossRef]
- Manirafasha, E.; Ndikubwimana, T.; Zeng, X.; Lu, Y.; Jing, K. Phycobiliprotein: Potential Microalgae Derived Pharmaceutical and Biological Reagent. Biochem. Eng. J. 2016, 109, 282–296. [Google Scholar] [CrossRef]
- Grossman, A.R.; Schaefer, M.R.; Chiang, G.G.; Collier, J.L. The Phycobilisome, a Light-Harvesting Complex Responsive to Environmental Conditions. Microbiol. Rev. 1993, 57, 725–749. [Google Scholar] [CrossRef]
- Tounsi, L.; Ben Hlima, H.; Hentati, F.; Hentati, O.; Derbel, H.; Michaud, P.; Abdelkafi, S. Microalgae: A Promising Source of Bioactive Phycobiliproteins. Mar. Drugs 2023, 21, 440. [Google Scholar] [CrossRef]
- Stadnichuk, I.N.; Krasilnikov, P.M.; Zlenko, D.V. Cyanobacterial Phycobilisomes and Phycobiliproteins. Microbiology 2015, 84, 101–111. [Google Scholar] [CrossRef]
- Hughes, R.-A.; Zhang, Y.; Zhang, R.; Williams, P.G.; Lindsey, J.S.; Miller, E.S. Genome Sequence and Composition of a Tolyporphin-Producing Cyanobacterium-Microbial Community. Appl. Environ. Microbiol. 2017, 83, e01068-17. [Google Scholar] [CrossRef] [PubMed]
- Dewick, P.M. Medicinal Natural Products; John Wiley & Sons, Ltd.: Chichester, UK, 2009. [Google Scholar]
- Funayama, S.; Cordell, J.A. Alkaloids Derived from Polyketides. In Alkaloids; Elsevier: Amsterdam, The Netherlands, 2015; pp. 257–261. [Google Scholar]
- Williamson, N.R.; Fineran, P.C.; Leeper, F.J.; Salmond, G.P.C. The Biosynthesis and Regulation of Bacterial Prodiginines. Nat. Rev. Microbiol. 2006, 4, 887–899. [Google Scholar] [CrossRef] [PubMed]
- Darshan, N.; Manonmani, H.K. Prodigiosin and Its Potential Applications. J. Food Sci. Technol. 2015, 52, 5393–5407. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Irace, C.; Costagliola, F.; Castelluccio, F.; Villani, G.; Calado, G.; Padula, V.; Cimino, G.; Lucas Cervera, J.; Santamaria, R.; et al. A New Cytotoxic Tambjamine Alkaloid from the Azorean Nudibranch Tambja ceutae. Bioorganic Med. Chem. Lett. 2010, 20, 2668–2670. [Google Scholar] [CrossRef] [PubMed]
- Picott, K.J.; Deichert, J.A.; DeKemp, E.M.; Snieckus, V.; Ross, A.C. Purification and Kinetic Characterization of the Essential Condensation Enzymes Involved in Prodiginine and Tambjamine Biosynthesis. Chembiochem A Eur. J. Chem. Biol. 2020, 21, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Sakai-Kawada, F.E.; Ip, C.G.; Hagiwara, K.A.; Awaya, J.D. Biosynthesis and Bioactivity of Prodiginine Analogs in Marine Bacteria, Pseudoalteromonas: A Mini Review. Front. Microbiol. 2019, 10, 1715. [Google Scholar] [CrossRef] [PubMed]
- Williamson, N.R.; Chawrai, S.; Leeper, F.J.; Salmond, G.P.C. Prodiginines and Their Potential Utility as Proapoptotic Anticancer Agents. In Emerging Cancer Therapy; Wiley: Hoboken, NJ, USA, 2010; pp. 333–366. [Google Scholar]
- Han, R.; Xiang, R.; Li, J.; Wang, F.; Wang, C. High-level Production of Microbial Prodigiosin: A Review. J. Basic Microbiol. 2021, 61, 506–523. [Google Scholar] [CrossRef]
- Kurbanoglu, E.B.; Ozdal, M.; Ozdal, O.G.; Algur, O.F. Enhanced Production of Prodigiosin by Serratia Marcescens MO-1 Using Ram Horn Peptone. Braz. J. Microbiol. 2015, 46, 631–637. [Google Scholar] [CrossRef]
- Islan, G.A.; Rodenak-Kladniew, B.; Noacco, N.; Duran, N.; Castro, G.R. Prodigiosin: A Promising Biomolecule with Many Potential Biomedical Applications. Bioengineered 2022, 13, 14227–14258. [Google Scholar] [CrossRef] [PubMed]
- Esatbeyoglu, T.; Wagner, A.E.; Schini-Kerth, V.B.; Rimbach, G. Betanin-A Food Colorant with Biological Activity. Mol. Nutr. Food Res. 2015, 59, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Azeredo, H.M.C. Betalains: Properties, Sources, Applications, and Stability—A Review. Int. J. Food Sci. Technol. 2009, 44, 2365–2376. [Google Scholar] [CrossRef]
- Polturak, G.; Aharoni, A. “La Vie En Rose”: Biosynthesis, Sources, and Applications of Betalain Pigments. Mol. Plant 2018, 11, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Gandía-Herrero, F.; García-Carmona, F. Escherichia coli Protein YgiD Produces the Structural Unit of Plant Pigments Betalains: Characterization of a Prokaryotic Enzyme with DOPA-Extradiol-Dioxygenase Activity. Appl. Microbiol. Biotechnol. 2014, 98, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Strack, D.; Vogt, T.; Schliemann, W. Recent Advances in Betalain Research. ChemInform 2003, 34, 247–269. [Google Scholar] [CrossRef]
- Velíšek, J.; Cejpek, K. Pigments of Higher Fungi—A Review. Czech J. Food Sci. 2011, 29, 87–102. [Google Scholar] [CrossRef]
- Khan, M.I.; Giridhar, P. Plant Betalains: Chemistry and Biochemistry. Phytochemistry 2015, 117, 267–295. [Google Scholar] [CrossRef]
- Grewal, P.S.; Modavi, C.; Russ, Z.N.; Harris, N.C.; Dueber, J.E. Bioproduction of a Betalain Color Palette in Saccharomyces Cerevisiae. Metab. Eng. 2018, 45, 180–188. [Google Scholar] [CrossRef]
- Soliev, A.B.; Hosokawa, K.; Enomoto, K. Bioactive Pigments from Marine Bacteria: Applications and Physiological Roles. Evid. Based Complement. Altern. Med. 2011, 2011, 670349. [Google Scholar] [CrossRef]
- Pierson, L.S.; Pierson, E.A. Metabolism and Function of Phenazines in Bacteria: Impacts on the Behavior of Bacteria in the Environment and Biotechnological Processes. Appl. Microbiol. Biotechnol. 2010, 86, 1659–1670. [Google Scholar] [CrossRef]
- Mentel, M.; Ahuja, E.G.; Mavrodi, D.V.; Breinbauer, R.; Thomashow, L.S.; Blankenfeldt, W. Of Two Make One: The Biosynthesis of Phenazines. ChemBioChem 2009, 10, 2295–2304. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, C.; Braña, A.F.; Méndez, C.; Salas, J.A. Reevaluation of the Violacein Biosynthetic Pathway and Its Relationship to Indolocarbazole Biosynthesis. ChemBioChem 2006, 7, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Yoon, K.; Lee, J.I.; Mitchell, R.J. Violacein: Properties and Production of a Versatile Bacterial Pigment. BioMed Res. Int. 2015, 2015, 465056. [Google Scholar] [CrossRef]
- Gayen, A.K.; Nichols, L.; Williams, G.J. An Artificial Pathway for Polyketide Biosynthesis. Nat. Catal 2020, 3, 536–538. [Google Scholar] [CrossRef]
- Dufossé, L. Microbial Production of Food Grade Pigments. Food Technol. Biotechnol. 2006, 44, 313–321. [Google Scholar]
- Sebak, M.; Molham, F.; Greco, C.; Tammam, M.A.; Sobeh, M.; El-Demerdash, A. Chemical Diversity, Medicinal Potentialities, Biosynthesis, and Pharmacokinetics of Anthraquinones and Their Congeners Derived from Marine Fungi: A Comprehensive Update. RSC Adv. 2022, 12, 24887–24921. [Google Scholar] [CrossRef] [PubMed]
- Medentsev, A.G.; Arinbasarova, A.Y.; Akimenko, V.K. Biosynthesis of Naphthoquinone Pigments by Fungi of the Genus Fusarium. Appl. Biochem. Microbiol. 2005, 41, 503–507. [Google Scholar] [CrossRef]
- Caro, Y.; Anamale, L.; Fouillaud, M.; Laurent, P.; Petit, T.; Dufosse, L. Natural Hydroxyanthraquinoid Pigments as Potent Food Grade Colorants: An Overview. Nat. Prod. Bioprospecting 2012, 2, 174–193. [Google Scholar] [CrossRef]
- Van den Berg, A.J.J. Biotechnology and Biosynthesis of Quinones. Pharm. Weekbl. 1991, 13, 74–77. [Google Scholar] [CrossRef]
- Yuliana, A.; Singgih, M.; Julianti, E.; Blanc, P.J. Derivates of Azaphilone Monascus Pigments. Biocatal. Agric. Biotechnol. 2017, 9, 183–194. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, J.; Huang, Y.; Xin, Q.; Wang, Z. Diversifying of Chemical Structure of Native Monascus Pigments. Front. Microbiol. 2018, 9, 3143. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Shao, Y.; Chen, F. Monascus Pigments. Appl. Microbiol. Biotechnol. 2012, 96, 1421–1440. [Google Scholar] [CrossRef] [PubMed]
- Pavesi, C.; Flon, V.; Mann, S.; Leleu, S.; Prado, S.; Franck, X. Biosynthesis of Azaphilones: A Review. Nat. Prod. Rep. 2021, 38, 1058–1071. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chen, R.; Liu, Q.; He, Y.; He, K.; Ding, X.; Kang, L.; Guo, X.; Xie, N.; Zhou, Y.; et al. Orange, Red, Yellow: Biosynthesis of Azaphilone Pigments in Monascus Fungi. Chem. Sci. 2017, 8, 4917–4925. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-K.; Yun, B.-S. Styrylpyrone-Class Compounds from Medicinal Fungi Phellinus and Inonotus Spp., and Their Medicinal Importance. J. Antibiot. 2011, 64, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Feng, X.; Liu, C.; Gao, J.; Qi, J. Diverse Metabolites and Pharmacological Effects from the Basidiomycetes Inonotus Hispidus. Antibiotics 2022, 11, 1097. [Google Scholar] [CrossRef]
- Palkina, K.A.; Balakireva, A.V.; Belozerova, O.A.; Chepurnykh, T.V.; Markina, N.M.; Kovalchuk, S.I.; Tsarkova, A.S.; Mishin, A.S.; Yampolsky, I.V.; Sarkisyan, K.S. Domain Truncation in Hispidin Synthase Orthologs from Non-Bioluminescent Fungi Does Not Lead to Hispidin Biosynthesis. Int. J. Mol. Sci. 2023, 24, 1317. [Google Scholar] [CrossRef]
- Luan, T. Research Progress on the Synthesis of Flavonoids by Saccharomyces cerevisiae. Int. J. Biol. Life Sci. 2023, 2, 51–53. [Google Scholar] [CrossRef]
- Fang, Z.; Jones, J.A.; Zhou, J.; Koffas, M.A.G. Engineering Escherichia Coli Co-Cultures for Production of Curcuminoids from Glucose. Biotechnol. J. 2018, 13, 1700576. [Google Scholar] [CrossRef]
- Kang, S.-Y.; Heo, K.T.; Hong, Y.-S. Optimization of Artificial Curcumin Biosynthesis in E. Coli by Randomized 5′-UTR Sequences to Control the Multienzyme Pathway. ACS Synth. Biol. 2018, 7, 2054–2062. [Google Scholar] [CrossRef]
- Solano, F. Melanins: Skin Pigments and Much More—Types, Structural Models, Biological Functions, and Formation Routes. New J. Sci. 2014, 2014, 498276. [Google Scholar] [CrossRef]
- Jacobson, E.S. Pathogenic Roles for Fungal Melanins. Clin. Microbiol. Rev. 2000, 13, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Glagoleva, A.Y.; Shoeva, O.Y.; Khlestkina, E.K. Melanin Pigment in Plants: Current Knowledge and Future Perspectives. Front. Plant Sci. 2020, 11, 770. [Google Scholar] [CrossRef] [PubMed]
- Eisenman, H.C.; Casadevall, A. Synthesis and Assembly of Fungal Melanin. Appl. Microbiol. Biotechnol. 2012, 93, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wenderoth, M.; Doppler, M.; Schuhmacher, R.; Marko, D.; Fischer, R. Fungal Melanin Biosynthesis Pathway as Source for Fungal Toxins. mBio 2022, 13, e00219-22. [Google Scholar] [CrossRef]
- Narsing Rao, M.P.; Xiao, M.; Li, W.-J. Fungal and Bacterial Pigments: Secondary Metabolites with Wide Applications. Front. Microbiol. 2017, 8, 1113. [Google Scholar] [CrossRef]
- Pavan, M.E.; López, N.I.; Pettinari, M.J. Melanin Biosynthesis in Bacteria, Regulation and Production Perspectives. Appl. Microbiol. Biotechnol. 2020, 104, 1357–1370. [Google Scholar] [CrossRef] [PubMed]
- Hasanien, Y.A.; Nassrallah, A.A.; Zaki, A.G.; Abdelaziz, G. Optimization, Purification, and Structure Elucidation of Anthraquinone Pigment Derivative from Talaromyces purpureogenus as a Novel Promising Antioxidant, Anticancer, and Kidney Radio-Imaging Agent. J. Biotechnol. 2022, 356, 30–41. [Google Scholar] [CrossRef]
- Ghiffary, M.R.; Prabowo, C.P.S.; Sharma, K.; Yan, Y.; Lee, S.Y.; Kim, H.U. High-Level Production of the Natural Blue Pigment Indigoidine from Metabolically Engineered Corynebacterium glutamicum for Sustainable Fabric Dyes. ACS Sustain. Chem. Eng. 2021, 9, 6613–6622. [Google Scholar] [CrossRef]
- Zhou, R.; Ma, L.; Qin, X.; Zhu, H.; Chen, G.; Liang, Z.; Zeng, W. Efficient Production of Melanin by Aureobasidium melanogenum Using a Simplified Medium and pH-Controlled Fermentation Strategy with the Cell Morphology Analysis. Appl. Biochem. Biotechnol. 2023, 1–20. [Google Scholar] [CrossRef]
- Tsouko, E.; Tolia, E.; Sarris, D. Microbial Melanin: Renewable Feedstock and Emerging Applications in Food-Related Systems. Sustainability 2023, 15, 7516. [Google Scholar] [CrossRef]
- Owary, J. Microbial Colourants in Food Industry: A New Dimension. In Life Sciences: Trends and Technology; Scieng Publications: Chennai, India, 2022; Volume 1, pp. 47–55. [Google Scholar]
- Elkhateeb, W.; Daba, G. Fungal Pigments: Their Diversity, Chemistry, Food and Non-Food Applications. Appl. Microbiol. 2023, 3, 735–751. [Google Scholar] [CrossRef]
- Agboyibor, C.; Kong, W.-B.; Chen, D.; Zhang, A.-M.; Niu, S.-Q. Monascus Pigments Production, Composition, Bioactivity and Its Application: A Review. Biocatal. Agric. Biotechnol. 2018, 16, 433–447. [Google Scholar] [CrossRef]
- Srilekha, V.; Krishna, G.; Sreelatha, B.; Jagadeesh Kumar, E.; Rajeshwari, K.V.N. Prodigiosin: A Fascinating and the Most Versatile Bioactive Pigment with Diverse Applications. Syst. Microbiol. Biomanufacturing 2023, 1–11. [Google Scholar] [CrossRef]
- Da Silva Ferreira, V.; Sant’Anna, C. Impact of Culture Conditions on the Chlorophyll Content of Microalgae for Biotechnological Applications. World J. Microbiol. Biotechnol. 2017, 33, 20. [Google Scholar] [CrossRef] [PubMed]
- Jacob-Lopes, E.; Maroneze, M.M.; Deprá, M.C.; Sartori, R.B.; Dias, R.R.; Zepka, L.Q. Bioactive Food Compounds from Microalgae: An Innovative Framework on Industrial Biorefineries. Curr. Opin. Food Sci. 2019, 25, 1–7. [Google Scholar] [CrossRef]
- Sluijs, I.; Cadier, E.; Beulens, J.W.J.; Van Der, A.D.L.; Spijkerman, A.M.W.; Van Der Schouw, Y.T. Dietary Intake of Carotenoids and Risk of Type 2 Diabetes. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wang, Y.; He, Y.; Liu, B.; Mou, H.; Chen, F.; Yang, S. Microalgae-Derived Pigments for the Food Industry. Mar. Drugs 2023, 21, 82. [Google Scholar] [CrossRef]
- Govindaraj, C.; Ugamoorthi, R.; Ramarethinam, S. Isolation of Pseudomonas Aeruginosa for Bacterial Pigment Production and Its Application on Synthetic Knitted Fabric. Indian J. Fibre Text. Res. 2021, 46, 168–173. [Google Scholar]
- Pérez-García, F.; Klein, V.J.; Brito, L.F.; Brautaset, T. From Brown Seaweed to a Sustainable Microbial Feedstock for the Production of Riboflavin. Front. Bioeng. Biotechnol. 2022, 10, 863690. [Google Scholar] [CrossRef]
- Chu, R.; Li, R.; Wang, C.; Ban, R. Production of Vitamin B2 (Riboflavin) by Bacillus subtilis. J Chem. Technol. Biotechnol. 2022, 97, 1941–1949. [Google Scholar] [CrossRef]
- Molelekoa, T.B.J.; Augustyn, W.; Regnier, T.; Da Silva, L.S. Chemical Characterization and Toxicity Evaluation of Fungal Pigments for Potential Application in Food, Phamarceutical and Agricultural Industries. Saudi J. Biol. Sci. 2023, 30, 103630. [Google Scholar] [CrossRef]
- Cao, K.; Cui, Y.; Sun, F.; Zhang, H.; Fan, J.; Ge, B.; Cao, Y.; Wang, X.; Zhu, X.; Wei, Z.; et al. Metabolic Engineering and Synthetic Biology Strategies for Producing High-Value Natural Pigments in Microalgae. Biotechnol. Adv. 2023, 68, 108236. [Google Scholar] [CrossRef]
- Xu, S.; Gao, S.; An, Y. Research Progress of Engineering Microbial Cell Factories for Pigment Production. Biotechnol. Adv. 2023, 65, 108150. [Google Scholar] [CrossRef]
- Orlandi, V.T.; Martegani, E.; Giaroni, C.; Baj, A.; Bolognese, F. Bacterial Pigments: A Colorful Palette Reservoir for Biotechnological Applications. Biotechnol. Appl. Biochem. 2022, 69, 981–1001. [Google Scholar] [CrossRef] [PubMed]
- Herráiz-Gil, S.; Del Carmen De Arriba, M.; Escámez, M.J.; León, C. Multi-Omic Data Integration in Food Science and Analysis. Curr. Opin. Food Sci. 2023, 52, 101049. [Google Scholar] [CrossRef]
- Helmy, M.; Elhalis, H.; Liu, Y.; Chow, Y.; Selvarajoo, K. Perspective: Multiomics and Machine Learning Help Unleash the Alternative Food Potential of Microalgae. Adv. Nutr. 2023, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Makaranga, A.; Jutur, P.P. Dynamic Metabolomic Crosstalk between Chlorella Saccharophila and Its New Symbiotic Bacteria Enhances Lutein Production in Microalga without Compromising Its Biomass. Enzym. Microb. Technol. 2023, 170, 110291. [Google Scholar] [CrossRef]
- Chen, D.; Yuan, X.; Zheng, X.; Fang, J.; Lin, G.; Li, R.; Chen, J.; He, W.; Huang, Z.; Fan, W.; et al. Multi-Omics Analyses Provide Insight into the Biosynthesis Pathways of Fucoxanthin in Isochrysis Galbana. Genom. Proteom. Bioinform. 2022, 20, 1138–1153. [Google Scholar] [CrossRef] [PubMed]
- Fathi, Z.; Tramontin, L.R.R.; Ebrahimipour, G.; Borodina, I.; Darvishi, F. Metabolic Engineering of Saccharomyces cerevisiae for Production of β-Carotene from Hydrophobic Substrates. FEMS Yeast Res. 2021, 21, foaa068. [Google Scholar] [CrossRef]
- Coussement, P.; Bauwens, D.; Maertens, J.; De Mey, M. Direct Combinatorial Pathway Optimization. ACS Synth. Biol. 2017, 6, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Shi, B.; Ye, Z.; Li, X.; Liu, M.; Chen, Y.; Xia, J.; Nielsen, J.; Deng, Z.; Liu, T. Lipid Engineering Combined with Systematic Metabolic Engineering of Saccharomyces cerevisiae for High-Yield Production of Lycopene. Metab. Eng. 2019, 52, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, J.; Cai, X.; Zhang, S.; Liang, Y.; Lin, Q. Regulation of Secondary Metabolite Biosynthesis in Monascus purpureus via Cofactor Metabolic Engineering Strategies. Food Microbiol. 2021, 95, 103689. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Zhang, X.; Wu, Z.; Wang, Z. Accumulation of Yellow Monascus Pigments by Extractive Fermentation in Nonionic Surfactant Micelle Aqueous Solution. Appl. Microbiol. Biotechnol. 2015, 99, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zeng, D.; Xu, C.; Gao, M. Effect of Low-Frequency Magnetic Field on Formation of Pigments of Monascus purpureus. Eur. Food Res. Technol. 2015, 240, 577–582. [Google Scholar] [CrossRef]
- Cho, S.; Shin, J.; Cho, B.-K. Applications of CRISPR/Cas System to Bacterial Metabolic Engineering. Int. J. Mol. Sci. 2018, 19, 1089. [Google Scholar] [CrossRef]
- Nishigandha Upasani, H.V.; Wagh, P.S. Microbial Pigments: Natural Colorants and Their Industrial Applications. Int. J. Curr. Microbiol. Appl. Sci. 2021, 10, 631–645. [Google Scholar] [CrossRef]
- Usmani, Z.; Sharma, M.; Awasthi, A.K.; Sivakumar, N.; Lukk, T.; Pecoraro, L.; Thakur, V.K.; Roberts, D.; Newbold, J.; Gupta, V.K. Bioprocessing of Waste Biomass for Sustainable Product Development and Minimizing Environmental Impact. Bioresour. Technol. 2021, 322, 124548. [Google Scholar] [CrossRef]
- Allied Market Research. Carotenoids Market Global Opportunity Analysis and Industry Forecast, 2021–2031. 2023. Available online: https://www.marketsandmarkets.com/Market-Reports/carotenoid-market-158421566.html?gad_source=1&gclid=Cj0KCQiAgqGrBhDtARIsAM5s0_kUyjexHN4la3DCUisevvPZ9llWUnhGS0qLQFsRuoCp4XrZNaFsyDYaAm0lEALw_wcB (accessed on 30 September 2023).
- Kiki, M.J. Biopigments of Microbial Origin and Their Application in the Cosmetic Industry. Cosmetics 2023, 10, 47. [Google Scholar] [CrossRef]
- Nalini, S.; Inbakandan, D.; Dhas, T.S.; Riyaz, S.U.M.; Manikandan, S. Current Progress in the Solid-State Fermentation and Utilization of Agroindustrial Residues for the Production of Biologically Active Secondary Metabolites. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Elsevier: Amsterdam, The Netherlands, 2021; pp. 75–94. [Google Scholar]
- De Medeiros, T.D.M.; Dufossé, L.; Bicas, J.L. Lignocellulosic Substrates as Starting Materials for the Production of Bioactive Biopigments. Food Chem. X 2022, 13, 100223. [Google Scholar] [CrossRef] [PubMed]
- Rocha Balbino, T.; Sánchez-Muñoz, S.; Díaz-Ruíz, E.; Moura Rocha, T.; Mier-Alba, E.; Custódio Inácio, S.; Jose Castro-Alonso, M.; De Carvalho Santos-Ebinuma, V.; Fernando Brandão Pereira, J.; César Santos, J.; et al. Lignocellulosic Biorefineries as a Platform for the Production of High-Value Yeast Derived Pigments—A Review. Bioresour. Technol. 2023, 386, 129549. [Google Scholar] [CrossRef] [PubMed]
- Jameel, M.; Umar, K.; Parveen, T.; Ismail, I.M.I.; Qari, H.A.; Yaqoob, A.A.; Ibrahim, M.N.M. Extraction of Natural Dyes from Agro-Industrial Waste. In Extraction of Natural Products from Agro-Industrial Wastes; Elsevier: Amsterdam, The Netherlands, 2023; pp. 197–216. [Google Scholar]
- Cipolatti, E.P.; Remedi, R.D.; Sá, C.D.S.; Rodrigues, A.B.; Gonçalves Ramos, J.M.; Veiga Burkert, C.A.; Furlong, E.B.; Fernandes De Medeiros Burkert, J. Use of Agroindustrial Byproducts as Substrate for Production of Carotenoids with Antioxidant Potential by Wild Yeasts. Biocatal. Agric. Biotechnol. 2019, 20, 101208. [Google Scholar] [CrossRef]
- Liu, Z.; Natalizio, F.; Dragone, G.; Mussatto, S.I. Maximizing the Simultaneous Production of Lipids and Carotenoids by Rhodosporidium toruloides from Wheat Straw Hydrolysate and Perspectives for Large-Scale Implementation. Bioresour. Technol. 2021, 340, 125598. [Google Scholar] [CrossRef]
- Basto, B.; Da Silva, N.R.; Teixeira, J.A.; Silvério, S.C. Production of Natural Pigments by Penicillium brevicompactum Using Agro-Industrial Byproducts. Fermentation 2022, 8, 536. [Google Scholar] [CrossRef]
- Thevarajah, B.; Nishshanka, G.K.S.H.; Premaratne, M.; Wasath, W.A.J.; Nimarshana, P.H.V.; Malik, A.; Ariyadasa, T.U. Cyanobacterial Pigment Production in Wastewaters Treated for Heavy Metal Removal: Current Status and Perspectives. J. Environ. Chem. Eng. 2023, 11, 108999. [Google Scholar] [CrossRef]
- Dursun, D.; Dalgıç, A.C. Optimization of Astaxanthin Pigment Bioprocessing by Four Different Yeast Species Using Wheat Wastes. Biocatal. Agric. Biotechnol. 2016, 7, 1–6. [Google Scholar] [CrossRef]
- Valduga, E.; Rausch Ribeiro, A.H.; Cence, K.; Colet, R.; Tiggemann, L.; Zeni, J.; Toniazzo, G. Carotenoids Production from a Newly Isolated Sporidiobolus Pararoseus Strain Using Agroindustrial Substrates. Biocatal. Agric. Biotechnol. 2014, 3, 207–213. [Google Scholar] [CrossRef]
- Liu, J.; Luo, Y.; Guo, T.; Tang, C.; Chai, X.; Zhao, W.; Bai, J.; Lin, Q. Cost-Effective Pigment Production by Monascus Purpureus Using Rice Straw Hydrolysate as Substrate in Submerged Fermentation. J. Biosci. Bioeng. 2020, 129, 229–236. [Google Scholar] [CrossRef]
- Sundaramahalingam, M.A.; Sivashanmugam, P. Production of Microbial Carotenoid Using Innate Inherent of the Food Industry Wastewater. J. Mol. Liq. 2023, 388, 122772. [Google Scholar] [CrossRef]
- Umesh, M.; Suresh, S.; Santosh, A.S.; Prasad, S.; Chinnathambi, A.; Al Obaid, S.; Jhanani, G.K.; Shanmugam, S. Valorization of Pineapple Peel Waste for Fungal Pigment Production Using Talaromyces albobiverticillius: Insights into Antibacterial, Antioxidant and Textile Dyeing Properties. Environ. Res. 2023, 229, 115973. [Google Scholar] [CrossRef]
- Morales-Oyervides, L.; Ruiz-Sánchez, J.P.; Oliveira, J.C.; Sousa-Gallagher, M.J.; Morales-Martínez, T.K.; Albergamo, A.; Salvo, A.; Giuffrida, D.; Dufossé, L.; Montañez, J. Medium Design from Corncob Hydrolyzate for Pigment Production by Talaromyces atroroseus GH2: Kinetics Modeling and Pigments Characterization. Biochem. Eng. J. 2020, 161, 107698. [Google Scholar] [CrossRef]
- Uğurlu, Ş.; Günan Yücel, H.; Aksu, Z. Valorization of Food Wastes with a Sequential Two-Step Process for Microbial β-Carotene Production: A Zero Waste Approach. J. Environ. Manag. 2023, 340, 118003. [Google Scholar] [CrossRef]
- Sharmila, G.; Nidhi, B.; Muthukumaran, C. Sequential Statistical Optimization of Red Pigment Production by Monascus Purpureus (MTCC 369) Using Potato Powder. Ind. Crops Prod. 2013, 44, 158–164. [Google Scholar] [CrossRef]
- Varmira, K.; Habibi, A.; Moradi, S.; Bahramian, E. Statistical Optimization of Airlift Photobioreactor for High Concentration Production of Torularhodin Pigment. Biocatal. Agric. Biotechnol. 2016, 8, 197–203. [Google Scholar] [CrossRef]
- González-Vega, R.I.; Cárdenas-López, J.L.; López-Elías, J.A.; Ruiz-Cruz, S.; Reyes-Díaz, A.; Perez-Perez, L.M.; Cinco-Moroyoqui, F.J.; Robles-Zepeda, R.E.; Borboa-Flores, J.; Del-Toro-Sánchez, C.L. Optimization of Growing Conditions for Pigments Production from Microalga Navicula incerta Using Response Surface Methodology and Its Antioxidant Capacity. Saudi J. Biol. Sci. 2021, 28, 1401–1416. [Google Scholar] [CrossRef] [PubMed]
- Reshma, R.; Chitra Devi, K.; Dinesh Kumar, S.; Santhanam, P.; Perumal, P.; Krishnaveni, N.; Begum, A.; Pragnya, M.; Arthikha, R.; Dhanalakshmi, B.; et al. Enhancement of Pigments Production in the Green Microalga Dunaliella salina (PSBDU05) under Optimized Culture Condition. Bioresour. Technol. Rep. 2021, 14, 100672. [Google Scholar] [CrossRef]
- Manon Mani, V.; Parimala Gnana Soundari, A.; Salin, K.P.; Mohankumar, R.; Preethi, K.; Al Obaid, S.; Ali Alharbi, S.; Jhanani, G.K.; Shanmugam, S. Optimization Parameters for the Production of Dimer of Epicatechin from an Endophytic Fungus Curvularia Australiensis FC2AP Using Response Surface Methodology (RSM). Environ. Res. 2023, 231, 115962. [Google Scholar] [CrossRef]
- Majumdar, S.; Priyadarshinee, R.; Kumar, A.; Mandal, T.; Dasgupta Mandal, D. Exploring Planococcus sp. TRC1, a Bacterial Isolate, for Carotenoid Pigment Production and Detoxification of Paper Mill Effluent in Immobilized Fluidized Bed Reactor. J. Clean. Prod. 2019, 211, 1389–1402. [Google Scholar] [CrossRef]
- Bauer, L.M.; Rodrigues, E.; Rech, R. Potential of Immobilized Chlorella Minutissima for the Production of Biomass, Proteins, Carotenoids and Fatty Acids. Biocatal. Agric. Biotechnol. 2020, 25, 101601. [Google Scholar] [CrossRef]
- Qiao, J.; Zeng, H.; Ye, W.; Qiu, R.; Zeng, X.; Xin, B.; Xie, T. Integrative Addition of Sucrose Esters and Immobilisation Technology for Enhancing Yellow Pigment Yield of Monascus Purpureus HBSD08 under Submerged Fermentation Conditions and Its Molecular Mechanism. LWT 2023, 186, 115233. [Google Scholar] [CrossRef]
- Liu, J.; Guo, T.; Luo, Y.; Chai, X.; Wu, J.; Zhao, W.; Jiao, P.; Luo, F.; Lin, Q. Enhancement of Monascus Pigment Productivity via a Simultaneous Fermentation Process and Separation System Using Immobilized-Cell Fermentation. Bioresour. Technol. 2019, 272, 552–560. [Google Scholar] [CrossRef]
- Cheng, S.-H.; Khoo, H.E.; Kong, K.W.; Prasad, K.N.; Galanakis, C.M. Extraction of Carotenoids and Applications. In Carotenoids: Properties, Processing and Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 259–288. [Google Scholar]
- López, G.-D.; Álvarez-Rivera, G.; Carazzone, C.; Ibáñez, E.; Leidy, C.; Cifuentes, A. Bacterial Carotenoids: Extraction, Characterization, and Applications. Crit. Rev. Anal. Chem. 2023, 53, 1239–1262. [Google Scholar] [CrossRef]
- Pailliè-Jiménez, M.E.; Stincone, P.; Brandelli, A. Natural Pigments of Microbial Origin. Front. Sustain. Food Syst. 2020, 4, 590439. [Google Scholar] [CrossRef]
- Islam, M.T.; Farhan, M.S.; Mahmud, M.H. Green Extraction of Dyes and Pigments from Natural Resources. In Renewable Dyes and Pigments; Elsevier: Amsterdam, The Netherlands, 2024; pp. 19–36. [Google Scholar]
- Sanjeewa, K.K.A.; Herath, K.H.I.N.M.; Kim, Y.-S.; Jeon, Y.-J.; Kim, S.-K. Enzyme-Assisted Extraction of Bioactive Compounds from Seaweeds and Microalgae. TrAC Trends Anal. Chem. 2023, 167, 117266. [Google Scholar] [CrossRef]
- Singh, S.; Nimse, S.B.; Mathew, D.E.; Dhimmar, A.; Sahastrabudhe, H.; Gajjar, A.; Ghadge, V.A.; Kumar, P.; Shinde, P.B. Microbial Melanin: Recent Advances in Biosynthesis, Extraction, Characterization, and Applications. Biotechnol. Adv. 2021, 53, 107773. [Google Scholar] [CrossRef] [PubMed]
- Lafarga, T.; Aguiló-Aguayo, I. Pulsed Electric Fields for the Extraction of Lipids, Pigments, and Polyphenols from Cultured Microalgae. In Innovative and Emerging Technologies in the Bio-Marine Food Sector; Elsevier: Amsterdam, The Netherlands, 2022; pp. 197–221. [Google Scholar]
- Pagels, F.; Pereira, R.N.; Vicente, A.A.; Guedes, A.C. Extraction of Pigments from Microalgae and Cyanobacteria—A Review on Current Methodologies. Appl. Sci. 2021, 11, 5187. [Google Scholar] [CrossRef]
- Luengo, E.; Condón-Abanto, S.; Álvarez, I.; Raso, J. Effect of Pulsed Electric Field Treatments on Permeabilization and Extraction of Pigments from Chlorella Vulgaris. J. Membr. Biol. 2014, 247, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Luengo, E.; Martínez, J.M.; Coustets, M.; Álvarez, I.; Teissié, J.; Rols, M.-P.; Raso, J. A Comparative Study on the Effects of Millisecond- and Microsecond-Pulsed Electric Field Treatments on the Permeabilization and Extraction of Pigments from Chlorella Vulgaris. J. Membr. Biol. 2015, 248, 883–891. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, J.; Castagnini, J.M.; Berrada, H.; Barba, F.J. Pulsed Electric Field (PEF) Recovery of Biomolecules from Chlorella: Extract Efficiency, Nutrient Relative Value, and Algae Morphology Analysis. Food Chem. 2023, 404, 134615. [Google Scholar] [CrossRef]
- Wang, X.; Tao, J.; Wei, D.; Shen, Y.; Tong, W. Development of an Adsorption Procedure for the Direct Separation and Purification of Prodigiosin from Culture Broth. Biotech. Appl. Biochem. 2004, 40, 277–280. [Google Scholar] [CrossRef]
- Shen, N.; Ren, J.; Liu, Y.; Sun, W.; Li, Y.; Xin, H.; Cui, Y. Natural Edible Pigments: A Comprehensive Review of Resource, Chemical Classification, Biosynthesis Pathway, Separated Methods and Application. Food Chem. 2023, 403, 134422. [Google Scholar] [CrossRef]
- Huang, L.; Zuo, S.; Gao, X.; Li, Z.; Wang, S.; Chen, B.; Li, X.; Zhu, L.; Zhang, Y. Extraction and Functional Properties of Pigment from Pumpkin Peels by a Novel Green Deep Eutectic Alcohol Two-Phase System. Sustain. Chem. Pharm. 2023, 33, 101067. [Google Scholar] [CrossRef]
- Machado, F.R.S.; Trevisol, T.C.; Boschetto, D.L.; Burkert, J.F.M.; Ferreira, S.R.S.; Oliveira, J.V.; Burkert, C.A.V. Technological Process for Cell Disruption, Extraction and Encapsulation of Astaxanthin from Haematococcus Pluvialis. J. Biotechnol. 2016, 218, 108–114. [Google Scholar] [CrossRef]
- Hamed, I.; Moradi, M.; Ezati, P.; O’Higgins, L.; Meléndez-Martínez, A.J.; Frleta Matas, R.; Šimat, V.; McClements, D.J.; Jakobsen, A.N.; Lerfall, J. Encapsulation of Microalgal-Based Carotenoids: Recent Advances in Stability and Food Applications. Trends Food Sci. Technol. 2023, 138, 382–398. [Google Scholar] [CrossRef]
- Vieira, M.V.; Pastrana, L.M.; Fuciños, P. Microalgae Encapsulation Systems for Food, Pharmaceutical and Cosmetics Applications. Mar. Drugs 2020, 18, 644. [Google Scholar] [CrossRef]
- Ning, S.; Wang, C.; Zhao, L.; Yang, J.; Shi, X.; Zheng, Y. Lecithin/Chitosan Nanoparticle Drug Carrier Improves Anti-Tumor Efficacy of Monascus Pigment Rubropunctatin. Int. J. Biol. Macromol. 2023, 242, 125058. [Google Scholar] [CrossRef]
- Shi, J.-Y.; Cai, W.-Q.; Luo, X.-T.; Su, B.-L.; Xiao, J.-W.; Zhang, G.-R.; Yang, Q.-Q.; Zhang, B.-B. Delivery of Natural Monascus Yellow Pigment Using Zein-Lecithin Nanoparticles: Fabrication, Characterization, and in Vitro Release Properties. Biochem. Eng. J. 2023, 197, 108992. [Google Scholar] [CrossRef]
- Majumdar, S.; Mandal, T.; Mandal, D.D. Chitosan Based Micro and Nano-Particulate Delivery Systems for Bacterial Prodigiosin: Optimization and Toxicity in Animal Model System. Int. J. Biol. Macromol. 2022, 222, 2966–2976. [Google Scholar] [CrossRef]
- Arab, M.; Hosseini, S.M.; Nayebzadeh, K.; Khorshidian, N.; Yousefi, M.; Razavi, S.H.; Mortazavian, A.M. Microencapsulation of Microbial Canthaxanthin with Alginate and High Methoxyl Pectin and Evaluation the Release Properties in Neutral and Acidic Condition. Int. J. Biol. Macromol. 2019, 121, 691–698. [Google Scholar] [CrossRef]
- Venil, C.K.; Aruldass, C.A.; Abd Halim, M.H.; Khasim, A.R.; Zakaria, Z.A.; Ahmad, W.A. Spray Drying of Violet Pigment from Chromobacterium Violaceum UTM 5 and Its Application in Food Model Systems. Int. Biodeterior. Biodegrad. 2015, 102, 324–329. [Google Scholar] [CrossRef]
- Dufossé, L. Microbial Pigments from Bacteria, Yeasts, Fungi, and Microalgae for the Food and Feed Industries. In Natural and Artificial Flavoring Agents and Food Dyes; Elsevier: Amsterdam, The Netherlands, 2018; pp. 113–132. [Google Scholar]
- Contreras-Machuca, P.I.; Avello, M.; Pastene, E.; Machuca, Á.; Aranda, M.; Hernández, V.; Fernández, M. Chemical Characterization and Microencapsulation of Extracellular Fungal Pigments. Appl. Microbiol. Biotechnol. 2022, 106, 8021–8034. [Google Scholar] [CrossRef]
- Caldas, M.; Santos, A.C.; Veiga, F.; Rebelo, R.; Reis, R.L.; Correlo, V.M. Melanin Nanoparticles as a Promising Tool for Biomedical Applications—A Review. Acta Biomater. 2020, 105, 26–43. [Google Scholar] [CrossRef]
- Seelam, S.D.; Agsar, D.; Halmuthur, M.S.K.; Reddy Shetty, P.; Vemireddy, S.; Reddy, K.M.; Umesh, M.K.; Rajitha, C. Characterization and Photoprotective Potentiality of Lime Dwelling Pseudomonas Mediated Melanin as Sunscreen Agent against UV-B Radiations. J. Photochem. Photobiol. B Biol. 2021, 216, 112126. [Google Scholar] [CrossRef]
- Manochkumar, J.; Doss, C.G.P.; Efferth, T.; Ramamoorthy, S. Tumor Preventive Properties of Selected Marine Pigments against Colon and Breast Cancer. Algal Res. 2022, 61, 102594. [Google Scholar] [CrossRef]
- Rajendran, P.; Somasundaram, P.; Dufossé, L. Microbial Pigments: Eco-Friendly Extraction Techniques and Some Industrial Applications. J. Mol. Struct. 2023, 1290, 135958. [Google Scholar] [CrossRef]
- Cikoš, A.-M.; Šubarić, D.; Roje, M.; Babić, J.; Jerković, I.; Jokić, S. Recent Advances on Macroalgal Pigments and Their Biological Activities (2016–2021). Algal Res. 2022, 65, 102748. [Google Scholar] [CrossRef]
- Tunca Koyun, M.; Sirin, S.; Aslim, B.; Taner, G.; Nigdelioglu Dolanbay, S. Characterization of Prodigiosin Pigment by Serratia Marcescens and the Evaluation of Its Bioactivities. Toxicol. In Vitro 2022, 82, 105368. [Google Scholar] [CrossRef]
- Mumtaz, R.; Bashir, S.; Numan, M.; Shinwari, Z.K.; Ali, M. Pigments from Soil Bacteria and Their Therapeutic Properties: A Mini Review. Curr. Microbiol. 2019, 76, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Milosevic, E.; Stanisavljevic, N.; Boskovic, S.; Stamenkovic, N.; Novkovic, M.; Bavelloni, A.; Cenni, V.; Kojic, S.; Jasnic, J. Antitumor Activity of Natural Pigment Violacein against Osteosarcoma and Rhabdomyosarcoma Cell Lines. J. Cancer Res. Clin. Oncol. 2023, 149, 10975–10987. [Google Scholar] [CrossRef]
- Rivero Berti, I.; Islan, G.A.; Castro, G.R. Enzymes and Biopolymers. The Opportunity for the Smart Design of Molecular Delivery Systems. Bioresour. Technol. 2021, 322, 124546. [Google Scholar] [CrossRef]
- Dogancı, M.A.; Ay Sal, F.; Guler, H.I.; Katı, H.; Ceylan, E.; Belduz, A.O.; Bozdal, G.; Yaylı, N.; Canakcı, S. Investigation of Potential Inhibitor Properties of Violacein against HIV-1 RT and CoV-2 Spike RBD:ACE-2. World J. Microbiol. Biotechnol. 2022, 38, 161. [Google Scholar] [CrossRef]
- Nawaz, A.; Chaudhary, R.; Shah, Z.; Dufossé, L.; Fouillaud, M.; Mukhtar, H.; Ul Haq, I. An Overview on Industrial and Medical Applications of Bio-Pigments Synthesized by Marine Bacteria. Microorganisms 2020, 9, 11. [Google Scholar] [CrossRef]
- Durán, N.; Castro, G.R.; Portela, R.W.D.; Fávaro, W.J.; Durán, M.; Tasic, L.; Nakazato, G. Violacein and Its Antifungal Activity: Comments and Potentialities. Lett. Appl. Microbiol. 2022, 75, 796–803. [Google Scholar] [CrossRef]
- Devkota, S.; Poudel, P.; Parajuli, T.; Bhatt, P. Evaluation of Effectiveness of Fungicides against Fusarium oxysporum f. Sp. Lentis in Laboratory. Acta Sci. Agric. 2023, 7, 35–38. [Google Scholar] [CrossRef]
- Ayyolath, A.; Kallingal, A.; Thachan Kundil, V.; Variyar, E.J. Studies on the Bioactive Properties of Penicillium Mallochi ARA-1 Pigment Isolated from Coffee Plantation. Biocatal. Agric. Biotechnol. 2020, 30, 101841. [Google Scholar] [CrossRef]
- Karbalaei-Heidari, H.R.; Partovifar, M.; Memarpoor-Yazdi, M. Evaluation of the Bioactive Potential of Secondary Metabolites Produced by a New Marine Micrococcus Species Isolated from the Persian Gulf. Avicenna J. Med. Biotechnol. 2020, 12, 61–65. [Google Scholar] [PubMed]
- Vasanthabharathi, V.; Lakshminarayanan, R.; Jayalakshmi, S. Melanin Production from Marine Streptomyces. Afr. J. Biotechnol. 2011, 10, 11224–11234. [Google Scholar]
- Ghattavi, K.; Homaei, A.; Kamrani, E.; Kim, S.-K. Melanin Pigment Derived from Marine Organisms and Its Industrial Applications. Dyes Pigments 2022, 201, 110214. [Google Scholar] [CrossRef]
- Lourith, N.; Kanlayavattanakul, M. Sustainable Approach to Natural Makeup Cosmetics Containing Microencapsulated Butterfly Pea Anthocyanins. Sustain. Chem. Pharm. 2023, 32, 101005. [Google Scholar] [CrossRef]
- Boo, H.-O.; Hwang, S.-J.; Bae, C.-S.; Park, S.-H.; Heo, B.-G.; Gorinstein, S. Extraction and Characterization of Some Natural Plant Pigments. Ind. Crops Prod. 2012, 40, 129–135. [Google Scholar] [CrossRef]
- Charoensit, P.; Sawasdipol, F.; Tibkawin, N.; Suphrom, N.; Khorana, N. Development of Natural Pigments from Tectona Grandis (Teak) Leaves: Agricultural Waste Material from Teak Plantations. Sustain. Chem. Pharm. 2021, 19, 100365. [Google Scholar] [CrossRef]
- Arad, S.; Yaron, A. Natural Pigments from Red Microalgae for Use in Foods and Cosmetics. Trends Food Sci. Technol. 1992, 3, 92–97. [Google Scholar] [CrossRef]
- Sandybayeva, S.K.; Kossalbayev, B.D.; Zayadan, B.K.; Sadvakasova, A.K.; Bolatkhan, K.; Zadneprovskaya, E.V.; Kakimov, A.B.; Alwasel, S.; Leong, Y.K.; Allakhverdiev, S.I.; et al. Prospects of Cyanobacterial Pigment Production: Biotechnological Potential and Optimization Strategies. Biochem. Eng. J. 2022, 187, 108640. [Google Scholar] [CrossRef]
- Cheirsilp, B.; Maneechote, W.; Srinuanpan, S.; Angelidaki, I. Microalgae as Tools for Bio-Circular-Green Economy: Zero-Waste Approaches for Sustainable Production and Biorefineries of Microalgal Biomass. Bioresour. Technol. 2023, 387, 129620. [Google Scholar] [CrossRef] [PubMed]
- Meruvu, H.; Dos Santos, J.C. Colors of Life: A Review on Fungal Pigments. Crit. Rev. Biotechnol. 2021, 41, 1153–1177. [Google Scholar] [CrossRef]
- Bayram, S.; Aygün, B.; Karadayi, M.; Alaylar, B.; Güllüce, M.; Karabulut, A. Determination of Toxicity and Radioprotective Properties of Bacterial and Fungal Eumelanin Pigments. Int. J. Radiat. Biol. 2023, 99, 1785–1793. [Google Scholar] [CrossRef] [PubMed]
- Antony, T.M.P.; Krishna, A.R.; Jayalekshmi, S.K.; Chockalingam, J.; Ramasamy, S. Isolation and Elucidation of Bacterial Melanin’s Sun Protection Factor (SPF) for Photoprotection in Cosmetics. J. Pure Appl. Microbiol. 2023, 17, 449–455. [Google Scholar] [CrossRef]
- Sánchez-Muñoz, S.; Mariano-Silva, G.; Leite, M.O.; Mura, F.B.; Verma, M.L.; Da Silva, S.S.; Chandel, A.K. Production of Fungal and Bacterial Pigments and Their Applications. In Biotechnological Production of Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2020; pp. 327–361. [Google Scholar]
- Colorado Gómez, V.K.; Ruiz-Sánchez, J.P.; Méndez-Zavala, A.; Morales-Oyervides, L.; Montañez, J. Biotechnological Production of Microbial Pigments: Recent Findings. In Handbook of Natural Colorants; Stevens, C., Bechtold, T., Manian, A., Pham, T., Eds.; Wiley: Hoboken, NJ, USA, 2023; pp. 439–457. [Google Scholar]
- Oh, J.-J.; Kim, J.Y.; Son, S.H.; Jung, W.-J.; Kim, D.H.; Seo, J.-W.; Kim, G.-H. Fungal Melanin as a Biocompatible Broad-Spectrum Sunscreen with High Antioxidant Activity. RSC Adv. 2021, 11, 19682–19689. [Google Scholar] [CrossRef] [PubMed]
- Weiss, V.; Okun, Z.; Shpigelman, A. Utilization of Hydrocolloids for the Stabilization of Pigments from Natural Sources. Curr. Opin. Colloid Interface Sci. 2023, 68, 101756. [Google Scholar] [CrossRef]
- Silva, T.M.; Silva Neto, A.B.D.; Teixeira, J.M.; Cerqueira-Silva, C.B.M.; Gualberto, S.A.; Freitas, J.S.D. Optimization of Pigment Production by Rhodotorula Minuta URM 5197 and Rhodotorula Mucilaginosa URM 7409 Using Yellow Passion Fruit Peel (Passiflora Edulis). Res. Soc. Dev. 2021, 10, e152101724311. [Google Scholar] [CrossRef]
- Nigam, P.S.; Luke, J.S. Food Additives: Production of Microbial Pigments and Their Antioxidant Properties. Curr. Opin. Food Sci. 2016, 7, 93–100. [Google Scholar] [CrossRef]
- Lu, Q.; Li, H.; Zou, Y.; Liu, H.; Yang, L. Astaxanthin as a Microalgal Metabolite for Aquaculture: A Review on the Synthetic Mechanisms, Production Techniques, and Practical Application. Algal Res. 2021, 54, 102178. [Google Scholar] [CrossRef]
- Cerezal-Mezquita, P.; Espinosa-Álvarez, C.; Jáuregui-Tirado, M.; Jaime-Matus, C.; Palma-Ramírez, J.; Ruiz-Domínguez, M.C. Physical-Chemical Characteristics of “Red Meal”, a Novel Non-Defatted Additive in the Fish Feed from Cracked Biomass of Haematococcus Pluvialis. Anim. Feed Sci. Technol. 2022, 285, 115247. [Google Scholar] [CrossRef]
- Zorriehzahra, M.J. Efficacy of Astaxanthin Fed to Rainbow Trout (Oncorhynchus mykiss): Effect on Growth, Pigmentation and Blood Indices. Int. J. Oceanogr. Aquac. 2020, 4, 000197. [Google Scholar] [CrossRef]
- Rekha, R.; Nimsi, K.A.; Manjusha, K.; Sirajudheen, T.K. Marine Yeast Rhodotorula Paludigena VA 242 a Pigment Enhancing Feed Additive for the Ornamental Fish Koi Carp. Aquac. Fish. 2024, 9, 66–70. [Google Scholar] [CrossRef]
- Pulcini, D.; Capoccioni, F.; Franceschini, S.; Martinoli, M.; Faccenda, F.; Secci, G.; Perugini, A.; Tibaldi, E.; Parisi, G. Muscle Pigmentation in Rainbow Trout (Oncorhynchus mykiss) Fed Diets Rich in Natural Carotenoids from Microalgae and Crustaceans. Aquaculture 2021, 543, 736989. [Google Scholar] [CrossRef]
- Araújo, I.C.S.; Lara, L.J.C. Perspectives on Vitamin E, Canthaxanthin and Selenium to Chick Embryo Antioxidant Protection. World’s Poult. Sci. J. 2023, 79, 265–283. [Google Scholar] [CrossRef]
- Fawzy, S.; Wang, W.; Wu, M.; Yi, G.; Huang, X. Effects of Dietary Different Canthaxanthin Levels on Growth Performance, Antioxidant Capacity, Biochemical and Immune-Physiological Parameters of White Shrimp (Litopenaeus vannamei). Aquaculture 2022, 556, 738276. [Google Scholar] [CrossRef]
- Wen, C.; Xu, X.; Zhou, D.; Yu, Q.; Wang, T.; Zhou, Y. The Effects of Canthaxanthin Microencapsulation on Yolk Color and Canthaxanthin Deposition in Egg Yolk of Laying Hens. Poult. Sci. 2022, 101, 101889. [Google Scholar] [CrossRef]
- Srivastava, A.; Kalwani, M.; Chakdar, H.; Pabbi, S.; Shukla, P. Biosynthesis and Biotechnological Interventions for Commercial Production of Microalgal Pigments: A Review. Bioresour. Technol. 2022, 352, 127071. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, L.; Nobre, B.P.; Marcelo, F.M.; Mrejen, S.; Cardoso, M.T.; Palavra, A.F.; Mendes, R.L. Functional Food Oil Coloured by Pigments Extracted from Microalgae with Supercritical CO2. Food Chem. 2007, 101, 717–723. [Google Scholar] [CrossRef]
- Gong, Z.; Jiao, P.; Huang, F.; Zhang, S.; Zhou, B.; Lin, Q.; Liu, J.; Liang, Y. Separation and Antioxidant Activity of the Water-Soluble Yellow Monascus Pigment and Its Application in the Preparation of Functional Rice Noodles. LWT 2023, 185, 115172. [Google Scholar] [CrossRef]
- Kim, D.; Ku, S. Beneficial Effects of Monascus Sp. KCCM 10093 Pigments and Derivatives: A Mini Review. Molecules 2018, 23, 98. [Google Scholar] [CrossRef] [PubMed]
- Hyde, K.D.; Xu, J.; Rapior, S.; Jeewon, R.; Lumyong, S.; Niego, A.G.T.; Abeywickrama, P.D.; Aluthmuhandiram, J.V.S.; Brahamanage, R.S.; Brooks, S.; et al. The Amazing Potential of Fungi: 50 Ways We Can Exploit Fungi Industrially. Fungal Divers. 2019, 97, 1–136. [Google Scholar] [CrossRef]
- Abel, G.; Amobonye, A.; Bhagwat, P.; Pillai, S. Diversity, Stability and Applications of Mycopigments. Process Biochem. 2023, 133, 270–284. [Google Scholar] [CrossRef]
- Poorniammal, R.; Prabhu, S.; Dufossé, L.; Kannan, J. Safety Evaluation of Fungal Pigments for Food Applications. J. Fungi 2021, 7, 692. [Google Scholar] [CrossRef]
- Nabi, B.G.; Mukhtar, K.; Ahmed, W.; Manzoor, M.F.; Ranjha, M.M.A.N.; Kieliszek, M.; Bhat, Z.F.; Aadil, R.M. Natural Pigments: Anthocyanins, Carotenoids, Chlorophylls, and Betalains as Colorants in Food Products. Food Biosci. 2023, 52, 102403. [Google Scholar] [CrossRef]
- Singh, S.; Aeri, V.; Sharma, V. Encapsulated Natural Pigments: Techniques and Applications. J. Food Process Eng. 2023, e14311. [Google Scholar] [CrossRef]
- Mishra, S.; Mishra, N.; Pradesh, U. Food-related microbial pigments: Current issues and future prospects. Acta Biomed. 2023, 94, 471–483. [Google Scholar]
- Kramar, A.; Kostic, M.M. Bacterial Secondary Metabolites as Biopigments for Textile Dyeing. Textiles 2022, 2, 252–264. [Google Scholar] [CrossRef]
- Anahas, A.M.P.; Kumaran, S.; Kandeel, M.; Muralitharan, G.; Silviya, J.; Adhimoolam, G.L.; Panagal, M.; Pugazhvendan, S.R.; Suresh, G.; Wilson Aruni, A.; et al. Applications of Natural Violet Pigments from Halophilic Chromobacterium violaceum PDF23 for Textile Dyeing with Antimicrobial and Antioxidant Potentials. J. Nanomater. 2022, 2022, 3885396. [Google Scholar] [CrossRef]
- Perumal, K.; Stalin, V.; Chandrasekarenthiran, S.; Sumathi, E.; Saravanakumar, A. Extraction and Characterization of Pigment from Sclerotinia sp. and Its Use in Dyeing Cotton. Text. Res. J. 2009, 79, 1178–1187. [Google Scholar] [CrossRef]
- Bisht, G.; Srivastava, S.; Kulshreshtha, R.; Sourirajan, A.; Baumler, D.J.; Dev, K. Applications of Red Pigments from Psychrophilic Rhodonellum Psychrophilum GL8 in Health, Food and Antimicrobial Finishes on Textiles. Process Biochem. 2020, 94, 15–29. [Google Scholar] [CrossRef]
- Lagashetti, A.C.; Dufossé, L.; Singh, S.K.; Singh, P.N. Fungal Pigments and Their Prospects in Different Industries. Microorganisms 2019, 7, 604. [Google Scholar] [CrossRef]
- Chadni, Z.; Rahaman, M.H.; Jerin, I.; Hoque, K.M.F.; Reza, M.A. Extraction and Optimisation of Red Pigment Production as Secondary Metabolites from Talaromyces verruculosus and Its Potential Use in Textile Industries. Mycology 2017, 8, 48–57. [Google Scholar] [CrossRef]
- Suwannarach, N.; Kumla, J.; Nishizaki, Y.; Sugimoto, N.; Meerak, J.; Matsui, K.; Lumyong, S. Optimization and Characterization of Red Pigment Production from an Endophytic Fungus, Nigrospora Aurantiaca CMU-ZY2045, and Its Potential Source of Natural Dye for Use in Textile Dyeing. Appl. Microbiol. Biotechnol. 2019, 103, 6973–6987. [Google Scholar] [CrossRef]
- Mutaf-Kılıc, T.; Demir, A.; Elibol, M.; Oncel, S.S. Microalgae Pigments as a Sustainable Approach to Textile Dyeing: A Critical Review. Algal Res. 2023, 76, 103291. [Google Scholar] [CrossRef]
- Aman Mohammadi, M.; Ahangari, H.; Mousazadeh, S.; Hosseini, S.M.; Dufossé, L. Microbial Pigments as an Alternative to Synthetic Dyes and Food Additives: A Brief Review of Recent Studies. Bioprocess Biosyst. Eng. 2022, 45, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pombeirono-Sponchiado, S.R.; Sousa, G.S.; Andrade, J.C.R.; Lisboa, H.F.; Gonçalves, R.C.R. Production of Melanin Pigment by Fungi and Its Biotechnological Applications. In Melanin; Blumenberg, M., Ed.; InTech: London, UK, 2017. [Google Scholar]

















| Parameters | Mineral | Vegetable | Microbial |
|---|---|---|---|
| Price/production costs | ✓ | X | X |
| Biodegradability | X | ✓ | ✓ |
| Contamination free | X | X | ✓ |
| Renewable | X | ✓ | ✓ |
| Use of substrate | X | ✓ | ✓ |
| Resistant to climate change | ✓ | X | ✓ |
| Genetic manipulation | X | ✓ | ✓ |
| Microorganisms | Carotenoids and Their Structural Formula |
|---|---|
| Bacteria: Rhodococcus maris Micrococcus roseus Microbacterium sp. LEMMJ01 Gordonia jacobaea Bradyrhizobium sp. Cyanobacteria: Anabaena variabilis Aphanizomenon flos-aquae Nostoc commune | Canthaxanthin  |
| Bacteria: Microbacterium sp. LEMMJ01 Paracoccus sp. Halobacterium salinarium Microalgae: Haematococcus pluvialis Yeast: Phaffia rhodozyma (Xanthophyllomyces dendrohous) | Astaxanthin  |
| Bacteria: Pseudomonas putida Mycobacterium kansasii Microalgae: Dunaliella salina Spirulina Filamentous fungi: Blakeslea trispora Phycomyces blaskeleeanus Mucor circinelloides Yeast: Rhodotorula glutinis | β-carotene  |
| Pigment/ Chemical Class | Color | Biological Activity | Microorganism | Status | References |
|---|---|---|---|---|---|
| 99mTc-Anthraquinone pigment complex | Red | Antioxidant and anticancer | Talaromyces purpureogenus (Fungi) | Research pharmaceutical use | [136] |
| Indigoidine | Blue | Antioxidant | Corynebacterium insidiosum; Corynebacterium glutamicum; Streptomyces chromofuscus (Bacteria) | Research and industrial production | [25,46,137] |
| Melanin | Black | Antimicrobial, antibiofilm, and antioxidant | Colletotrichum lagenarium, Aspergillus fumigatus, Aureobasidium melanogenum (fungi) Vibrio cholerae, Shewanella colwelliana, Alteromonas nigrifaciens, (Bacteria) | Research | [138,139,140] |
| Monascorrubramin | Red | Antioxidant and anticancer | Monascus sp. (Fungi) | Research and industrial production | [141,142] |
| Prodigiosin | Red | Anticancer, antimicrobial, and immunosuppressant | Serratia marcescens; Pseudoalteromonas rubra (Bacteria) | Research and industrial production | [3,91,143] |
| Chlorophylls | Green | Improving immune system antioxidant and anticancer | Chlorella sp. Scenedesmus dimorphus Chlamydomonas reinhardtii (microalgae) | Research and industrial production | [144] |
| Astaxanthin | Red | Antioxidant, photoprotector, anti-inflammatory, anticancer, antimicrobial, and antihyperlipidemia that increases serum adiponectin. | Haematococcus pluvialis Chlorella sp. (microalgae) | Research and industrial production | [145,146,147] |
| Pyocanin | Blue-green | Antioxidant | Pseudomonas aeruginosa (Bacteria) | Research | [148] |
| Riboflavin | Yellow-orange | Nutritional supplement | Bacillus sp.; Ashbya gossypii (Bacteria) | Research and industrial production | [149,150] |
| Alternative Agro-Waste/ByProducts | Pigment Studied | Microorganisms | Pigment Yield | References |
|---|---|---|---|---|
| Wheat wastes | Astaxanthin | Yamadazyma guilliermondii, Xanthophyllomyces dendrorhous, Yarrowia lipolytica and Sporidiobolus salmonicolor | 109.23 μg/gram of waste maximum | [178] |
| Glycerol, Corn steep liquor, parboiled rice water | Carotenoids (B-carotene) | Sporidiobolus pararoseus | 843 μg/L total (346 ug/L b-carotene) | [179] |
| Rice straw hydrolysate with glucose medium | Extracellular azaphilones | Monascus sp. (mutant strain) | 20.86 U/mL | [180] |
| Food industry wastewater | Carotenoid | R. mucilaginosa | 810 μg/g | [181] |
| Pineapple peel waste | - | Talaromyces albobiverticillius | 0.523 mg/g | [182] |
| Corncob Hydrolysates | monascorubrin and rubropunctamine | T. atroroseus | 16.17 OD500 nm | [183] |
| Orange and grape wastes | β-carotene | R. glutinis | 5.9 g/L maximmum | [184] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barreto, J.V.d.O.; Casanova, L.M.; Junior, A.N.; Reis-Mansur, M.C.P.P.; Vermelho, A.B. Microbial Pigments: Major Groups and Industrial Applications. Microorganisms 2023, 11, 2920. https://doi.org/10.3390/microorganisms11122920
Barreto JVdO, Casanova LM, Junior AN, Reis-Mansur MCPP, Vermelho AB. Microbial Pigments: Major Groups and Industrial Applications. Microorganisms. 2023; 11(12):2920. https://doi.org/10.3390/microorganisms11122920
Chicago/Turabian StyleBarreto, João Vitor de Oliveira, Livia Marques Casanova, Athayde Neves Junior, Maria Cristina Pinheiro Pereira Reis-Mansur, and Alane Beatriz Vermelho. 2023. "Microbial Pigments: Major Groups and Industrial Applications" Microorganisms 11, no. 12: 2920. https://doi.org/10.3390/microorganisms11122920
APA StyleBarreto, J. V. d. O., Casanova, L. M., Junior, A. N., Reis-Mansur, M. C. P. P., & Vermelho, A. B. (2023). Microbial Pigments: Major Groups and Industrial Applications. Microorganisms, 11(12), 2920. https://doi.org/10.3390/microorganisms11122920