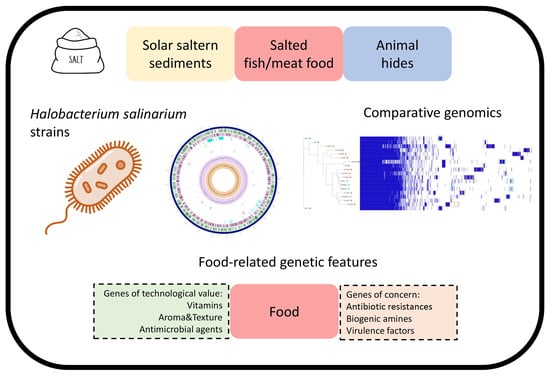
Comparative Genomics of Halobacterium salinarum Strains Isolated from Salted Foods Reveals Protechnological Genes for Food Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Molecular Fingerprinting of Halophilic Archaea from Salted Foods
2.2. Whole-Genome Sequencing and Comparative Genomics of Selected Halobacterium salinarum Strains
3. Results and Discussion
3.1. Molecular Fingerprinting of Halophilic Archaea Isolates
3.2. Comparative Genomic Analysis of H. salinarum Strains
3.2.1. Functional and Protechnological Genes in H. salinarum Food Strains
3.2.2. Genes of Concern in H. salinarum Food Strains
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Oren, A.; Ventosa, A.; Grant, W.D. Proposed Minimal Standards for Description of New Taxa in the Order Halobacteriales. Int. J. Syst. Evol. Microbiol. 1997, 47, 233–238. [Google Scholar] [CrossRef] [Green Version]
- Corral, P.; Corcelli, A.; Ventosa, A. Halostagnicola Bangensis Sp. Nov., an Alkaliphilic Haloarchaeon from a Soda Lake. Int. J. Syst. Evol. Microbiol. 2015, 65, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, M.; Kannan, V.; Pašić, L. Diversity of Microorganisms in Solar Salterns of Tamil Nadu, India. World J. Microbiol. Biotechnol. 2009, 25, 1007–1017. [Google Scholar] [CrossRef]
- Pfeifer, F. Haloarchaea and the Formation of Gas Vesicles. Life 2015, 5, 385–402. [Google Scholar] [CrossRef]
- Roh, S.W.; Lee, M.L.; Bae, J.W. Haladaptatus Cibarius Sp. Nov., an Extremely Halophilic Archaeon from Seafood, and Emended Description of the Genus Haladaptatus. Int. J. Syst. Evol. Microbiol. 2010, 60, 1187–1190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roh, S.W.; Bae, J.W. Halorubrum Cibi Sp. Nov., an Extremely Halophilic Archaeon from Salt-Fermented Seafood. J. Microbiol. 2009, 47, 162–166. [Google Scholar] [CrossRef]
- Tapingkae, W.; Tanasupawat, S.; Itoh, T.; Parkin, K.L.; Benjakul, S.; Visessanguan, W.; Valyasevi, R. Natrinema Gari Sp. Nov., a Halophilic Archaeon Isolated from Fish Sauce in Thailand. Int. J. Syst. Evol. Microbiol. 2008, 58, 2378–2383. [Google Scholar] [CrossRef] [PubMed]
- DeLong, E.F. Archaea in Coastal Marine Environments. Proc. Natl. Acad. Sci. USA 1992, 89, 5685–5689. [Google Scholar] [CrossRef] [Green Version]
- Brown, A.D. Microbial Water Stress. Bacteriol. Rev. 1976, 40, 803–846. [Google Scholar] [CrossRef]
- Pitt, J.I. Xerophilic Fungi and the Spoilage of Foods of Plant Origin. In Water Relations of Foods; Academic Press: Cambridge, MA, USA, 1975. [Google Scholar]
- Lee, C.J.D.; McMullan, P.E.; O’Kane, C.J.; Stevenson, A.; Santos, I.C.; Roy, C.; Ghosh, W.; Mancinelli, R.L.; Mormile, M.R.; McMullan, G.; et al. NaCl-Saturated Brines Are Thermodynamically Moderate, Rather than Extreme, Microbial Habitats. FEMS Microbiol. Rev. 2018, 42, 672–693. [Google Scholar] [CrossRef] [Green Version]
- Stevenson, A.; Cray, J.A.; Williams, J.P.; Santos, R.; Sahay, R.; Neuenkirchen, N.; McClure, C.D.; Grant, I.R.; Houghton, J.D.; Quinn, J.P.; et al. Is There a Common Water-Activity Limit for the Three Domains of Life. ISME J. 2015, 9, 1333–1351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oren, A. Microbial Life at High Salt Concentrations: Phylogenetic and Metabolic Diversity. Saline Syst. 2008, 4, 2. [Google Scholar] [CrossRef] [Green Version]
- Karan, R.; Capes, M.D.; DasSarma, S. Function and Biotechnology of Extremophilic Enzymes in Low Water Activity. Aquat. Biosyst. 2012, 8, 4. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, S.P.; Ng, W.V.; Salzberg, S.L.; Hood, L.; DasSarma, S. Understanding the Adaptation of Halobacterium Species NRC-1 to Its Extreme Environment through Computational Analysis of Its Genome Sequence. Genome Res. 2001, 11, 1641–1650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capes, M.D.; DasSarma, P.; DasSarma, S. The Core and Unique Proteins of Haloarchaea. BMC Genom. 2012, 13, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vauclare, P.; Marty, V.; Fabiani, E.; Martinez, N.; Jasnin, M.; Gabel, F.; Peters, J.; Zaccai, G.; Franzetti, B. Molecular Adaptation and Salt Stress Response of Halobacterium salinarum Cells Revealed by Neutron Spectroscopy. Extremophiles 2015, 19, 1099–1107. [Google Scholar] [CrossRef]
- Akolkar, A.V.; Durai, D.; Desai, A.J. Halobacterium Sp. SP1(1) as a Starter Culture for Accelerating Fish Sauce Fermentation. J. Appl. Microbiol. 2010, 109, 44–53. [Google Scholar] [CrossRef]
- Aponte, M.; Blaiotta, G.; Francesca, N.; Moschetti, G. Could Halophilic Archaea Improve the Traditional Salted Anchovies (Engraulis Encrasicholus L.) Safety and Quality? Lett. Appl. Microbiol. 2010, 51, 697–703. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.S. Diversity of Halophilic Archaea in Fermented Foods and Human Intestines and Their Application. J. Microbiol. Biotechnol. 2013, 23, 1645–1653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ventosa, A.; Oren, A. Halobacterium salinarum Nom. Corrig., a Name to Replace Halobacterium salinarium (Elazari-Volcani) and to Include Halobacterium halobium and Halobacterium cutirubrum. Int. J. Syst. Evol. Microbiol. 1996, 46, 347. [Google Scholar] [CrossRef] [Green Version]
- DasSarma, P.; DasSarma, S. On the Origin of Prokaryotic “Species”: The Taxonomy of Halophilic Archaea. Saline Syst. 2008, 4, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matarredona, L.; Camacho, M.; Zafrilla, B.; Bonete, M.J.; Esclapez, J. The Role of Stress Proteins in Haloarchaea and Their Adaptive Response to Environmental Shifts. Biomolecules 2020, 10, 1390. [Google Scholar] [CrossRef] [PubMed]
- Bonneau, R.; Facciotti, M.T.; Reiss, D.J.; Schmid, A.K.; Pan, M.; Kaur, A.; Thorsson, V.; Shannon, P.; Johnson, M.H.; Bare, J.C.; et al. A Predictive Model for Transcriptional Control of Physiology in a Free Living Cell. Cell 2007, 131, 1354–1365. [Google Scholar] [CrossRef] [Green Version]
- Pfeifer, K.; Ergal, İ.; Koller, M.; Basen, M.; Schuster, B.; Rittmann, S.K.M.R. Archaea Biotechnology. Biotechnol. Adv. 2021, 47, 107668. [Google Scholar] [CrossRef]
- Margesin, R.; Schinner, F. Potential of Halotolerant and Halophilic Microorganisms for Biotechnology. Extremophiles 2001, 5, 73–83. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, B.; van Belkum, M.J.; Diep, D.B.; Chikindas, M.L.; Ermakov, A.M.; Tiwari, S.K. Halocins, Natural Antimicrobials of Archaea: Exotic or Special or Both? Biotechnol. Adv. 2021, 53, 107834. [Google Scholar] [CrossRef]
- Versalovic, J.; Schneider, M.; de Bruijn, F.J.; Lupski, J.R. Genomic Fingerprinting of Bacteria Using Repetitive Sequence-Based Polymerase Chain Reaction. Methods Mol. Cell. Biol. 1994, 5, 25–40. [Google Scholar]
- Henriet, O.; Fourmentin, J.; Delincé, B.; Mahillon, J. Exploring the Diversity of Extremely Halophilic Archaea in Food-Grade Salts. Int. J. Food Microbiol. 2014, 191, 36–44. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [Green Version]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving Bacterial Genome Assemblies from Short and Long Sequencing Reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef] [Green Version]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality Assessment Tool for Genome Assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parks, D.H.; Imelfort, M.; Skennerton, C.T.; Hugenholtz, P.; Tyson, G.W. CheckM: Assessing the Quality of Microbial Genomes Recovered from Isolates, Single Cells, and Metagenomes. Genome Res. 2015, 25, 1043–1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meier-Kolthoff, J.P.; Göker, M. TYGS Is an Automated High-Throughput Platform for State-of-the-Art Genome-Based Taxonomy. Nat. Commun. 2019, 10, 2182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seemann, T. Prokka: Rapid Prokaryotic Genome Annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef] [Green Version]
- Page, A.J.; Cummins, C.A.; Hunt, M.; Wong, V.K.; Reuter, S.; Holden, M.T.G.; Fookes, M.; Falush, D.; Keane, J.A.; Parkhill, J. Roary: Rapid Large-Scale Prokaryote Pan Genome Analysis. Bioinformatics 2015, 31, 3691–3693. [Google Scholar] [CrossRef] [Green Version]
- Fontana, A.; Zacconi, C.; Morelli, L. Genetic Signatures of Dairy Lactobacillus casei Group. Front. Microbiol. 2018, 9, 2611. [Google Scholar] [CrossRef]
- Bourdichon, F.; Patrone, V.; Fontana, A.; Milani, G.; Morelli, L. Safety Demonstration of a Microbial Species for Use in the Food Chain: Weissella confusa. Int. J. Food Microbiol. 2021, 339, 109028. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. The Relative Contribution of Human Past: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontologia Electronica. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- de Jong, A.; van Hijum, S.A.F.T.; Bijlsma, J.J.E.; Kok, J.; Kuipers, O.P. BAGEL: A Web-Based Bacteriocin Genome Mining Tool. Nucleic Acids Res. 2006, 34, 273–279. [Google Scholar] [CrossRef]
- Jia, B.; Raphenya, A.R.; Alcock, B.; Waglechner, N.; Guo, P.; Tsang, K.K.; Lago, B.A.; Dave, B.M.; Pereira, S.; Sharma, A.N.; et al. CARD 2017: Expansion and Model-Centric Curation of the Comprehensive Antibiotic Resistance Database. Nucleic Acids Res. 2017, 45, D566–D573. [Google Scholar] [CrossRef]
- Chen, L.; Zheng, D.; Liu, B.; Yang, J.; Jin, Q. VFDB 2016: Hierarchical and Refined Dataset for Big Data Analysis—10 Years On. Nucleic Acids Res. 2016, 44, D694–D697. [Google Scholar] [CrossRef] [PubMed]
- Gaba, S.; Kumari, A.; Medema, M.; Kaushik, R. Pan-Genome Analysis and Ancestral State Reconstruction of Class Halobacteria: Probability of a New Super-Order. Sci. Rep. 2020, 10, 21205. [Google Scholar] [CrossRef] [PubMed]
- Inglin, R.C.; Meile, L.; Stevens, M.J.A. Clustering of Pan- and Core-Genome of Lactobacillus Provides Novel Evolutionary Insights for Differentiation. BMC Genom. 2018, 19, 284. [Google Scholar] [CrossRef] [PubMed]
- Fontana, A.; Falasconi, I.; Molinari, P.; Treu, L.; Basile, A.; Vezzi, A.; Campanaro, S.; Morelli, L. Genomic Comparison of Lactobacillus helveticus strains Highlights Probiotic Potential. Front. Microbiol. 2019, 10, 1380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McInerney, J.O.; Whelan, F.J.; Domingo-Sananes, M.R.; McNally, A.; O’Connell, M.J. Pangenomes and Selection: The Public Goods Hypothesis. In The Pangenome: Diversity, Dynamics and Evolution of Genomes; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Fiorentino, G.; Ronca, R.; Cannio, R.; Rossi, M.; Bartolucci, S. MarR-like Transcriptional Regulator Involved in Detoxification of Aromatic Compounds in Sulfolobus solfataricus. J. Bacteriol. 2007, 189, 7351–7360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nandan, A.; Nampoothiri, K.M. Therapeutic and Biotechnological Applications of Substrate Specific Microbial Aminopeptidases. Appl. Microbiol. Biotechnol. 2020, 104, 5243–5257. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Cao, S.M. Study on Endogenous Protease and Protein Degradation of Dry-Salted Decapterus Maruadsi. J. Food 2018, 16, 350–356. [Google Scholar] [CrossRef] [Green Version]
- Udomsil, N.; Pongjanla, S.; Rodtong, S.; Tanasupawat, S.; Yongsawatdigul, J. Extremely Halophilic Strains of Halobacterium salinarum as a Potential Starter Culture for Fish Sauce Fermentation. J. Food Sci. 2022, 87, 5375–5389. [Google Scholar] [CrossRef]
- Rawat, M.; Maupin-Furlow, J.A. Redox and Thiols in Archaea. Antioxidants 2020, 9, 381. [Google Scholar] [CrossRef]
- Smirnov, A.; Suzina, N.; Chudinova, N.; Kulakovskaya, T.; Kulaev, I. Formation of Insoluble Magnesium Phosphates during Growth of the Archaea Halorubrum distributum and Halobacterium salinarium and the Bacterium Brevibacterium antiquum. FEMS Microbiol. Ecol. 2005, 52, 129–137. [Google Scholar] [CrossRef]
- Leblanc, J.G.; Laiño, J.E.; del Valle, M.J.; Vannini, V.; van Sinderen, D.; Taranto, M.P.; de Valdez, G.F.; de Giori, G.S.; Sesma, F. B-Group Vitamin Production by Lactic Acid Bacteria—Current Knowledge and Potential Applications. J. Appl. Microbiol. 2011, 111, 1297–1309. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Shah, N.; Prajapati, J.B. Biosynthesis of Vitamins and Enzymes in Fermented Foods by Lactic Acid Bacteria and Related Genera—A Promising Approach. Croat. J. Food Sci. Technol. 2013, 5, 85–91. [Google Scholar]
- Woodson, J.D.; Reynolds, A.A.; Escalante-Semerena, J.C. ABC Transporter for Corrinoids in Halobacterium sp. Strain NRC-1. J. Bacteriol. 2005, 187, 5901–5909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, H.; Kang, J.; Zhang, D. Microbial Production of Vitamin B12: A Review and Future Perspectives. Microb. Cell Fact. 2017, 16, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodson, J.D.; Peck, R.F.; Krebs, M.P.; Escalante-Semerena, J.C. The CobY Gene of the Archaeon Halobacterium sp. Strain NRC-1 Is Required for de Novo Cobamide Synthesis. Proc. J. Bacteriol. 2003, 185, 311–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deptula, P.; Chamlagain, B.; Edelmann, M.; Sangsuwan, P.; Nyman, T.A.; Savijoki, K.; Piironen, V.; Varmanen, P. Food-like Growth Conditions Support Production of Active Vitamin B12 by Propionibacterium freudenreichii 2067 without DMBI, the Lower Ligand Base, or Cobalt Supplementation. Front. Microbiol. 2017, 8, 368. [Google Scholar] [CrossRef] [Green Version]
- Capozzi, V.; Russo, P.; Dueñas, M.T.; López, P.; Spano, G. Lactic Acid Bacteria Producing B-Group Vitamins: A Great Potential for Functional Cereals Products. Appl. Microbiol. Biotechnol. 2012, 96, 1383–1394. [Google Scholar] [CrossRef]
- Burgess, C.M.; Smid, E.J.; van Sinderen, D. Bacterial Vitamin B2, B11 and B12 Overproduction: An Overview. Int. J. Food Microbiol. 2009, 133, 1–7. [Google Scholar] [CrossRef]
- Xiao, Z.; Lu, J.R. Generation of Acetoin and Its Derivatives in Foods. J. Agric. Food Chem. 2014, 62, 6487–6497. [Google Scholar] [CrossRef]
- Hong, S.B.; Choi, J.H.; Chang, Y.K.; Mun, S. Production of High-Purity Fucose from the Seaweed of Undaria Pinnatifida through Acid-Hydrolysis and Simulated-Moving Bed Purification. Sep. Purif. Technol. 2019, 213, 133–141. [Google Scholar] [CrossRef]
- Xiao, M.; Ren, X.; Yu, Y.; Gao, W.; Zhu, C.; Sun, H.; Kong, Q.; Fu, X.; Mou, H. Fucose-Containing Bacterial Exopolysaccharides: Sources, Biological Activities, and Food Applications. Food Chem. X 2022, 13, 100233. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ruiz, C.; Srivastava, G.K.; Carranza, D.; Mata, J.A.; Llamas, I.; Santamaría, M.; Quesada, E.; Molina, I.J. An Exopolysaccharide Produced by the Novel Halophilic Bacterium Halomonas stenophila Strain B100 Selectively Induces Apoptosis in Human T Leukaemia Cells. Appl. Microbiol. Biotechnol. 2011, 89, 345–355. [Google Scholar] [CrossRef]
- Li, J.; Xu, H.; Chen, X.; Xu, L.; Cheng, R.; Zhang, J.; Wang, S. Characterization of an Exopolysaccharide with Distinct Rheological Properties from Paenibacillus edaphicus NUST16. Int. J. Biol. Macromol. 2017, 105, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rani, R.P.; Anandharaj, M.; David Ravindran, A. Characterization of a Novel Exopolysaccharide Produced by Lactobacillus gasseri FR4 and Demonstration of Its in Vitro Biological Properties. Int. J. Biol. Macromol. 2018, 109, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Yan, J.; Yang, H. Inositol Nutritional Supplementation for the Prevention of Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2022, 14, 2831. [Google Scholar] [CrossRef]
- Dinicola, S.; Unfer, V.; Facchinetti, F.; Soulage, C.O.; Greene, N.D.; Bizzarri, M.; Laganà, A.S.; Chan, S.Y.; Bevilacqua, A.; Pkhaladze, L.; et al. Inositols: From Established Knowledge to Novel Approaches. Int. J. Mol. Sci. 2021, 22, 10575. [Google Scholar] [CrossRef] [PubMed]
- Croze, M.L.; Soulage, C.O. Potential Role and Therapeutic Interests of Myo-Inositol in Metabolic Diseases. Biochimie 2013, 95, 1811–1827. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xiang, H.; Liu, J.; Zhou, M.; Tan, H. Purification and Biological Characterization of Halocin C8, a Novel Peptide Antibiotic from Halobacterium Strain AS7092. Extremophiles 2003, 7, 401–407. [Google Scholar] [CrossRef]
- Shand, R.F.; Leyva, K.J. Archaeal Antimicrobials: An Undiscovered Country. In Archaea: New Models for Prokaryotic Biology; Caister Academic Press: Norfolk, UK, 2008. [Google Scholar]
- Galal, A.; Abou Elhassan, S.; Saleh, A.H.; Ahmed, A.I.; Abdelrahman, M.M.; Kamal, M.M.; Khalel, R.S.; Ziko, L. A Survey of the Biosynthetic Potential and Specialized Metabolites of Archaea and Understudied Bacteria. Curr. Res. Biotechnol. 2023, 5, 100117. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Li, G.; Yang, Y.; Ding, W. Current Advancements in Sactipeptide Natural Products. Front. Chem. 2021, 9, 595991. [Google Scholar] [CrossRef] [PubMed]
- van Wolferen, M.; Orell, A.; Albers, S.V. Archaeal Biofilm Formation. Nat. Rev. Microbiol. 2018, 16, 699–713. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Rebuffat, S. The Manifold Roles of Microbial Ribosomal Peptide-Based Natural Products in Physiology and Ecology. J. Biol. Chem. 2020, 295, 34–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Price, M.T.; Fullerton, H.; Moyer, C.L. Biogeography and Evolution of Thermococcus Isolates from Hydrothermal Vent Systems of the Pacific. Front. Microbiol. 2015, 6, 968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckburg, P.B.; Lepp, P.W.; Relman, D.A. Archaea and Their Potential Role in Human Disease. Infect Immun. 2003, 71, 591–596. [Google Scholar] [CrossRef] [Green Version]
- Falb, M.; Müller, K.; Königsmaier, L.; Oberwinkler, T.; Horn, P.; von Gronau, S.; Gonzalez, O.; Pfeiffer, F.; Bornberg-Bauer, E.; Oesterhelt, D. Metabolism of Halophilic Archaea. Extremophiles 2008, 12, 177–196. [Google Scholar] [CrossRef] [Green Version]
- Higashibata, H.; Fujiwara, S.; Ezaki, S.; Takagi, M.; Fukui, K.; Imanaka, T. Effect of Polyamines on Histone-Induced DNA Compaction of Hyperthermophilic Archaea. J. Biosci. Bioeng. 2000, 89, 103–106. [Google Scholar] [CrossRef]
- Alfonzo, A.; Randazzo, W.; Barbera, M.; Sannino, C.; Corona, O.; Settanni, L.; Moschetti, G.; Santulli, A.; Francesca, N. Effect of Salt Concentration and Extremely Halophilic Archaea on the Safety and Quality Characteristics of Traditional Salted Anchovies. J. Aquat. Food Prod. Technol. 2017, 26, 620–637. [Google Scholar] [CrossRef] [Green Version]
- Bay, D.C.; Rommens, K.L.; Turner, R.J. Small Multidrug Resistance Proteins: A Multidrug Transporter Family that Continues to Grow. Biochim. Biophys. Acta Biomembr. 2008, 1778, 1814–1838. [Google Scholar] [CrossRef] [Green Version]
- EFSA Statement on the Requirements for Whole Genome Sequence Analysis of Microorganisms Intentionally Used in the Food Chain. EFSA J. 2021, 19, e06506. [CrossRef]
- Patenge, N.; Berendes, A.; Engelhardt, H.; Schuster, S.C.; Oesterhelt, D. The Fla Gene Cluster Is Involved in the Biogenesis of Flagella in Halobacterium salinarum. Mol. Microbiol. 2001, 41, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Losensky, G.; Vidakovic, L.; Klingl, A.; Pfeifer, F.; Frölsl, S. Novel Pili-like Surface Structures of Halobacterium salinarum Strain R1 Are Crucial for Surface Adhesion. Front. Microbiol. 2014, 5, 755. [Google Scholar] [CrossRef]
- Duan, Q.; Zhou, M.; Zhu, L.; Zhu, G. Flagella and Bacterial Pathogenicity. J. Basic Microbiol. 2013, 53, 1–8. [Google Scholar] [CrossRef]
- Niessen, N.; Soppa, J. Regulated Iron Siderophore Production of the Halophilic Archaeon Haloferax volcanii. Biomolecules 2020, 10, 1072. [Google Scholar] [CrossRef] [PubMed]
- Sandy, M.; Butler, A. Microbial Iron Acquisition: Marine and Terrestrial Siderophores. Chem. Rev. 2009, 109, 4580–4595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischbach, M.A.; Lin, H.; Liu, D.R.; Walsh, C.T. How Pathogenic Bacteria Evade Mammalian Sabotage in the Battle for Iron. Nat. Chem. Biol. 2006, 2, 132–138. [Google Scholar] [CrossRef]
- Correnti, C.; Strong, R.K. Mammalian Siderophores, Siderophore-Binding Lipocalins, and the Labile Iron Pool. J. Biol. Chem. 2012, 287, 13524–13531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dave, B.P.; Anshuman, K.; Hajela, P. Siderophores of Halophilic Archaea and Their Chemical Characterization. Indian J. Exp. Biol. 2006, 44, 340–344. [Google Scholar] [PubMed]




| Strain | Isolation Source | Isolation Category | Genome Source |
|---|---|---|---|
| CECT 395 1 | salted codfish | Food | This study |
| UC4235 | salted codfish | ||
| UC4236 | salted codfish | ||
| UC4237 | salted codfish | ||
| UC4238 | bacon | ||
| UC4239 | bacon | ||
| UC4240 | bacon | ||
| UC4241 | sausage casing | ||
| UC4242 | sausage casing | ||
| UC4243 | sausage casing | ||
| DSM 3754T 2 | salted cowhide | Animal | NCBI |
| DSM 669 | salted buffalo hide | ||
| DSM 671 | salted hide | ||
| NRC-1 | salted hide | ||
| AS2 | solar saltern sediment | Environmental | NCBI |
| AS8 | solar saltern sediment | ||
| AS11 | solar saltern sediment | ||
| AS19 | solar saltern sediment | ||
| CBA1132 | solar saltern sediment |
| Functional and Protechnological Categories | Gene Annotation | CECT 395 | UC 4235 | UC 4236 | UC 4237 | UC 4238 | UC 4239 | UC 4240 | UC 4241 | UC 4242 | UC 4243 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Folate biosynthesis | GTP cyclohydrolase I (EC 3.5.4.16) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 |
| 6-carboxy-5,6,7,8-tetrahydropterin synthase (EC 4.1.2.50) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | |
| 7-carboxy-7-deazaguanine synthase (EC 4.3.99.3) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | |
| 7-cyano-7-deazaguanine synthase (EC 6.3.4.20) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | |
| Pantothenate and CoA biosynthesis | Ketol-acid reductoisomerase (NADP(+)) (EC 1.1.1.86) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 |
| Dihydroxy-acid dehydratase (EC 4.2.1.9) | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | |
| Acetolactate synthase (EC 2.2.1.6) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | |
| Menaquinone biosynthesis | Isochorismate synthase (EC 5.4.4.2) | 1 | 2 | 2 | 1 | 1 | 2 | 2 | 2 | 1 | 1 |
| 2-succinyl-5-enolpyruvyl-6-hydroxy-3-cyclohexene-1-carboxylic-acid synthase (EC 2.2.1.9) | 1 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 1 | 1 | |
| O-succinylbenzoate synthase (EC 4.2.1.113) | 0 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | |
| O-succinylbenzoic acid–CoA ligase (EC 6.2.1.26) | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | |
| Naphthoate synthase (EC 4.1.3.36) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 1,4-dihydroxy-2-naphthoate polyprenyltransferase (EC 2.5.1.74) | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Demethylmenaquinone methyltransferase (EC 2.1.1.163) | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 2 | |
| Vitamin B12 coenzyme 1 | Cob(I)alamin adenosyltransferase (EC 2.5.1.17) | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Cobalamin synthase (EC 2.7.8.26) | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 2 | 1 | 2 | |
| Aroma and texture | Acetolactate synthase (EC 2.2.1.6) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 |
| GDP-L-fucose synthetase (EC 1.1.1.271) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | |
| Polyol biosynthesis | Inositol-1-monophosphatase (EC 3.1.3.25) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Antimicrobial compounds | Halocin-C8 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Sactipeptide | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Categories of Concern | Gene Annotation | CECT 395 | UC 4235 | UC 4236 | UC 4237 | UC 4238 | UC 4239 | UC 4240 | UC 4241 | UC 4242 | UC 4243 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Biogenic amines | Pyruvoyl-dependent arginine decarboxylase (EC 4.1.1.19) | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Agmatinase (EC 3.5.3.11) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Tyrosine decarboxylase (EC 4.1.1.25) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Antibiotic resistance | Multidrug efflux SMR transporter protein HsmR/QacG | 0 | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 1 | 1 |
| Putative virulence factors | Flagellin (flgA1, flgA2) | 4 | 5 | 7 | 6 | 6 | 6 | 7 | 2 | 5 | 6 |
| Flagella E | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Flagellar protein G | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |
| Putative flagella-related protein H (FlaH) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | |
| Type IV pilin | 1 | 3 | 4 | 1 | 2 | 2 | 1 | 1 | 1 | 1 | |
| Prepilin peptidase | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Flp pilus assembly complex ATPase component (TadA) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| IucA/IucC family siderophore biosynthesis protein | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fontana, A.; Falasconi, I.; Bellassi, P.; Fanfoni, E.; Puglisi, E.; Morelli, L. Comparative Genomics of Halobacterium salinarum Strains Isolated from Salted Foods Reveals Protechnological Genes for Food Applications. Microorganisms 2023, 11, 587. https://doi.org/10.3390/microorganisms11030587
Fontana A, Falasconi I, Bellassi P, Fanfoni E, Puglisi E, Morelli L. Comparative Genomics of Halobacterium salinarum Strains Isolated from Salted Foods Reveals Protechnological Genes for Food Applications. Microorganisms. 2023; 11(3):587. https://doi.org/10.3390/microorganisms11030587
Chicago/Turabian StyleFontana, Alessandra, Irene Falasconi, Paolo Bellassi, Elisabetta Fanfoni, Edoardo Puglisi, and Lorenzo Morelli. 2023. "Comparative Genomics of Halobacterium salinarum Strains Isolated from Salted Foods Reveals Protechnological Genes for Food Applications" Microorganisms 11, no. 3: 587. https://doi.org/10.3390/microorganisms11030587
APA StyleFontana, A., Falasconi, I., Bellassi, P., Fanfoni, E., Puglisi, E., & Morelli, L. (2023). Comparative Genomics of Halobacterium salinarum Strains Isolated from Salted Foods Reveals Protechnological Genes for Food Applications. Microorganisms, 11(3), 587. https://doi.org/10.3390/microorganisms11030587