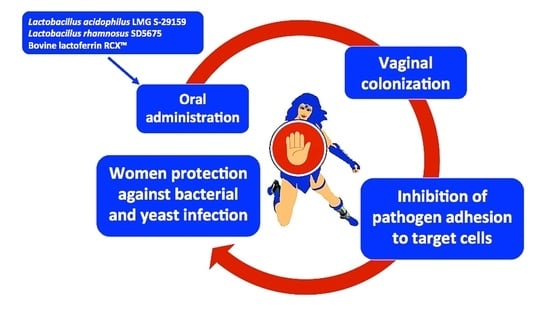
Warding Off Recurrent Yeast and Bacterial Vaginal Infections: Lactoferrin and Lactobacilli
Abstract
:1. Introduction
2. Preclinical Research: Evaluation of Effects in Cells and Animals
2.1. Lactoferrin
2.2. Lactobacilli
2.3. Combination of Bovine Lactoferrin with Lactoferrin-Resistant Probiotics
3. Clinical Studies
3.1. Lactoferrin Treatment in Women with Bacterial and Yeast Vaginal Diseases
3.2. Lactobacilli Treatment in Women with Uro-Genital Infections
3.3. Combined Lactoferrin and Lactobacilli Treatment in Woman with Vaginal Bacterial and Fungal Infections
3.4. Factors Affecting Safety and Efficacy of Probiotics
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ma, B.; Forney, L.J.; Ravel, J. Vaginal microbiome: Rethinking health and disease. Annu. Rev. Microbiol. 2012, 66, 371–389. [Google Scholar] [CrossRef] [Green Version]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2011, 108, 4680–4687. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Brown, C.J.; Abdo, Z.; Davis, C.C.; Hansmann, M.A.; Joyce, P.; Foster, J.A.; Forney, L.J. Differences in the composition of vaginal microbial communities found in healthy Caucasian and black women. ISME J. 2007, 1, 121–133. [Google Scholar] [CrossRef]
- Mastromarino, P.; Vitali, B.; Mosca, L. Bacterial vaginosis: A review on clinical trials with probiotics. New Microbiol. 2013, 36, 229–238. [Google Scholar]
- Mulu, W.; Yimer, M.; Zenebe, Y.; Abera, B. Common causes of vaginal infections and antibiotic susceptibility of aerobic bacterial isolates in women of reproductive age attending at Felegehiwot Referral Hospital, Ethiopia: A cross sectional study. BMC Womens Health 2015, 15, 42. [Google Scholar] [CrossRef] [Green Version]
- Donders, G.G.; Van Calsteren, K.; Bellen, G.; Reybrouck, R.; Van den Bosch, T.; Riphagen, I.; Van Lierde, S. Predictive value for preterm birth of abnormal vaginal flora, bacterial vaginosis and aerobic vaginitis during the first trimester of pregnancy. BJOG 2009, 116, 1315–1324. [Google Scholar] [CrossRef]
- Jahic, M.; Mulavdic, M.; Nurkic, J.; Jahic, E.; Nurkic, M. Clinical characteristics of aerobic vaginitis and its association to vaginal candidiasis, trichomonas vaginitis and bacterial vaginosis. Med. Arch. 2013, 67, 428–430. [Google Scholar] [CrossRef] [Green Version]
- Turovskiy, Y.; Noll, K.S.; Chikindas, M.L. The etiology of bacterial vaginosis. J. Appl. Microbiol. 2011, 110, 1105–1128. [Google Scholar] [CrossRef] [PubMed]
- Rampersaud, R.; Randis, T.M.; Ratner, A.J. Microbiota of the upper and lower genital tract. Semin. Fetal Neonatal Med. 2012, 17, 51–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarbo, G.; Coco, L.; Leanza, V.; Genovese, F.; Leanza, G.; D’Agati, A.; Giannone, T.T.; Giunta, M.R.; Palumbo, M.A.; Carbonaro, A.; et al. Aerobic vaginitis during pregnancy. Res. Obstet. Gynecol. 2013, 2, 7–11. [Google Scholar] [CrossRef]
- Srinivasan, U.; Misra, D.; Marazita, M.L.; Foxman, B. Vaginal and oral microbes, host genotype and preterm birth. Med. Hypotheses 2009, 73, 963–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donders, G.G. Definition and classification of abnormal vaginal flora. Best Pract. Res. Clin. Obstet. Gynaecol. 2007, 21, 355–373. [Google Scholar] [CrossRef] [PubMed]
- Donders, G.G.; Vereecken, A.; Bosmans, E.; Dekeersmaecker, A.; Salembier, G.; Spitz, B. Definition of a type of abnormal vaginal flora that is distinct from bacterial vaginosis: Aerobic vaginitis. BJOG Int. J. Obstet. Gynaecol. 2002, 109, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Lobos, O.; Padila, C. Phenotypic characterisation and genomic DNA polymorphisms of Escherichia coli strains isolated as the sole microorganisms from vaginal infections. Microbiology 2009, 3, 825–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobel, J.D.; Reichman, O.; Misra, D.; Yoo, W. Prognosis and treatment of desquamative inflammatory vaginitis. Obstet. Gynecol. 2011, 117, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Sobel, J.D. Vulvovaginal candidosis. Lancet 2007, 369, 1961–1971. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention-2015 Sexually Transmitted Diseases Treatment Guidelines Diseases Characterized by Vaginal Discharge-CDC Bacterial Vaginosis. Available online: https://www.cdc.gov/std/tg2015/bv.htm (accessed on 14 February 2018).
- Borges, S.; Silva, J.; Teixeira, P. The role of lactobacilli and probiotics in maintaining vaginal health. Arch. Gynecol. Obstet. 2014, 289, 479–489. [Google Scholar] [CrossRef]
- Beigi, R.H.; Austin, M.N.; Meyn, L.A.; Krohn, M.A.; Hillier, S.L. Antimicrobial resistance associated with the treatment of bacterial vaginosis. Am. J. Obstet. Gynecol. 2004, 191, 1124–1129. [Google Scholar] [CrossRef]
- Ferris, M.J.; Masztal, A.; Aldridge, K.E.; Fortenberry, J.D.; Fidel, P.L., Jr.; Martin, D.H. Association of Atopobium vaginae, a recently described metronidazole resistant anaerobe, with bacterial vaginosis. BMC Infect. Dis. 2004, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Bradshaw, C.S.; Tabrizi, S.N.; Fairley, C.K.; Morton, A.N.; Rudland, E.; Garland, S.M. The association of Atopobium vaginae and Gardnerella vaginalis with bacterial vaginosis and recurrence after oral metronidazole therapy. J. Infect. Dis. 2006, 194, 828–836. [Google Scholar] [CrossRef] [Green Version]
- Bradshaw, C.S.; Morton, A.N.; Hocking, J.; Garland, S.M.; Morris, M.B.; Moss, L.M.; Horvath, L.B.; Kuzevska, I.; Fairley, C.K. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J. Infect. Dis. 2006, 193, 1478–1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cribby, S.; Taylor, M.; Reid, G. Vaginal microbiota and the use of probiotics. Interdiscip. Perspect. Infect. Dis. 2008, 2008, 256490. [Google Scholar] [CrossRef] [PubMed]
- Homayouni, A.; Bastani, P.; Ziyadi, S.; Mohammad-Alizadeh-Charandabi, S.; Ghalibaf, M.; Mortazavian, A.M.; Mehrabany, E.V. Effects of probiotics on the recurrence of bacterial vaginosis: A review. Low Genit. Tract Dis. 2014, 18, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Nyirjesy, P.; Robinson, J.; Mathew, L.; Lev-Sagie, A.; Reyes, I.; Culhane, J.F. Alternative therapies in women with chronic vaginitis. Obstet. Gynecol. 2011, 117, 856–861. [Google Scholar] [CrossRef]
- Health and Nutrition Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria; Food and Agriculture Organization of the United Nations and World Health Organization: Córdoba, Argentina, 2001; Available online: http://www.fao.org/3/a-a0512e.pdf (accessed on 1 May 2006).
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [Green Version]
- Bertuccini, L.; Russo, R.; Iosi, F.; Superti, F. Lactobacilli and lactoferrin: Biotherapeutic effects for vaginal health. J. Funct. Foods 2018, 45, 86–94. [Google Scholar] [CrossRef]
- González-Chávez, S.A.; Arévalo-Gallegos, S.; Rascón-Cruz, Q. Lactoferrin: Structure, function and applications. Int. J. Antimicrob. Agents 2009, 33, 301.e1–301.e8. [Google Scholar] [CrossRef]
- Kim, W.S.; Ohashi, M.; Tanaka, T.; Kumura, H.; Kim, G.Y.; Kwon, I.K.; Goh, J.S.; Shimazaki, K. Growth-promoting effects of lactoferrin on L. acidophilus and Bifidobacterium spp. Biometals 2004, 17, 279–283. [Google Scholar] [CrossRef]
- Rahman, M.M.; Kim, W.S.; Ito, T.; Kumura, H.; Shimazaki, K. Growth promotion and cell binding ability of bovine lactoferrin to Bifidobacterium longum. Anaerobe 2009, 15, 133–137. [Google Scholar] [CrossRef] [Green Version]
- Orsi, N. The antimicrobial activity of lactoferrin: Current status and perspectives. Biometals 2004, 17, 189–196. [Google Scholar] [CrossRef]
- Baker, H.M.; Baker, E.N. Lactoferrin and iron: Structural and dynamic aspects of binding and release. Biometals 2004, 17, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Superti, F.; Berlutti, F.; Paesano, R.; Valenti, P. Structure and activity of lactoferrin-a multi functional protective agent for human health. In Iron Metabolism and Disease; Fuchs, H., Ed.; Publisher Research Signpost: Trivandrum, India, 2008; Volume 8, pp. 1–32. [Google Scholar]
- Liu, B.; Newburg, D.S. Human Milk Glycoproteins Protect Infants Against Human Pathogens. Breastfeed. Med. 2013, 8, 354–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lupetti, A.; Paulusma-Annema, A.; Welling, M.M.; Dogterom-Ballering, H.; Brouwer, C.P.; Senesi, S.; Van Dissel, J.T.; Nibbering, P.H. Synergistic activity of the N-terminal peptide of human lactoferrin and fluconazole against Candida species. Antimicrob. Agents Chemother. 2003, 47, 262–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrés, M.T.; Viejo-Díaz, M.; Fierro, J.F. Human lactoferrin induces apoptosis-like cell death in Candida albicans: Critical role of K+-channel-mediated K+ efflux. Antimicrob. Agents Chemother. 2008, 52, 4081–4088. [Google Scholar] [CrossRef] [Green Version]
- Sessa, R.; Di Pietro, M.; Filardo, S.; Bressan, A.; Mastromarino, P.; Biasucci, A.V.; Rosa, L.; Cutone, A.; Berlutti, F.; Paesano, R.; et al. Lactobacilli-lactoferrin interplay in Chlamydia trachomatis infection. Pathog. Dis. 2017, 75. [Google Scholar] [CrossRef] [Green Version]
- Naidu, A.S.; Miedzobrodzki, J.; Musser, J.M.; Rosdahl, V.T.; Hedström, S.A.; Forsgren, A. Human lactoferrin binding in clinical isolates of Staphylococcus aureus. J. Med. Microbiol. 1991, 34, 323–328. [Google Scholar] [CrossRef]
- Otsuki, K.; Yakuwa, K.; Sawada, M.; Hasegawa, A.; Sasaki, Y.; Mitsukawa, K.; Chiba, H.; Nagatsuka, M.; Saito, H.; Okai, T. Recombinant human lactoferrin has preventive effects on lipopolysaccharide-induced preterm delivery and production of inflammatory cytokines in mice. J. Perinat. Med. 2005, 33, 320–323. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Abe, S.; Okutomi, T.; Tansho, S.; Kawase, K.; Yamaguchi, H. Cooperative anti-Candida effects of lactoferrin or its peptides in combination with azole antifungal agents. Microbiol. Immunol. 1996, 40, 821–825. [Google Scholar] [CrossRef]
- Fernandes, K.E.; Carter, D.A. The Antifungal Activity of Lactoferrin and Its Derived Peptides: Mechanisms of Action and Synergy with Drugs against Fungal Pathogens. Front. Microbiol. 2017, 8, 2. [Google Scholar] [CrossRef] [Green Version]
- Yakuwa, K.; Otsuki, K.; Nakayama, K.; Hasegawa, A.; Sawada, M.; Mitsukawa, K.; Chiba, H.; Nagatsuka, M.; Okai, T. Recombinant human lactoferrin has a potential to suppresses uterine cervical ripening in preterm delivery in animal model. Arch. Gynecol. Obstet. 2007, 275, 331–334. [Google Scholar] [CrossRef]
- Falagas, M.; Betsi, G.I.; Athanasiou, S. Probiotics for the treatment of women with bacterial vaginosis. Clin. Microbiol. Infect. 2007, 13, 657–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abad, C.L.; Safdar, N. The role of lactobacillus probiotics in the treatment or prevention of urogenital infections—A systematic review. J. Chemother. 2009, 21, 243–352. [Google Scholar] [CrossRef] [PubMed]
- Ventura, M.; O’Flaherty, S.; Claesson, M.J.; Turroni, F.; Klaenhammer, T.R.; van Sinderen, D.; O’Toole, P.W. Genome-scale analyses of health-promoting bacteria: Probiogenomics. Nat. Rev. Microbiol. 2009, 7, 61–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boris, S.; Suárez, J.E.; Vázquez, F.; Barbés, C. Adherence of human vaginal lactobacilli to vaginal epithelial cells and interaction with uropathogens. Infect. Immun. 1998, 66, 1985–1989. [Google Scholar] [CrossRef] [Green Version]
- Pino, A.; Bartolo, E.; Caggia, C.; Cianci, A.; Randazzo, C.L. Detection of vaginal lactobacilli as probiotic candidates. Sci. Rep. 2019, 9, 3355. [Google Scholar] [CrossRef]
- Allonsius, C.N.; van den Broek, M.F.L.; De Boeck, I.; Kiekens, S.; Oerlemans, E.F.M.; Kiekens, F.; Foubert, K.; Vandenheuvel, D.; Cos, P.; Delputte, P.; et al. Interplay between Lactobacillus rhamnosus GG and Candida and the involvement of exopolysaccharides. Microb. Biotechnol. 2017, 10, 1753–1763. [Google Scholar] [CrossRef]
- Coudeyras, S.; Jugie, G.; Vermerie, M.; Forestier, C. Adhesion of human probiotic Lactobacillus rhamnosus to cervical and vaginal cells and interaction with vaginosis-associated pathogens. Infect. Dis. Obstet. Gynecol. 2008, 2008, 549640. [Google Scholar] [CrossRef] [Green Version]
- Calonghi, N.; Parolin, C.; Sartor, G.; Verardi, L.; Giordani, B.; Frisco, G.; Marangoni, A.; Vitali, B. Interaction of vaginal Lactobacillus strains with HeLa cells plasma membrane. Benef. Microbes 2017, 8, 625–633. [Google Scholar] [CrossRef]
- Atassi, F.; Brassart, D.; Grob, P.; Graf, F.; Servin, A.L. Lactobacillus strains isolated from the vaginal microbiota of healthy women inhibit Prevotella bivia and Gardnerella vaginalis in coculture and cell culture. FEMS Immunol. Med. Microbiol. 2006, 48, 424–432. [Google Scholar] [CrossRef] [Green Version]
- Parolin, C.; Marangoni, A.; Laghi, L.; Foschi, C.; Ñahui Palomino, R.A.; Calonghi, N.; Cevenini, R.; Vitali, B. Isolation of Vaginal Lactobacilli and Characterization of Anti-Candida Activity. PLoS ONE 2015, 10, e0131220. [Google Scholar] [CrossRef]
- Castro, J.; Henriques, A.; Machado, A.; Henriques, M.; Jefferson, K.K.; Cerca, N. Reciprocal interference between Lactobacillus spp. and Gardnerella vaginalis on initial adherence to epithelial cells. Int. J. Med. Sci. 2013, 10, 1193–1198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, R.D.; Johnson, S.J. Probiotic Lactobacillus and estrogen effects on vaginal epithelial gene expression responses to Candida albicans. J. Biomed. Sci. 2012, 19, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertuccini, L.; Russo, R.; Iosi, F.; Superti, F. Effects of Lactobacillus rhamnosus and Lactobacillus acidophilus on bacterial vaginal pathogens. Int. J. Immunopathol. Pharmacol. 2017, 30, 163–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, S.E.; Jeong, J.J.; Choi, S.Y.; Kim, H.; Han, M.J.; Kim, D.H. Lactobacillus rhamnosus HN001 and Lactobacillus acidophilus La-14 Attenuate Gardnerella vaginalis-Infected Bacterial Vaginosis in Mice. Nutrients 2017, 9, 531. [Google Scholar] [CrossRef] [Green Version]
- Gill, H.S.; Rutherfurd, K.J.; Prasad, J.; Gopal, P.K. Enhancement of natural and acquired immunity by Lactobacillus rhamnosus (HN001), Lactobacillus acidophilus (HN017) and Bifidobacterium lactis (HN019). Br. J. Nutr. 2000, 83, 167–176. [Google Scholar] [CrossRef] [Green Version]
- De Gregorio, P.R.; Silva, J.A.; Marchesi, A.; Nader-Macías, M.E.F. Anti-Candida activity of beneficial vaginal lactobacilli in in vitro assays and in a murine experimental model. FEMS Yeast Res. 2019, 19, foz008. [Google Scholar] [CrossRef] [Green Version]
- Siroli, L.; Patrignani, F.; Serrazanetti, D.I.; Parolin, C.; Ñahui Palomino, R.A.; Vitali, B.; Lanciotti, R. Determination of Antibacterial and Technological Properties of Vaginal Lactobacilli for Their Potential Application in Dairy Products. Front. Microbiol. 2017, 8, 166. [Google Scholar] [CrossRef] [Green Version]
- Shu, Q.; Gill, H.S. Immune protection mediated by the probiotic Lactobacillus rhamnosus HN001 (DR20) against Escherichia coli O157:H7 infection in mice. FEMS Immunol. Med. Microbiol. 2002, 34, 59–64. [Google Scholar] [CrossRef]
- Chen, P.W.; Jheng, T.T.; Shyu, C.L.; Mao, F.C. Antimicrobial potential for the combination of bovine lactoferrin or its hydrolysate with lactoferrin-resistant probiotics against foodborne pathogens. J. Dairy Sci. 2013, 96, 1438–1446. [Google Scholar] [CrossRef]
- Chen, P.W.; Jheng, T.T.; Shyu, C.L.; Mao, F.C. Synergistic antibacterial efficacies of the combination of bovine lactoferrin or its hydrolysate with probiotic secretion in curbing the growth of meticillin-resistant Staphylococcus aureus. J. Med. Microbiol. 2013, 62, 1845–1851. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.W.; Ku, Y.W.; Chu, F.Y. Influence of bovine lactoferrin on the growth of selected probiotic bacteria under aerobic conditions. Biometals 2014, 27, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Maddox, I.S.; Ferguson, L.R.; Shu, Q. Influence of bovine lactoferrin on selected probiotic bacteria and intestinal pathogens. Biometals 2010, 23, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.W.; Liu, Z.S.; Kuo, T.C.; Hsieh, M.C.; Li, Z.W. Prebiotic effects of bovine lactoferrin on specific probiotic bacteria. Biometals 2017, 30, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Sherman, M.P.; Bennett, S.H.; Hwang, F.F.; Yu, C. Neonatal small bowel epithelia: Enhancing anti-bacterial defense with lactoferrin and Lactobacillus GG. Biometals 2004, 17, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Liu, S.; Wang, H.; Su, H.; Liu, Z. Enhanced antifungal activity of bovine lactoferrin-producing probiotic Lactobacillus casei in the murine model of vulvovaginal candidiasis. BMC Microbiol. 2019, 19, 7. [Google Scholar] [CrossRef] [PubMed]
- Otsuki, K.; Tokunaka, M.; Oba, T.; Nakamura, M.; Shirato, N.; Okai, T. Administration of oral and vaginal prebiotic lactoferrin for a woman with a refractory vaginitis recurring preterm delivery: Appearance of lactobacillus in vaginal flora followed by term delivery. J. Obstet. Gynaecol. Res. 2014, 40, 583–585. [Google Scholar] [CrossRef] [Green Version]
- Otsuki, K.; Imai, N. Effects of lactoferrin in 6 patients with refractory bacterial vaginosis. Biochem. Cell Biol. 2017, 95, 31–33. [Google Scholar] [CrossRef]
- Pino, A.; Giunta, G.; Randazzo, C.L.; Caruso, S.; Caggia, C.; Cianci, A. Bacterial biota of women with bacterial vaginosis treated with lactoferrin: An open prospective randomized trial. Microb. Ecol. Health Dis. 2017, 28, 1357417. [Google Scholar] [CrossRef] [Green Version]
- Costantino, D.; Guaraldi, C. Preliminary evaluation of a vaginal cream containing lactoferrin in the treatment of vulvovaginal candidosis. Minerva Ginecol. 2008, 60, 121–125. [Google Scholar]
- Marelli, G.; Papaleo, E.; Ferrari, A. Lactobacilli for prevention of urogenital infections: A review. Eur. Rev. Med. Pharmacol. Sci. 2004, 8, 87–95. [Google Scholar]
- Mastromarino, P.; Macchia, S.; Meggiorini, L.; Trinchieri, V.; Mosca, L.; Perluigi, M.; Midulla, C. Effectiveness of Lactobacillus-containing vaginal tablets in the treatment of symptomatic bacterial vaginosis. Clin. Microbiol. Infect. 2009, 15, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Barrons, R.; Tassone, D. Use of Lactobacillus probiotics for bacterial genitourinary infections in women: A review. Clin. Ther. 2008, 30, 453–468. [Google Scholar] [CrossRef]
- Dover, S.E.; Aroutcheva, A.A.; Faro, S.; Chikindas, M.L. Natural antimicrobials and their role in vaginal health: A short review. Int. J. Probiotics Prebiotics 2008, 3, 219–230. [Google Scholar] [PubMed]
- Reid, G.; Bruce, A.W.; Fraser, N.; Heinemann, C.; Owen, J.; Henning, B. Oral probiotics can resolve urogenital infections. FEMS Immunol. Med. Microbiol. 2001, 30, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Del Popolo, G.; Nelli, F. Recurrent bacterial symptomatic cystitis: A pilot study on a new natural option for treatment. Arch. Ital. Urol. Androl. 2018, 90, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Cadieux, P.A.; Burton, J.; Devillard, E.; Reid, G. Lactobacillus by-products inhibit the growth and virulence of uropathogenic Escherichia coli. J. Physiol Pharmacol. 2009, 60, 13–18. [Google Scholar] [PubMed]
- Van de Wijgert, J.H.H.M.; Verwijs, M.C. Lactobacilli-containing vaginal probiotics to cure or prevent bacterial or fungal vaginal dysbiosis: A systematic review and recommendations for future trial designs. BJOG 2019. [Google Scholar] [CrossRef]
- Xie, H.Y.; Feng, D.; Wei, D.M.; Mei, L.; Chen, H.; Wang, X.; Fang, F. Probiotics for vulvovaginal candidiasis in non-pregnant women. Cochrane Database Syst. Rev. 2017, 11, CD010496. [Google Scholar] [CrossRef]
- Shukla, A.; Sobel, J.D. Vulvovaginitis Caused by Candida Species Following Antibiotic Exposure. Curr. Infect. Dis. Rep. 2019, 21, 44. [Google Scholar] [CrossRef]
- Senok, A.C.; Verstraelen, H.; Temmerman, M.; Botta, G.A. Probiotics for the treatment of bacterial vaginosis. Cochrane Database Syst. Rev. 2009, 4, CD006289. [Google Scholar] [CrossRef] [PubMed]
- Gille, C.; Böer, B.; Marschal, M.; Urschitz, M.S.; Heinecke, V.; Hund, V.; Speidel, S.; Tarnow, I.; Mylonas, I.; Franz, A.; et al. Effect of probiotics on vaginal health in pregnancy. EFFPRO, a randomized controlled trial. Am. J. Obstet. Gynecol. 2016, 215, 608.e1–608.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Alberti, D.; Russo, R.; Terruzzi, F.; Nobile, V.; Ouwehand, A.C. Lactobacilli vaginal colonisation after oral consumption of Respecta (®) complex: A randomised controlled pilot study. Arch. Gynecol. Obstet. 2015, 292, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Russo, R.; Edu, A.; De Seta, F. Study on the effects of an oral lactobacilli and lactoferrin complex in women with intermediate vaginal microbiota. Arch. Gynecol Obstet. 2018, 298, 139–145. [Google Scholar] [CrossRef]
- Russo, R.; Karadja, E.; De Seta, F. Evidence-based mixture containing Lactobacillus strains and lactoferrin to prevent recurrent bacterial vaginosis: A double blind, placebo controlled, randomised clinical trial. Benef. Microbes 2019, 10, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Russo, R.; Superti, F.; Karadja, E.; De Seta, F. Randomised clinical trial in women with Recurrent Vulvovaginal Candidiasis: Efficacy of probiotics and lactoferrin as maintenance treatment. Mycoses 2019, 62, 328–335. [Google Scholar] [CrossRef] [PubMed]
- De Simone, C. The unregulated probiotic market. Clin. Gastroenterol. Hepatol. 2019, 17, 809–817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolaček, S.; Hojsak, I.; Berni Canani, R.; Guarino, A.; Indrio, F.; Orel, R.; Pot, B.; Shamir, R.; Szajewska, H.; Vandenplas, Y.; et al. Commercial Probiotic Products: A Call for Improved Quality Control. A Position Paper by the ESPGHAN Working Group for Probiotics and Prebiotics. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marrazzo, J.M.; Cook, R.L.; Wiesenfeld, H.C.; Murray, P.J.; Busse, B.; Krohn, M.; Hillier, S.L. Women’s satisfaction with an intravaginal Lactobacillus capsule for the treatment of bacterial vaginosis. J. Womens Health (Larchmt) 2006, 15, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Hemmerling, A.; Harrison, W.; Schroeder, A.; Park, J.; Korn, A.; Shiboski, S.; Foster-Rosales, A.; Cohen, C.R. Phase 2a study assessing colonization efficiency, safety, and acceptability of Lactobacillus crispatus CTV-05 in women with bacterial vaginosis. Sex. Transm. Dis. 2010, 37, 745–750. [Google Scholar] [CrossRef]
- Czaja, C.A.; Stapleton, A.E.; Yarova-Yarovaya, Y.; Stamm, W.E. Phase I trial of a Lactobacillus crispatus vaginal suppository for prevention of recurrent urinary tract infection in women. Infect. Dis. Obstet. Gynecol. 2007, 2007, 35387. [Google Scholar] [CrossRef] [Green Version]
- Stapleton, A.E.; Au-Yeung, M.; Hooton, T.M.; Fredricks, D.N.; Roberts, P.L.; Czaja, C.A.; Yarova-Yarovaya, Y.; Fiedler, T.; Cox, M.; Stamm, W.E. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin. Infect. Dis. 2011, 52, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- Grin, P.M.; Kowalewska, P.M.; Alhazzan, W.; Fox-Robichaud, A.E. Lactobacillus for preventing recurrent urinary tract infections in women: Meta-analysis. Can. J. Urol. 2013, 20, 6607–6614. [Google Scholar] [PubMed]
- Ho, M.; Chang, Y.Y.; Chang, W.C.; Lin, H.C.; Wang, M.H.; Lin, W.C.; Chiu, T.H. Oral Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 to reduce Group B Streptococcus colonization in pregnant women: A randomized controlled trial. Taiwan J. Obstet. Gynecol. 2016, 55, 515–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsen, P.; Williamson, M.; Traynor, V.; Georgiou, C. The impact of oral probiotics on vaginal Group B Streptococcal colonisation rates in pregnant women: A pilot randomised control study. Women Birth 2018, 31, 31–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Effect | Experimental Model | References |
|---|---|---|
| Bacteriostatic effect | Gram +, Gram – | [32,33] |
| Bactericidal effect | Gram +, Gram – | [32] |
| Free iron sequestration | Gram +, Gram – | [32,33] |
| Interaction with lipoteichoic acid | Gram + | [34] |
| Interaction with LPS | Gram – | [34] |
| Interaction with cell membrane | Candida spp. | [34,36,37] |
| Inhibition of bacterial adhesion to the host tissue | Chlamydia trachomatis Staphylococcus aureus | [38,39] |
| Enhancer of biofilm formation | Lactobacillus acidophilus, Lactobacillus rhamnosus | [28] |
| Suppression of TNFα and IL-6 expression | LPS-treated pregnant mice | [40] |
| Species | Effect | Experimental Model | References |
|---|---|---|---|
| L. acidophilus | Adhesion | HeLa and | [28] |
| Vaginal epithelial cells | [47] | ||
| Adhesion and pathogen displacement | Vaginal epithelial cells | [47] | |
| Bactericidal effect | G. vaginalis, A. vaginae, E. coli, S. aureus | [56,57] | |
| Immunostimulation | Mice | [58] | |
| L. rhmanosus | Adhesion | HeLa | [28] |
| Vaginal epithelial cells | [48,49] | ||
| Cervical and vaginal cells | [50] | ||
| Bactericidal effect | G. vaginalis, A. vaginae, E. coli, S. aureus, P. bivia | [50,56,57] | |
| Anti-candida effect | Vaginal epithelial cells | [49,50,55] | |
| Mice | [59] | ||
| Immunostimulation | Mice | [50] | |
| L. gasseri | Adhesion | HeLa cells | [51,52] |
| Vaginal epithelial cells | [47] | ||
| Adhesion and pathogen displacement | HeLa cells | [53] | |
| Vaginal epithelial cells | [47] | ||
| Bactericidal effect | G. vaginalis, P. bivia | [52] | |
| Anticandida effect | HeLa cells | [53] | |
| L. crispatus | Adhesion | HeLa cells | [51] |
| Adhesion and pathogen displacement | HeLa cells | [53,54] | |
| Anticandida effect | HeLa cells | [53] | |
| L. jensenii | Adhesion | Vaginal epithelial cells | [47] |
| Adhesion and pathogen displacement | Vaginal epithelial cells | [47] | |
| L. reuteri | Anticandida effect | Vaginal epithelial cells | [55] |
| Mice | [59] | ||
| L. vaginalis | Adhesion | HeLa cells | [51] |
| Substance | Effect | Target Population | References |
|---|---|---|---|
| Lactoferrin | Vaginal microbiota balancing | Women with refractory vaginitis and vaginosis | [69,70] |
| Women with bacterial vaginosis | [71] | ||
| Women with vulvovaginal candidiasis | [72] | ||
| Lactobacillus spp. | Vaginal microbiota balancing | Women with uro-genital infections | [73,74,75,76,77,78] |
| Women with vulvovaginal candidiasis | [79,80,81] |
| Effect | Experimental model | References |
|---|---|---|
| Inhibition of NF-κB activation, IL-1β, TNF-α and IL-17 expression | Mice | [57] |
| Vaginal colonization | Mice | [57] |
| Healthy women | [85] | |
| Dysbiotic women | [86] | |
| Women with bacterial vaginosi | [87] | |
| Women with vulvovaginal candidiasis | [88] | |
| Vaginal microbiota balancing | Women | [86] |
| Bacterial vaginosis inhibition | Mice | [57] |
| Women | [87] | |
| Vulvovaginal candidiasis inhibition | Women | [88] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Superti, F.; De Seta, F. Warding Off Recurrent Yeast and Bacterial Vaginal Infections: Lactoferrin and Lactobacilli. Microorganisms 2020, 8, 130. https://doi.org/10.3390/microorganisms8010130
Superti F, De Seta F. Warding Off Recurrent Yeast and Bacterial Vaginal Infections: Lactoferrin and Lactobacilli. Microorganisms. 2020; 8(1):130. https://doi.org/10.3390/microorganisms8010130
Chicago/Turabian StyleSuperti, Fabiana, and Francesco De Seta. 2020. "Warding Off Recurrent Yeast and Bacterial Vaginal Infections: Lactoferrin and Lactobacilli" Microorganisms 8, no. 1: 130. https://doi.org/10.3390/microorganisms8010130
APA StyleSuperti, F., & De Seta, F. (2020). Warding Off Recurrent Yeast and Bacterial Vaginal Infections: Lactoferrin and Lactobacilli. Microorganisms, 8(1), 130. https://doi.org/10.3390/microorganisms8010130