Oligosaccharides from Palm Kernel Cake Enhances Adherence Inhibition and Intracellular Clearance of Salmonella enterica Serovar Enteritidis In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Size Exclusion Chromatography
2.3. Pooling Fractions Based on HPLC Profiles
2.4. Molecular Weight Determination
2.5. Cells and Bacterial Culture Conditions
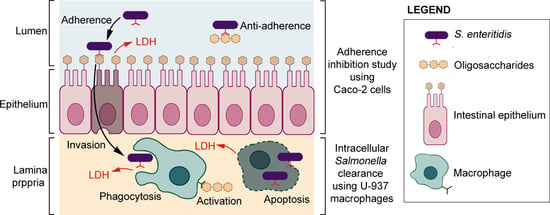
2.6. Adherence Inhibition Study Using Caco-2 Cells
2.7. LDH Analysis of Caco-2 Cells
2.8. Intracellular Salmonella Clearance Using U-937
2.9. Statistical Analysis
3. Results and Discussion
3.1. HPLC Profiles of PKC Oligosaccharides
3.2. Molecular Weights of Oligosaccharides
3.3. Salmonella Enteritidis Adherence Inhibition to Caco-2 Cells
3.4. Effects of Oligosaccharides on the Release of LDH in LPS-Induced Caco-2 Cells
3.5. Intracellular Clearance of Salmonella Enteritidis in U-937 Macrophages
3.6. LDH Measurement and Correlation between LDH Levels and Intracellular Salmonella Enteritidis Clearance in U-937 When Treated with Non-Digestible Oligosaccharides
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Binns, N. Probiotics, Prebiotics and the Gut Microbiota; ILSI Europe: Brussels, Belgium, 2013; p. 4. [Google Scholar]
- Sender, R.; Fuchs, S.; Milo, R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [Green Version]
- Murphy, K.M. Janeway’s Immunobiology; Taylor & Francis Group: Third Avenue, NY, USA, 2011; p. 466. [Google Scholar]
- Derrien, M.; van Hylckama Vlieg, J.E. Fate, activity, and impact of ingested bacteria within the human gut microbiota. Trends Microbiol. 2015, 23, 354–366. [Google Scholar] [CrossRef] [Green Version]
- Gorbach, S. Chapter 95: Microbiology of the Gastrointestinal Tract. In Medical Microbiology, 4th ed.; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996; Available online: http://www.ncbi.nlm.nih.gov/books/NBK7670/#_ncbi_dlg_citbx_NBK7670 (accessed on 1 May 2018).
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current understanding of dysbiosis in disease in human and animal models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef] [Green Version]
- Stecher, B.; Maier, L.; Hardt, W.-D. ‘Blooming’ in the gut: How dysbiosis might contribute to pathogen evolution. Nat. Rev. Microbiol. 2013, 11, 277. [Google Scholar] [CrossRef] [Green Version]
- Hendriksen, R.S.; Vieira, A.R.; Karlsmose, S.; Lo Fo Wong, D.M.; Jensen, A.B.; Wegener, H.C.; Aarestrup, F.M. Global monitoring of Salmonella serovar distribution from the World Health Organization Global Foodborne Infections Network Country Data Bank: Results of quality assured laboratories from 2001 to 2007. Foodborne Pathog. Dis. 2011, 8, 887–900. [Google Scholar] [CrossRef] [Green Version]
- Thung, T.; Mahyudin, N.; Basri, D.F.; Wan Mohamed Radzi, C.; Nakaguchi, Y.; Nishibuchi, M.; Radu, S. Prevalence and antibiotic resistance of Salmonella Enteritidis and Salmonella Typhimurium in raw chicken meat at retail markets in Malaysia. Poult. Sci. 2016, 95, 1888–1893. [Google Scholar] [CrossRef]
- Thai, T.H.; Hirai, T.; Lan, N.T.; Yamaguchi, R. Antibiotic resistance profiles of Salmonella serovars isolated from retail pork and chicken meat in North Vietnam. Int. J. Food Microbiol. 2012, 156, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Qu, D.; Zhang, X.; Shen, J.; Cui, S.; Shi, Y.; Xi, M.; Sheng, M.; Zhi, S.; Meng, J. Prevalence and characterization of Salmonella serovars in retail meats of marketplace in Shaanxi, China. Int. J. Food Microbiol. 2010, 141, 63–72. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority EFSA; European Centre for Disease Prevention and Control ECDC. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSa J. 2018, 16, e05500. [Google Scholar]
- Gal-Mor, O.; Boyle, E.C.; Grassl, G.A. Same species, different diseases: How and why typhoidal and non-typhoidal Salmonella enterica serovars differ. Front. Microbiol. 2014, 5, 391. [Google Scholar] [CrossRef] [Green Version]
- Khan, C. The dynamic interactions between Salmonella and the microbiota, within the challenging niche of the gastrointestinal tract. Int. Sch. Res. Not. 2014, 2014, 1–23. [Google Scholar]
- Thiennimitr, P.; Winter, S.E.; Winter, M.G.; Xavier, M.N.; Tolstikov, V.; Huseby, D.L.; Sterzenbach, T.; Tsolis, R.M.; Roth, J.R.; Bäumler, A.J. Intestinal inflammation allows Salmonella to use ethanolamine to compete with the microbiota. Proc. Natl. Acad. Sci. USA 2011, 108, 17480–17485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcus, S.L.; Brumell, J.H.; Pfeifer, C.G.; Finlay, B.B. Salmonella pathogenicity islands: Big virulence in small packages. Microbes Infect. 2000, 2, 145–156. [Google Scholar] [CrossRef]
- Coppa, G.V.; Zampini, L.; Galeazzi, T.; Facinelli, B.; Ferrante, L.; Capretti, R.; Orazio, G. Human milk oligosaccharides inhibit the adhesion to Caco-2 cells of diarrheal pathogens: Escherichia coli, Vibrio cholerae, and Salmonella fyris. Pediatr. Res. 2006, 59, 377–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibuki, M.; Kovacs-Nolan, J.; Fukui, K.; Kanatani, H.; Mine, Y. β 1-4 mannobiose enhances Salmonella-killing activity and activates innate immune responses in chicken macrophages. Vet. Immunol. Immunopathol. 2011, 139, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Allen, V.; Fernandez, F.; Hinton, M. Evaluation of the influence of supplementing the diet with mannose or palm kernel meal on Salmonella colonisation in poultry. Br. Poult. Sci. 1997, 38, 485–488. [Google Scholar] [CrossRef]
- Ricke, S.C. Focus: Nutrition and Food Science: Impact of Prebiotics on Poultry Production and Food Safety. Yale J. Biol. Med. 2018, 91, 151. [Google Scholar]
- Phillips, G.O.; Williams, P.A. Handbook of Hydrocolloids; Elsevier Science: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Charalampopoulos, D.; Rastall, R.A. Prebiotics and Probiotics Science and Technology; Springer: Spring Street, NY, USA, 2009; p. 354. [Google Scholar]
- Bunning, S.; De Pauw, E. Land Resource Planning for Sustainable Land Management; Eng No. 14; Land and Water Division Working Paper; FAO: Rome, Italy, 2017; pp. 9–15. [Google Scholar]
- Soni, H.; Kango, N. Microbial Mannanases: Properties and Applications. In Advances in Enzyme Biotechnology; Shukla, P., Pletschke, B.I., Eds.; Springer: Haryana, India, 2013; pp. 42–52. [Google Scholar]
- Otieno, D.O.; Ahring, B.K. The potential for oligosaccharide production from the hemicellulose fraction of biomasses through pretreatment processes: Xylooligosaccharides (XOS), arabinooligosaccharides (AOS), and mannooligosaccharides (MOS). Carbohydr. Res. 2012, 360, 84–92. [Google Scholar] [CrossRef]
- Hishamuddin, M.A. Malaysian Palm Kernel Cake as Animal Feed. Palm Oil Dev. 2001, 34, 4–6. [Google Scholar]
- Jahromi, M.F.; Liang, J.B.; Abdullah, N.; Goh, Y.M.; Ebrahimi, R.; Shokryazdan, P. Extraction and Characterization of Oligosaccharides from Palm Kernel Cake as Prebiotic. BioResources 2016, 11, 674–695. [Google Scholar]
- Biggs, P.; Parsons, C.; Fahey, G. The effects of several oligosaccharides on growth performance, nutrient digestibilities, and cecal microbial populations in young chicks. Poult. Sci. 2007, 86, 2327–2336. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Takemura, N.; Sonoyama, K.; Kawagishi, H.; Topping, D.L.; Conlon, M.A.; Morita, T. Degree of polymerization of inulin-type fructans differentially affects number of lactic acid bacteria, intestinal immune functions, and immunoglobulin A secretion in the rat cecum. J. Agric. Food Chem. 2011, 59, 5771–5778. [Google Scholar] [CrossRef] [PubMed]
- Anadón, A.; Martínez-Larrañaga, M.R.; Ares, I.; Martínez, M.A. Prebiotics: Safety and toxicity considerations. In Nutraceuticals; Elsevier: Amsterdam, The Netherlands, 2016; pp. 757–775. [Google Scholar]
- Thammana, M. A review on high performance liquid chromatography (HPLC). RRJPA 2016, 5, 22–28. [Google Scholar]
- Bello, B.; Mustafa, S.; Tan, J.S.; Ibrahim, T.A.T.; Tam, Y.J.; Ariff, A.B.; Manap, M.Y.; Abbasiliasi, S. Evaluation of the effect of soluble polysaccharides of palm kernel cake as a potential prebiotic on the growth of probiotics. 3 Biotech 2018, 8, 346. [Google Scholar] [CrossRef]
- Kalidas, N.R.; Saminathan, M.; Ismail, I.S.; Abas, F.; Maity, P.; Islam, S.S.; Manshoor, N.; Shaari, K. Structural characterization and evaluation of prebiotic activity of oil palm kernel cake mannanoligosaccharides. Food Chem. 2017, 234, 348–355. [Google Scholar] [CrossRef]
- Henanjunda. Factory Direct Food Grade [Mannan Oligosaccharides] Quality Mannan Oligosaccharide Purity 99% Large Quantity Favorably. Available online: https://detail.1688.com/offer/45031592549.html (accessed on 17 June 2018).
- Lesage, G.; Bussey, H. Cell wall assembly in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2006, 70, 317–343. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.; Mazumdar, S. Electrospray ionization mass spectrometry: A technique to access the information beyond the molecular weight of the analyte. Int. J. Anal. Chem. 2012, 2012, 1–40. [Google Scholar] [CrossRef] [Green Version]
- Uzal, F.; Plattner, B.; Hostetter, J. Alimentary System. In Jubb, Kennedy and Palmer’s Pathology of Domestic Animals, 6th ed.; Caswell, J., Williams, K., Maxie, M., Eds.; Elsevier: St. Louis, MO, USA, 2007; Volume 2, pp. 167–168. [Google Scholar]
- Shoaf-Sweeney, K.D.; Hutkins, R.W. Adherence, Anti-Adherence, and Oligosaccharides: Preventing Pathogens from Sticking to the Host. Adv. Food Nutr. Res. 2008, 55, 101–161. [Google Scholar]
- Grzymajło, K.; Kuźmińska-Bajor, M.; Jaworski, J.; Dobryszycki, P.; Ugorski, M. The high-adhesive properties of the FimH adhesin of Salmonella enterica serovar Enteritidis are determined by a single F118S substitution. Microbiology 2010, 156, 1738–1748. [Google Scholar] [CrossRef] [Green Version]
- Old, D. Inhibition of the interaction between fimbrial haemagglutinins and erythrocytes by D-mannose and other carbohydrates. Microbiology 1972, 71, 149–157. [Google Scholar] [CrossRef] [Green Version]
- Determination of the Generally Recognized as Safe (Gras) Status of Fructooligosaccharides as Food Ingredient; FDA: College Park, MD, USA, 2016.
- Smith, D.L., Jr.; Nagy, T.R.; Wilson, L.S.; Dong, S.; Barnes, S.; Allison, D.B. The effect of mannan oligosaccharide supplementation on body weight gain and fat accrual in C57Bl/6J mice. Obesity 2010, 18, 995–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grieshop, C.; Flickinger, E.; Bruce, K.; Patil, A.; Czarnecki-Maulden, G.; Fahey, G., Jr. Gastrointestinal and immunological responses of senior dogs to chicory and mannan-oligosaccharides. Arch. Anim. Nutr. 2004, 58, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Faseleh Jahromi, M.; Shokryazdan, P.; Idrus, Z.; Ebrahimi, R.; Bashokouh, F.; Liang, J.B. Modulation of Immune Function in Rats Using Oligosaccharides Extracted from Palm Kernel Cake. BioMed Res. Int. 2017, 2017, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, S.; Jahromi, M.F.; Liang, J.B.; Zulkifli, I.; Farjam, A.S.; Laudadio, V.; Tufarelli, V. Effect of oligosaccharides extract from palm kernel expeller on growth performance, gut microbiota and immune response in broiler chickens. Poult. Sci. 2015, 94, 2414–2420. [Google Scholar] [CrossRef]
- Nishio, K.; Horie, M.; Akazawa, Y.; Shichiri, M.; Iwahashi, H.; Hagihara, Y.; Yoshida, Y.; Niki, E. Attenuation of lipopolysaccharide (LPS)-induced cytotoxicity by tocopherols and tocotrienols. Redox Biol. 2013, 1, 97–103. [Google Scholar] [CrossRef] [Green Version]
- Ramana, K.V.; Fadl, A.A.; Tammali, R.; Reddy, A.B.; Chopra, A.K.; Srivastava, S.K. Aldose reductase mediates the lipopolysaccharide-induced release of inflammatory mediators in RAW264. 7 murine macrophages. J. Biol. Chem. 2006, 281, 33019–33029. [Google Scholar] [CrossRef] [Green Version]
- Riss, T.L.; Moravec, R.A. Cell proliferation assays: Improved homogeneous methods used to measure the number of cells in culture. In Cell Biology; Elsevier: Amsterdam, The Netherlands, 2006; pp. 25–31. [Google Scholar]
- Solano, C.; Sesma, B.; Alvarez, M.; Urdaneta, E.; Garcia-Ros, D.; Calvo, A.; Gamazo, C. Virulent strains of Salmonella enteritidis disrupt the epithelial barrier of Caco-2 and HEp-2 cells. Arch. Microbiol. 2001, 175, 46–51. [Google Scholar] [CrossRef]
- Pasqualetti, V.; Altomare, A.; Guarino, M.P.L.; Locato, V.; Cocca, S.; Cimini, S.; Palma, R.; Alloni, R.; De Gara, L.; Cicala, M. Antioxidant activity of inulin and its role in the prevention of human colonic muscle cell impairment induced by lipopolysaccharide mucosal exposure. PLoS ONE 2014, 9, e98031. [Google Scholar] [CrossRef] [Green Version]
- Hou, Y.; Ding, X.; Hou, W. Composition and antioxidant activity of water-soluble oligosaccharides from Hericium erinaceus. Mol. Med. Rep. 2015, 11, 3794–3799. [Google Scholar] [CrossRef] [Green Version]
- Van den Ende, W.; Peshev, D.; De Gara, L. Disease prevention by natural antioxidants and prebiotics acting as ROS scavengers in the gastrointestinal tract. Trends Food Sci. Technol. 2011, 22, 689–697. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958. [Google Scholar] [CrossRef]
- Jin, M.; Iwamoto, T.; Yamada, K.; Satsu, H.; Totsuka, M.; Shimizu, M. Effects of chondroitin sulfate and its oligosaccharides on Toll-like receptor-mediated IL-6 secretion by macrophage-like J774. 1 cells. Biosci. Biotechnol. Biochem. 2011, 75, 1283–1289. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Luo, F.; Ding, C.; Albeituni, S.; Hu, X.; Ma, Y.; Cai, Y.; McNally, L.; Sanders, M.A.; Jain, D. Dectin-1 activation by a natural product β-glucan converts immunosuppressive macrophages into an M1-like phenotype. J. Immunol. 2015, 195, 5055–5065. [Google Scholar] [CrossRef] [PubMed]
- Babu, U.S.; Sommers, K.; Harrison, L.M.; Balan, K.V. Effects of fructooligosaccharide-inulin on Salmonella-killing and inflammatory gene expression in chicken macrophages. Vet. Immunol. Immunopathol. 2012, 149, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Schnyder, J.; Baggiolini, M. Role of phagocytosis in the activation of macrophages. J. Exp. Med. 1978, 148, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.L.; Tsolis, R.M.; Bäumler, A.J.; Smith, R.; Adams, L.G. Salmonella enterica Serovar typhimurium induces cell death in bovine monocyte-derived macrophages by early sipb-dependent and delayedsipb-independent mechanisms. Infect. Immun. 2001, 69, 2293–2301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valle, E.; Guiney, D.G. Characterization of Salmonella-induced cell death in human macrophage-like THP-1 cells. Infect. Immun. 2005, 73, 2835–2840. [Google Scholar] [CrossRef] [Green Version]
- Jackson, S. Research Methods: A Modular Approach, 3th ed.; Cengage Learning: Stamford, CT, USA, 2007; p. 85. [Google Scholar]
- Fisher, R.A.; Gollan, B.; Helaine, S. Persistent bacterial infections and persister cells. Nat. Rev. Microbiol. 2017, 15, 453. [Google Scholar] [CrossRef]
- Fisher, R.A.; Cheverton, A.M.; Helaine, S. Analysis of Macrophage-Induced Salmonella Persisters. In Bacterial Persistence; Springer: Berlin/Heidelberg, Germany, 2016; pp. 177–187. [Google Scholar]





| Sugar | Molecular Formula | Expected Molecular Weight | Observed Molecular Weight | |||
|---|---|---|---|---|---|---|
| Small | Big | MOS | FOS | |||
| Monosaccharide | C6H12O6 | 180.2 | 179.1 | - | - | 179.1 |
| Disaccharide | C12H22O11 | 342.3 | 341.1 | - | - | 341.1 |
| Trisaccharide | C18H32O16 | 504.4 | 503.1 | - | - | 503.1 |
| Tetrasaccharide | C24H42O21 | 666.6 | 666.2 | - | - | 666.2 |
| Pentasaccharide | C30H52O26 | 828.7 | 828.3 | 828.3 | - | 828.3 |
| Hexasaccharide | C36H62O31 | 990.9 | 990.3 | 990.3 | - | 990.3 |
| Heptasaccharide | C42H72O36 | 1153 | - | 1152.4 | - | - |
| Octasaccharide | C48H82O41 | 1315.2 | - | 1314.4 | - | - |
| Nonasaccharide | C54H92O46 | 1477.3 | - | - | - | - |
| Decasaccharide | C60H102O51 | 1639.5 | - | 1638.5 | - | - |
| Oligosaccharides | Correlation Coefficient (r2) Values | |||
|---|---|---|---|---|
| 0 µg/ mL | 50 µg/ mL | 500 µg/ mL | 1000 µg/ mL | |
| Control | 0.10 | - | - | - |
| Small | - | 0.74 | 0.50 | 0.04 |
| Big | - | 0.57 | 0.15 | 0.54 |
| MOS | - | 0.51 | 0.46 | 0.11 |
| FOS | - | 0.78 | 0.93 | 0.67 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foo, R.Q.; Jahromi, M.F.; Chen, W.L.; Ahmad, S.; Lai, K.S.; Idrus, Z.; Liang, J.B. Oligosaccharides from Palm Kernel Cake Enhances Adherence Inhibition and Intracellular Clearance of Salmonella enterica Serovar Enteritidis In Vitro. Microorganisms 2020, 8, 255. https://doi.org/10.3390/microorganisms8020255
Foo RQ, Jahromi MF, Chen WL, Ahmad S, Lai KS, Idrus Z, Liang JB. Oligosaccharides from Palm Kernel Cake Enhances Adherence Inhibition and Intracellular Clearance of Salmonella enterica Serovar Enteritidis In Vitro. Microorganisms. 2020; 8(2):255. https://doi.org/10.3390/microorganisms8020255
Chicago/Turabian StyleFoo, Rui Qing, Mohammad Faseleh Jahromi, Wei Li Chen, Syahida Ahmad, Kok Song Lai, Zulkifli Idrus, and Juan Boo Liang. 2020. "Oligosaccharides from Palm Kernel Cake Enhances Adherence Inhibition and Intracellular Clearance of Salmonella enterica Serovar Enteritidis In Vitro" Microorganisms 8, no. 2: 255. https://doi.org/10.3390/microorganisms8020255
APA StyleFoo, R. Q., Jahromi, M. F., Chen, W. L., Ahmad, S., Lai, K. S., Idrus, Z., & Liang, J. B. (2020). Oligosaccharides from Palm Kernel Cake Enhances Adherence Inhibition and Intracellular Clearance of Salmonella enterica Serovar Enteritidis In Vitro. Microorganisms, 8(2), 255. https://doi.org/10.3390/microorganisms8020255