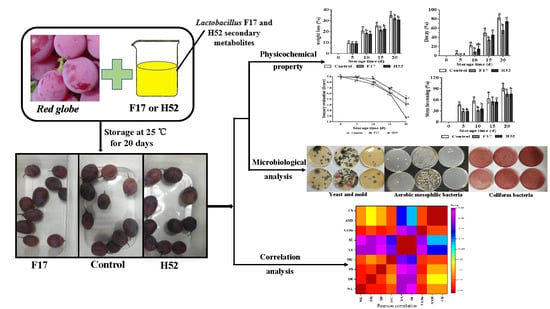
Products of Lactobacillus delbrueckii subsp. bulgaricus Strain F17 and Leuconostoc lactis Strain H52 Are Biopreservatives for Improving Postharvest Quality of ‘Red Globe’ Grapes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Preparation of Strains F17 and H52 Fermentation Supernatants
2.3. Determination of Weight Loss
2.4. Decay Assessment
2.5. Stem Browning Assessment
2.6. Chemical Analysis of Grape Juice: Measurement of Total SSC, pH, and TA
2.7. Assay of Total Phenols
2.8. Microbiological Analysis
2.9. Sensory Evaluation
2.10. Statistical Analysis
3. Results
3.1. Weight Loss
3.2. The Effects on Decay of Fruit
3.3. Grade of Stem Browning
3.4. Chemical Analysis of Grape Juice
3.5. Changes in Total Phenols
3.6. Microbiological Analysis
3.7. Sensory Evaluation
3.8. Pearson Correlation Analysis
4. Discussion
4.1. Weight Loss
4.2. Effects on the Decay of Fruit
4.3. Grade of Stem Browning
4.4. Chemical Analysis of Grape Juice
4.5. Changes in Total Phenols
4.6. Microbiological Analysis
4.7. Sensory Evaluation
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siroli, L.; Patrignani, F.; Serrazanetti, D.I.; Tabanelli, G.; Montanari, C.; Gardini, F.; Lanciotti, R. Lactic acid bacteria and natural antimicrobials to improve the safety and shelf-life of minimally processed sliced apples and lamb’s lettuce. Food Microbiol. 2015, 47, 74–84. [Google Scholar] [CrossRef]
- Krikorian, R.; Boespflug, E.L.; Fleck, D.E.; Stein, A.L.; Wightman, J.D.; Shidler, M.D.; Sadat-Hossieny, S. Concord grape juice supplementation and neurocognitive function in human aging. J. Agric. Food Chem. 2012, 60, 5736–5742. [Google Scholar] [CrossRef] [PubMed]
- Rasines-Perea, Z.; Teissedre, P.-L. Grape polyphenols’ effects in human cardiovascular diseases and diabetes. Molecules 2017, 22, 68. [Google Scholar] [CrossRef]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.W.; Fong, H.H.S.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer chemopreventive activity of resveratol, a natural product derived from grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, J.; Wu, R.; Strik, B.C.; Zhao, Y. Effect of edible coatings on the quality of fresh blueberries (Duke and Elliott) under commercial storage conditions. Postharvest Biol. Technol. 2011, 59, 71–79. [Google Scholar] [CrossRef]
- Pastor, C.; Sánchez-González, L.; Marcilla, A.; Chiralt, A.; Cháfer, M.; González-Martínez, C. Quality and safety of table grapes coated with hydroxypropylmethylcellulose edible coatings containing propolis extract. Postharvest Biol. Technol. 2011, 60, 64–70. [Google Scholar] [CrossRef]
- Badosa, E.; Trias, R.; Parés, D.; Pla, M.; Montesinos, E. Microbiological quality of fresh fruit and vegetable products in Catalonia (Spain) using normalised plate-counting methods and real time polymerase chain reaction (QPCR). J. Sci. Food Agric. 2008, 88, 605–611. [Google Scholar] [CrossRef]
- Abdolahi, A.; Hassani, A.; Ghosta, Y.; Bernousi, I.; Meshkatalsadat, M.H. Study on the potential use of essential oils for decay control and quality preservation of tabarzeh table grape. J. Plant Prot. Res. 2010, 50, 45–52. [Google Scholar] [CrossRef]
- Zhang, J.; Li, D.; Xu, W.; Fu, Y. Preservation of Kyoho grapes stored in active, slow-releasing pasteurizing packaging at room temperature. LWT Food Sci. Technol. 2014, 56, 440–444. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, M.; Bhandari, B.; Gao, Z. Recent developments in novel shelf life extension technologies of fresh-cut fruits and vegetables. Trends Food Sci. Technol. 2017, 64, 23–38. [Google Scholar] [CrossRef] [Green Version]
- Gol, N.B.; Patel, P.R.; Rao, T.V.R. Improvement of quality and shelf-life of strawberries with edible coatings enriched with chitosan. Postharvest Biol. Technol. 2013, 85, 185–195. [Google Scholar] [CrossRef]
- Serrano, M.; Valverde, J.M.; Guillén, F.; Castillo, S.; Martínez-Romero, D.; Valero, D. Use of Aloe vera gel coating preserves the functional properties of table grapes. J. Agric. Food Chem. 2006, 54, 3882–3886. [Google Scholar] [CrossRef]
- Droby, S.; Cohen, L.; Wiess, B.; Daus, A.; Wisniewski, M. Microbial control of postharvest diseases of fruits and vegetables-current status and future outlook. Acta Hortic. 2001, 553, 371–376. [Google Scholar] [CrossRef]
- Oh, Y.A.; Oh, Y.J.; Song, A.Y.; Won, J.S.; Song, K.B.; Min, S.C. Comparison of effectiveness of edible coatings using emulsions containing lemongrass oil of different size droplets on grape berry safety and preservation. LWT Food Sci. Technol. 2017, 75, 742–750. [Google Scholar] [CrossRef]
- Kim, I.-H.; Oh, Y.A.; Lee, H.; Song, K.B.; Min, S.C. Grape berry coatings of lemongrass oil-incorporating nanoemulsion. LWT Food Sci. Technol. 2014, 58, 1–10. [Google Scholar] [CrossRef]
- Sánchez-González, L.; Pastor, C.; Vargas, M.; Chiralt, A.; González-Martínez, C.; Cháfer, M. Effect of hydroxypropylmethylcellulose and chitosan coatings with and without bergamot essential oil on quality and safety of cold-stored grapes. Postharvest Biol. Technol. 2011, 60, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Settanni, L.; Corsetti, A. Application of bacteriocins in vegetable food biopreservation. Int. J. Food Microbiol. 2008, 121, 123–138. [Google Scholar] [CrossRef]
- Fang, X.; Li, Y.; Guo, W.; Ke, W.; Bi, S.; Guo, X.; Zhang, Y. Lactobacillus delbrueckii subsp. bulgaricus F17 and Leuconostoc lactis H52 supernatants delay the decay of strawberry fruits: A microbiome perspective. Food Funct. 2019, 10, 7767–7781. [Google Scholar] [CrossRef]
- Ding, W.; Wang, L.; Zhang, J.; Ke, W.; Zhou, J.; Zhu, J.; Guo, X.; Long, R. Characterization of antioxidant properties of lactic acid bacteria isolated from spontaneously fermented yak milk in the Tibetan Plateau. J. Funct. Foods 2017, 35, 481–488. [Google Scholar] [CrossRef]
- Plessas, S.; Bosnea, L.; Psarianos, C.; Koutinas, A.A.; Marchant, R.; Banat, I.M. Lactic acid production by mixed cultures of Kluyveromyces marxianus, Lactobacillus delbrueckii ssp. bulgaricus and Lactobacillus helveticus. Bioresour. Technol. 2008, 99, 5951–5955. [Google Scholar] [CrossRef]
- El Kafsi, H.; Binesse, J.; Loux, V.; Buratti, J.; Boudebbouze, S.; Dervyn, R.; Kennedy, S.; Galleron, N.; Quinquis, B.; Batto, J.-M.; et al. Lactobacillus delbrueckii ssp. lactis and ssp. bulgaricus: A chronicle of evolution in action. BMC Genom. 2014, 15, 407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhai, Z.; An, H.; Wang, G.; Luo, Y.; Hao, Y. Functional role of pyruvate kinase from Lactobacillus bulgaricus in acid tolerance and identification of its transcription factor by bacterial one-hybrid. Sci. Rep. 2015, 5, 17024. [Google Scholar] [CrossRef] [PubMed]
- Graça, A.; Esteves, E.; Nunes, C.; Abadias, M.; Quintas, C. Microbiological quality and safety of minimally processed fruits in the marketplace of southern Portugal. Food Control 2016, 73, 775–783. [Google Scholar]
- Vermeiren, L.; Devlieghere, F.; Debevere, J. Evaluation of meat born lactic acid bacteria as protective cultures for the biopreservation of cooked meat products. Int. J. Food Microbiol. 2004, 96, 149–164. [Google Scholar] [CrossRef]
- Jones, R.J.; Hussein, H.M.; Zagorec, M.; Brightwell, G.; Tagg, J.R. Isolation of lactic acid bacteria with inhibitory activity against pathogens and spoilage organisms associated with fresh meat. Food Microbiol. 2008, 25, 228–234. [Google Scholar] [CrossRef]
- Swetwiwathana, A.; Visessanguan, W. Potential of bacteriocin-producing lactic acid bacteria for safety improvements of traditional Thai fermented meat and human health. Meat Sci. 2015, 109, 101–105. [Google Scholar] [CrossRef]
- Woraprayote, W.; Malila, Y.; Sorapukdee, S.; Swetwiwathana, A.; Benjakul, S.; Visessanguan, W. Bacteriocins from lactic acid bacteria and their applications in meat and meat products. Meat Sci. 2016, 120, 118–132. [Google Scholar] [CrossRef]
- Meng, X.; Li, B.; Liu, J.; Tian, S. Physiological responses and quality attributes of table grape fruit to chitosan preharvest spray and postharvest coating during storage. Food Chem. 2008, 106, 501–508. [Google Scholar] [CrossRef]
- Meng, X.; Tian, S. Effects of preharvest application of antagonistic yeast combined with chitosan on decay and quality of harvested table grape fruit. J. Sci. Food Agric. 2009, 89, 1838–1842. [Google Scholar] [CrossRef]
- Candir, E.; Ozdemir, A.E.; Kamiloglu, O.; Soylu, E.M.; Dilbaz, R.; Ustun, D. Modified atmosphere packaging and ethanol vapor to control decay of ‘Red Globe’ table grapes during storage. Postharvest Biol. Technol. 2012, 63, 98–106. [Google Scholar] [CrossRef]
- Youssef, K.; Roberto, S.R.; Chiarotti, F.; Koyama, R.; Hussain, I.; de Souza, R.T. Control of Botrytis mold of the new seedless grape “BRS Vitoria” during cold storage. Sci. Hortic. Amst. 2015, 193, 316–321. [Google Scholar] [CrossRef] [Green Version]
- Guerra, I.C.D.; de Oliveira, P.D.L.; Santos, M.M.F.; Lúcio, A.S.S.C.; Tavares, J.F.; Barbosa-Filho, J.M.; Madruga, M.S.; de Souza, E.L. The effects of composite coatings containing chitosan and Mentha (piperita L. or x villosa Huds) essential oil on postharvest mold occurrence and quality of table grape cv. Isabella. Innov. Food Sci. Emerg. Technol. 2016, 34, 112–121. [Google Scholar] [CrossRef]
- dos Santos, N.S.T.; Aguiar, A.J.A.A.; de Oliveira, C.E.V.; de Sales, C.V.; e Silva, S.D.M.; da Silva, R.S.; Stamford, T.C.M.; de Souza, E.L. Efficacy of the application of a coating composed of chitosan and Origanum vulgare L. essential oil to control Rhizopus stolonifer and Aspergillus niger in grapes (Vitis labrusca L.). Food Microbiol. 2012, 32, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Lucera, A.; Conte, A.; Mastromatteo, M.; Speranza, B.; Antonacci, A.; Del Nobile, M.A. Effects of passive and active modified atmosphere packaging conditions on ready-to-eat table grape. J. Food Eng. 2011, 102, 115–121. [Google Scholar] [CrossRef]
- Melo, N.F.C.B.; de MendonçaSoares, B.L.; Diniz, K.M.; Leal, C.F.; Canto, D.; Flores, M.A.P.; Tavares-Filho, J.H.d.C.; Galembeck, A.; Stamford, T.L.M.; Stamford-Arnaud, T.M.; et al. Effects of fungal chitosan nanoparticles as eco-friendly edible coatings on the quality of postharvest table grapes. Postharvest Biol. Technol. 2018, 139, 56–66. [Google Scholar] [CrossRef]
- Sabir, F.K.; Sabir, A.; Unal, S.; Taytak, M.; Kucukbasmaci, A.; Bilgin, O.F. Postharvest quality extension of minimally processed table grapes by chitosan coating. Int. J. Fruit Sci. 2018, 19, 347–358. [Google Scholar] [CrossRef]
- Kraiem, M.; Kachouri, F.; Ghoul, M.; Hamdi, M. Antioxidative and bioprotective effect of lactic acid bacteria on postharvest strawberry: Intact and cell lysates. J. Food Sci. Technol. 2015, 52, 7345–7352. [Google Scholar] [CrossRef]
- Pearson, R.C.; Goheen, A.C. Compendium of grape diseases. Mycologia 1989, 81, 176–177. [Google Scholar]
- Dalié, D.K.D.; Deschamps, A.M.; Richard-forget, F. Lactic acid bacteria–potential for control of mould growth and mycotoxins: A review. Food Control 2010, 21, 370–380. [Google Scholar] [CrossRef]
- Bai, J.; Plotto, A.; Spotts, R.; Rattanapanone, N. Ethanol vapor and saprophytic yeast treatments reduce decay and maintain quality of intact and fresh-cut sweet cherries. Postharvest Biol. Technol. 2011, 62, 204–212. [Google Scholar] [CrossRef]
- Baxter, C.J.; Carrari, F.; Bauke, A.; Overy, S.; Hill, S.A.; Quick, P.W.; Fernie, A.R.; Sweetlove, L.J. Fruit carbohydrate metabolism in an introgression line of tomato with increased fruit soluble solids. Plant Cell Physiol. 2005, 46, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Zhu, Z.; Zhang, P. Effects of chitosan–glucose complex coating on postharvest quality and shelf life of table grapes. Carbohyd. Polym. 2013, 95, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Yagiz, Y.; Zhao, L.; Simonne, A.; Lu, J.; Marshall, M.R. Fruit quality, nutraceutical and antimicrobial properties of 58 muscadine grape varieties (Vitis rotundifolia Michx.) grown in United States. Food Chem. 2017, 215, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Zhao, Y.; Leonard, S.W.; Traber, M.G. Edible coatings to improve storability and enhance nutritional value of fresh and frozen strawberries (Fragaria × ananassa) and raspberries (Rubus ideaus). Postharvest Biol. Technol. 2004, 33, 67–78. [Google Scholar] [CrossRef]
- Martínez-Ferrer, M.; Harper, C.; Pérez-Muñoz, F.; Chaparro, M. Modified atmosphere packaging of minimally processed mango and pineapple fruits. J. Food Sci. 2002, 67, 3365–3371. [Google Scholar] [CrossRef]
- Nicolosi, E.; Ferlito, F.; Amenta, M.; Russo, T.; Rapisarda, P. Changes in the quality and antioxidant components of minimally processed table grapes during storage. Sci. Hortic. Amst. 2018, 232, 175–183. [Google Scholar] [CrossRef]
- Li, W.; Ji, J.; Rui, X.; Yu, J.; Tang, W.; Chen, X.; Jiang, M.; Dong, M. Production of exopolysaccharides by Lactobacillus helveticus MB2-1 and its functional characteristics in vitro. LWT Food Sci. Technol. 2014, 59, 732–739. [Google Scholar] [CrossRef]
- Yildiz, H.; Karatas, N. Microbial exopolysaccharides: Resources and bioactive properties. Process Biochem. 2018, 72, 41–46. [Google Scholar] [CrossRef]
- Champa, W.A.H.; Gill, M.I.S.; Mahajan, B.V.C.; Bedi, S. Exogenous treatment of spermine to maintain quality and extend postharvest life of table grapes (Vitis vinifera L.) cv. Flame Seedless under low temperature storage. LWT Food Sci. Technol. 2015, 60, 412–419. [Google Scholar] [CrossRef]
- Sabir, A.; Sabir, F.K.; Kara, Z. Effects of modified atmosphere packing and honey dip treatments on quality maintenance of minimally processed grape cv. Razaki (V. vinifera L.) during cold storage. J. Food Sci. Technol. 2011, 48, 312–318. [Google Scholar] [CrossRef] [Green Version]
- Pretel, M.T.; Martínez-Madrid, M.C.; Martínez, J.R.; Carreño, J.C.; Romojaro, F. Prolonged storage of ‘Aledo’ table grapes in a slightly CO2 enriched atmosphere in combination with generators of SO2. LWT Food Sci. Technol. 2006, 39, 1109–1116. [Google Scholar] [CrossRef]
- Rizzini, F.M.; Bonghi, C.; Tonutti, P. Postharvest water loss induces marked changes in transcript profiling in skins of wine grape berries. Postharvest Biol. Technol. 2009, 52, 247–253. [Google Scholar] [CrossRef]
- Vazquez-Hernandez, M.; Navarro, S.; Sanchez-Ballesta, M.T.; Merodio, C.; Escribano, M.I. Short-term high CO2 treatment reduces water loss and decay by modulating defense proteins and organic osmolytes in Cardinal table grape after cold storage and shelf-life. Sci. Hortic. Amst. 2018, 234, 27–35. [Google Scholar] [CrossRef]





| Indicators | Treatment | Storage Times (Days) | ||||
|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 20 | ||
| SSC | Control | 15.00 ± 0.10 a | 16.00 ± 0.71 a | 19.63 ± 0.65 a | 18.00 ± 0.61 a | 17.45 ± 0.36 a |
| F17 | 15.00 ± 0.10 a | 15.38 ± 0.41 a | 17.50 ± 0.35 b | 18.38 ± 1.08 a | 20.25 ± 0.25 b | |
| H52 | 15.00 ± 0.10 a | 15.75 ± 0.56 a | 18.25 ± 0.25 b | 19.88 ± 0.74 b | 19.50 ± 0.50 b | |
| TA | Control | 0.67 ± 0.02 a | 0.64 ± 0.01 a | 0.60 ± 0.01 a | 0.57 ± 0.02 a | 0.50 ± 0.01 a |
| F17 | 0.68 ± 0.02 a | 0.67 ± 0.01 a | 0.63 ± 0.01 a | 0.62 ± 0.03 b | 0.56 ± 0.02 b | |
| H52 | 0.68 ± 0.03 a | 0.65 ± 0.02 a | 0.61 ± 0.01 a | 0.59 ± 0.00 ab | 0.53 ± 0.01 ab | |
| SSC/TA | Control | 22.49 ± 0.65 a | 25.01 ± 1.33 a | 32.52 ± 1.45 a | 31.90 ± 1.67 ab | 34.73 ± 0.56 a |
| F17 | 22.13 ± 0.86 a | 23.04 ± 0.54 a | 27.89 ± 0.67 b | 29.80 ± 1.85 b | 36.19 ± 1.13 a | |
| H52 | 22.27 ± 1.12 a | 24.42 ± 0.61 a | 30.08 ± 1.16 ab | 33.59 ± 1.91 a | 36.83 ± 1.52 a | |
| pH | Control | 3.51 ± 0.02 a | 3.52 ± 0.03 a | 3.54 ± 0.02 a | 3.57 ± 0.01 a | 3.63 ± 0.02 a |
| F17 | 3.48 ± 0.02 a | 3.45 ± 0.02 b | 3.49 ± 0.03 a | 3.52 ± 0.02 b | 3.56 ± 0.05 b | |
| H52 | 3.48 ± 0.02 a | 3.46 ± 0.03 b | 3.51 ± 0.01 a | 3.55 ± 0.02 ab | 3.60 ± 0.02 ab | |
| Microorganism | Treatment | Storage Times (Days) | ||||
|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 20 | ||
| AMB | Control | 2.14 ± 0.10 a | 2.30 ± 0.03 a | 3.14 ± 0.34 a | 3.15 ± 0.04 a | 2.51 ± 0.12 a |
| F17 | 2.12 ± 0.19 a | 2.30 ± 0.00 a | 2.51 ± 0.13 b | 2.61 ± 0.08 b | 2.47 ± 0.06 a | |
| H52 | 2.14 ± 0.09 a | 2.30 ± 0.05 a | 2.85 ± 0.17 c | 2.91 ± 0.15 c | 2.35 ± 0.04 a | |
| YAMs | Control | 1.44 ± 0.07 a | 2.55 ± 0.16 a | 3.48 ± 0.15 a | 3.41 ± 0.11 a | 3.58 ± 0.16 a |
| F17 | 1.45 ± 0.04 a | 1.80 ± 0.05 b | 3.33 ± 0.18 a | 3.25 ± 0.16 a | 3.54 ± 0.04 a | |
| H52 | 1.44 ± 0.02 a | 1.89 ± 0.07 b | 2.26 ± 0.11 b | 2.99 ± 0.15 b | 3.10 ± 0.06 b | |
| CB | Control | 2.28 ± 0.06 a | 2.56 ± 0.11 a | 2.47 ± 0.04 a | 3.22 ± 0.07 a | 2.78 ± 0.07 a |
| F17 | 2.24 ± 0.08 a | 2.11 ± 0.07 b | 2.35 ± 0.02 a | 3.05 ± 0.04 b | 2.59 ± 0.06 b | |
| H52 | 2.28 ± 0.02 a | 2.00 ± 0.08 b | 1.97 ± 0.09 b | 2.70 ± 0.09 c | 2.51 ± 0.10 b | |
| Variables Compared | Pearson Correlation Coefficient (r) | ||
|---|---|---|---|
| Control Group | F17 Group | H52 Group | |
| WL vs. DR | 0.930 ** | 0.884 ** | 0.902 ** |
| WL vs. SSC | 0.768 ** | 0.938 ** | 0.953 ** |
| WL vs. SB | 0.926 ** | 0.941 ** | 0.957 ** |
| WL vs. SE | −0.879 ** | −0.862 ** | −0.910 ** |
| WL vs. AMB | 0.572 ** | 0.767 ** | 0.463 * |
| WL vs. YAMs | 0.907 ** | 0.933 ** | 0.956 ** |
| WL vs. CB | 0.647 ** | 0.600 ** | 0.474 * |
| DR vs. pH | 0.885 ** | 0.744 ** | 0.931 ** |
| DR vs. SB | 0.832 ** | 0.878 ** | 0.887 ** |
| DR vs. SE | −0.981 ** | −0.959 ** | −0.970 ** |
| DR vs. TP | −0.910 ** | −0.885 ** | −0.904 ** |
| DR vs. AMB | 0.627 ** | 0.756 ** | 0.568 ** |
| DR vs. YAMs | 0.829 ** | 0.843 ** | 0.908 ** |
| DR vs. CB | 0.763 ** | 0.852 ** | 0.620 ** |
| pH vs. TA | −0.821 ** | −0.457 * | −0.851 ** |
| pH vs. TP | −0.815 ** | −0.688 ** | −0.865 ** |
| SE vs. SSC | −0.489 * | −0.899 ** | −0.949 ** |
| SE vs. SB | −0.826 ** | −0.894 ** | −0.884 ** |
| SE vs. AMB | −0.613 ** | −0.721 ** | −0.594 ** |
| SE vs. YAMs | −0.823 ** | −0.866 ** | −0.926 ** |
| SE vs. CB | −0.489 * | −0.616 ** | −0.693 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, X.; Duan, Q.; Wang, Z.; Li, F.; Du, J.; Ke, W.; Liu, D.; Beier, R.C.; Guo, X.; Zhang, Y. Products of Lactobacillus delbrueckii subsp. bulgaricus Strain F17 and Leuconostoc lactis Strain H52 Are Biopreservatives for Improving Postharvest Quality of ‘Red Globe’ Grapes. Microorganisms 2020, 8, 656. https://doi.org/10.3390/microorganisms8050656
Fang X, Duan Q, Wang Z, Li F, Du J, Ke W, Liu D, Beier RC, Guo X, Zhang Y. Products of Lactobacillus delbrueckii subsp. bulgaricus Strain F17 and Leuconostoc lactis Strain H52 Are Biopreservatives for Improving Postharvest Quality of ‘Red Globe’ Grapes. Microorganisms. 2020; 8(5):656. https://doi.org/10.3390/microorganisms8050656
Chicago/Turabian StyleFang, Xiang, Qinchun Duan, Zhuo Wang, Fuyun Li, Jianxiong Du, Wencan Ke, Diru Liu, Ross C. Beier, Xusheng Guo, and Ying Zhang. 2020. "Products of Lactobacillus delbrueckii subsp. bulgaricus Strain F17 and Leuconostoc lactis Strain H52 Are Biopreservatives for Improving Postharvest Quality of ‘Red Globe’ Grapes" Microorganisms 8, no. 5: 656. https://doi.org/10.3390/microorganisms8050656
APA StyleFang, X., Duan, Q., Wang, Z., Li, F., Du, J., Ke, W., Liu, D., Beier, R. C., Guo, X., & Zhang, Y. (2020). Products of Lactobacillus delbrueckii subsp. bulgaricus Strain F17 and Leuconostoc lactis Strain H52 Are Biopreservatives for Improving Postharvest Quality of ‘Red Globe’ Grapes. Microorganisms, 8(5), 656. https://doi.org/10.3390/microorganisms8050656