Diversity of Leptogium (Collemataceae, Ascomycota) in East African Montane Ecosystems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling
2.2. Morphological Inspection and Molecular Methods
2.3. Data Analyses
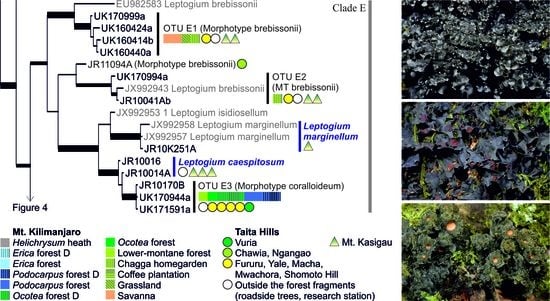
3. Results
3.1. mtSSU and nuITS Molecular Markers in Leptogium
3.2. Leptogium Morphotypes
3.3. Leptogium Diversity on the Studied East African Mountains
3.4. Diversity and Ecosystems
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Hemp, C.; Kehl, S.; Schultz, O.; Wägele, J.W.; Hemp, A. Climatic fluctuations and orogenesis as motors for speciation in East Africa: Case study on Parepistaurus Karsch, 1896 (Orthoptera). Syst. Entomol. 2015, 40, 17–34. [Google Scholar] [CrossRef]
- Merckx, V.S.F.T.; Hendriks, K.P.; Beentjes, K.K.; Mennes, C.B.; Becking, L.E.; Peijnenburg, K.T.C.A.; Afendy, A.; Arumugam, N.; de Boer, H.; Biun, A.; et al. Evolution of endemism on a young tropical mountain. Nature 2015, 524, 347–350. [Google Scholar] [CrossRef]
- Schwery, O.; Onstein, R.E.; Bouchenak-Khelladi, Y.; Xing, Y.; Carter, R.J.; Linder, H.P. As old as the mountains: The radiations of the Ericaceae. New Phytol. 2015, 207, 355–367. [Google Scholar] [CrossRef]
- Hemp, A.; Hemp, C. Broken bridges: The isolation of Kilimanjaro’s ecosystem. Glob. Chang. Biol. 2018, 24, 3499–3507. [Google Scholar] [CrossRef]
- Wasser, S.K.; Lovett, J.C. Introduction to the biography and ecology of the rain forests of eastern Africa. In Biogeography and Ecology of the Rain Forests of Eastern Africa; Lovett, J.C., Wasser, S.K., Eds.; Cambridge University Press: Cambridge, UK, 1993; pp. 3–8. ISBN 0-521-43083-6. [Google Scholar]
- Hemp, C.; Grzywacz, B.; Warchałowska-Śliwa, E.; Hemp, A. Topography and climatic fluctuations boosting speciation: Biogeography and a molecular phylogeny of the East African genera Afroanthracites Hemp & Ingrisch and Afroagraecia Ingrisch & Hemp (Orthoptera, Tettigoniidae, Conocephalinae, Agraeciini). Org. Divers. Evol. 2016, 16, 211–223. [Google Scholar] [CrossRef]
- Lovett, J.C.; Wasser, S.K. Biogeography and Ecology of the Rain Forests of Eastern Africa; Lovett, J.C., Wasser, S.K., Eds.; Cambridge University Press: Cambridge, UK, 1993; ISBN 0-521-43083-6. [Google Scholar]
- Medley, K.E.; Kalibo, H.W. Ethnobotanical Survey of ‘Wild’ Woody Plant Resources at Mount Kasigau, Kenya. J. East Afr. Nat. Hist. 2007, 96, 149–186. [Google Scholar] [CrossRef]
- Bytebier, B. Taita Hills Biodiversity Project Report; National Museums of Kenya: Nairobi, Kenya, 2001. [Google Scholar]
- Harper, E.B.; Measey, G.J.; Patrick, D.A.; Menegon, M.; Vonesh, J.R. Field Guide to the Amphibians of the Eastern Arc Mountains and Coastal Forests of Tanzania and Kenya; Camerapix Publishers International: Nairobi, Kenya, 2010; ISBN 9-781904-722489. [Google Scholar]
- Burgess, N.D.; Butynski, T.M.; Cordeiro, N.J.; Doggart, N.H.; Fjeldså, J.; Howell, K.M.; Kilahama, F.B.; Loader, S.P.; Lovett, J.C.; Mbilinyi, B.; et al. The biological importance of the Eastern Arc Mountains of Tanzania and Kenya. Biol. Conserv. 2007, 134, 209–231. [Google Scholar] [CrossRef]
- Hemp, A. Vegetation of Kilimanjaro: Hidden endemics and missing bamboo. Afr. J. Ecol. 2006, 44, 305–328. [Google Scholar] [CrossRef]
- Lewis, S.L.; Malhi, Y.; Phillips, O.L. Fingerprinting the impacts of global change on tropical forests. Philos. Trans. R. Soc. B. 2004, 359, 437–462. [Google Scholar] [CrossRef]
- Fisher, B. African exception to drivers of deforestation. Nat. Geosci. 2010, 3, 375–376. [Google Scholar] [CrossRef]
- Eva, H.D.; Brink, A.; Simonetti, D. Monitoring Land Cover Dynamics in Sub-Saharan Africa; Institute of Environment and Sustainability, European Commission: Ispra, Italy, 2006. [Google Scholar]
- Pellikka, P.K.; Lötjönen, M.; Siljander, M.; Lens, L. Airborne remote sensing of spatiotemporal change (1955–2004) in indigenous and exotic forest cover in the Taita Hills, Kenya. Int. J. Appl. Earth Obs. Geoinf. 2009, 11, 221–232. [Google Scholar] [CrossRef]
- Balmford, A.; Moore, J.L.; Brooks, T.; Burgess, N.; Hansen, L.A.; Williams, P.; Rahbek, C. Conservation conflicts across Africa. Science 2001, 291, 2616–2619. [Google Scholar] [CrossRef] [PubMed]
- Brooks, T.M.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Rylands, A.B.; Konstant, W.R.; Flick, P.; Pilgrim, J.; Oldfield, S.; Magin, G.; et al. Habitat Loss and Extinction in the Hotspots of Biodiversity. Conserv. Biol. 2002, 16, 909–923. [Google Scholar] [CrossRef] [Green Version]
- Hemp, A. Climate change-driven forest fires marginalize the impact of ice cap wasting on Kilimanjaro. Glob. Chang. Biol. 2005, 11, 1013–1023. [Google Scholar] [CrossRef]
- Pimm, S.L.; Raven, P. Biodiversity. Extinction by numbers. Nature 2000, 403, 843–845. [Google Scholar] [CrossRef]
- Laurance, W.F.; Useche, D.C.; Rendeiro, J.; Kalka, M.; Bradshaw, C.J.A.; Sloan, S.P.; Laurance, S.G.; Campbell, M.; Abernethy, K.; Alvarez, P.; et al. Averting biodiversity collapse in tropical forest protected areas. Nature 2012, 489, 290–294. [Google Scholar] [CrossRef] [Green Version]
- Alvarenga, L.D.P.; Pôrto, K.C.; de Oliveira, J.R.d.P.M. Habitat loss effects on spatial distribution of non-vascular epiphytes in a Brazilian Atlantic forest. Biodivers. Conserv. 2010, 19, 619–635. [Google Scholar] [CrossRef]
- Benítez, A.; Prieto, M.; González, Y.; Aragón, G. Effects of tropical montane forest disturbance on epiphytic macrolichens. Sci. Total Environ. 2012, 441, 169–175. [Google Scholar] [CrossRef]
- Aerts, R.; Thijs, K.W.; Lehouck, V.; Beentje, H.; Bytebier, B.; Matthysen, E.; Gulinck, H.; Lens, L.; Muys, B. Woody plant communities of isolated Afromontane cloud forests in Taita Hills, Kenya. Plant Ecol. 2011, 212, 639–649. [Google Scholar] [CrossRef] [Green Version]
- Lehouck, V.; Spanhove, T.; Colson, L.; Adringa-Davis, A.; Cordeiro, N.J.; Lens, L. Habitat disturbance reduces seed dispersal of a forest interior tree in a fragmented African cloud forest. Oikos 2009, 118, 1023–1034. [Google Scholar] [CrossRef]
- Omoro, L.M.; Pellikka, P.K.; Rogers, P.C. Tree species diversity, richness, and similarity between exotic and indigenous forests in the cloud forests of Eastern Arc Mountains, Taita Hills, Kenya. J. For. Res. 2010, 21, 255–264. [Google Scholar] [CrossRef]
- Otálora, M.A.G.; Jørgensen, P.M.; Wedin, M. A revised generic classification of the jelly lichens, Collemataceae. Fungal Divers. 2014, 64, 275–293. [Google Scholar] [CrossRef] [Green Version]
- Otálora, M.A.G.; Aragón, G.; Martínez, I.; Wedin, M. Cardinal characters on a slippery slope—A re-evaluation of phylogeny, character evolution, and evolutionary rates in the jelly lichens (Collemataceae s. str). Mol. Phylogenet. Evol. 2013, 68, 185–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wedin, M.; Wiklund, E.; Jørgensen, P.M.; Ekman, S. Slippery when wet: Phylogeny and character evolution in the gelatinous cyanobacterial lichens (Peltigerales, Ascomycetes). Mol. Phylogenet. Evol. 2009, 53, 862–871. [Google Scholar] [CrossRef]
- Košuthová, A.; Westberg, M.; Tálora, M.A.G.; Wedin, M. Rostania revised: Testing generic delimitations in Collemataceae (Peltigerales, Lecanoromycetes). MycoKeys 2019, 17–33. [Google Scholar] [CrossRef]
- Swinscow, T.D.V.; Krog, H. Macrolichens of East Africa; Natural History Museum Publications: London, UK, 1988; ISBN 0565010395. [Google Scholar]
- Alstrup, V.; Christensen, S.N. New records of lichens with cyanobacteria from Tanzania and Kenya. Cryptogam. Mycol. 2006, 27, 59–68. [Google Scholar]
- Frisch, A.; Hertel, H. Flora of macrolichens in the alpine and subalpine zones of Mount Kenya (Kenya). Sauteria 1998, 9, 363–370. [Google Scholar]
- Kirika, P.M.; Ndiritu, G.G.; Mugambi, G.K.; Newton, L.E.; Lumbsch, H.T. Diversity and Altitudinal Distribution of Understorey Corticolous lichens in a tropical montane forest in Kenya (East Africa). Cryptogam. Biodivers. Assess. 2018, 2018, 47–70. [Google Scholar] [CrossRef]
- Bjelland, T.; Bendiksby, M.; Frisch, A. Geographically disjunct phylogenetic lineages in Leptogium hibernicum reveal Leptogium krogiae sp. nov. from East Africa. Lichenologist 2017, 49, 239–251. [Google Scholar] [CrossRef]
- Kitaura, M.J.; Marcelli, M.P. A revision of Leptogium species with spherical-celled hairs (section Mallotium p.p.). Bryologist 2013, 116, 15–27. [Google Scholar] [CrossRef]
- Bytebier, B.; Chuah-Petiot, M. A preliminary checklist of the bryoflora of the Taita Hills, Kenya. Trop. Bryol. 2002, 22, 55–66. [Google Scholar]
- Beentje, H.J. An ecological and floristical study of the forests of the Taita Hills, Kenya. Utafiti 1988, 1, 23–66. [Google Scholar]
- Wilder, C.; Brooks, T.; Lens, L. Vegetation Structure and Composition of the Taita Hills Forests. J. East Afr. Nat. Hist. 1998, 87, 181–187. [Google Scholar] [CrossRef]
- Thijs, K.W. Tree Community Dynamics and Ecosystem Function in a Tropical Landscape under Deforestation Pressure. Ph.D. Thesis, KU Leuven, Leuven, Belgium, 2015. [Google Scholar]
- Thijs, K.W.; Roelen, I.; Musila, W.M. Field Guide to the Woody Plants of Taita Hills, Kenya. J. East Afr. Nat. Hist. 2013, 102, 1–272. [Google Scholar] [CrossRef]
- Adriaensen, F.; Githiru, M.; Mwang’ombe, J.; Matthysen, E.; Lens, L. Restoration and Increase of Connectivity among Fragmented Forest Patches in the Taita Hills, South-East Kenya; CEPF Project Report; Critical Ecosystem Partnership Fund: Arlington, VA, USA, 2006. [Google Scholar]
- Henkin, M.A.; Medley, K.E.; Maingi, J.K. Biophysical analysis of afromontane forest community types at Mount Kasigau, Kenya. Afr. J. Ecol. 2015, 53, 454–464. [Google Scholar] [CrossRef]
- Medley, K.E.; Maingi, J.K. Biogeographic Patterns of Forest Diversity at Mount Kasigau, Kenya. J. East Afr. Nat. Hist. 2015, 103, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Lambrechts, C.; Woodley, B.; Hemp, A.; Hemp, C.; Nnyiti, P. Aerial Survey of the Threats to Mt. Kilimanjaro Forests; UNDP: Dar es Salaam, Tanzania, 2002. [Google Scholar]
- Rutten, G.; Ensslin, A.; Hemp, A.; Fischer, M. Vertical and Horizontal Vegetation Structure across Natural and Modified Habitat Types at Mount Kilimanjaro. PLoS ONE 2015, 10, e0138822. [Google Scholar] [CrossRef] [Green Version]
- Appelhans, T.; Mwangomo, E.; Otte, I.; Detsch, F.; Nauss, T.; Hemp, A. Eco-meteorological characteristics of the southern slopes of Kilimanjaro, Tanzania. Int. J. Climatol. 2016, 36, 3245–3258. [Google Scholar] [CrossRef] [Green Version]
- Hemp, A. Continuum or zonation? Altitudinal gradients in the forest vegetation of Mt. Kilimanjaro. Plant Ecolog. 2006, 184, 27–42. [Google Scholar] [CrossRef]
- Enroth, J.; Nyqvist, P.; Malombe, I.; Pellikka, P.; Rikkinen, J. Additions to the moss flora of the Taita Hills and Mount Kasigau, Kenya. Pol. Bot. J. 2013, 58, 495–510. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PRC Protcols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: London, UK, 1990; pp. 315–322. ISBN 0-12-372181-4. [Google Scholar]
- Fedrowitz, K.; Kaasalainen, U.; Rikkinen, J. Genotype variability of Nostoc symbionts associated with three epiphytic Nephroma species in a boreal forest landscape. Bryologist 2011, 114, 220–230. [Google Scholar] [CrossRef] [Green Version]
- Zoller, S.; Scheidegger, C.; Sperisen, C. Pcr Primers for the Amplification of Mitochondrial Small Subunit Ribosomal DNA of Lichen-forming Ascomycetes. Lichenologist 1999, 31, 511–516. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acid Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Müller, K.; Müller, J.; Neinhuis, C.; Quandt, D. PhyDE; Phylogenetic Data Editor. 2010. Available online: http://www.phyde.de/index.html (accessed on 7 December 2020).
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef] [Green Version]
- Posada, D. jModelTest: Phylogenetic model averaging. Mol. Biol. Evol. 2008, 25, 1253–1256. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for Inference of Large Phylogenetic Trees. In Proceedings of the Gateway Computing Environments Workshop (GCE). Gateway Computing Environments Workshop, New Orleans, LA, USA, 14 November 2010. [Google Scholar]
- Olsson, S.; Kaasalainen, U.; Rikkinen, J. Reconstruction of structural evolution in the trnL intron P6b loop of symbiotic Nostoc (Cyanobacteria). Curr. Genet. 2012, 58, 49–58. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior Summarization in Bayesian Phylogenetics Using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef] [Green Version]
- Stöver, B.C.; Müller, K.F. TreeGraph 2: Combining and visualizing evidence from different phylogenetic analyses. BMC Bioinform. 2010, 11, 7. [Google Scholar] [CrossRef] [Green Version]
- Bungartz, F.; Hagedorn, G.; Rambold, G. LIAS Glossary—A Wiki-Based Online Dictionary for Ascomycete Terminology Used by LIAS, the Global Information System for Lichenized and Non-Lichenized Ascomycetes. Available online: http://glossary.lias.net/ (accessed on 7 December 2020).
- Dodge, C.W. The foliose and fruticose lichens of Costa Rica. I. Ann. Mo. Bot. Gard. 1933, 20, 373–467. [Google Scholar] [CrossRef]
- Tavares, C.N. Líquenes da Serra do Gerês. Port. Acta Biol. 1950, 3, 1–189. [Google Scholar]
- Kitaura, M.J.; Marcelli, M.P. The Leptogium juressianum complex in southeastern Brazil. Mycotaxon 2012, 120, 215–221. [Google Scholar] [CrossRef]
- Swartz, O.P. Nova Genera et Species Plantarum Seu Prodromus Descriptioneum Vegetabilium Maximam Parte Incognitorum qua sub Itinere in Indiam Occidentalem Annis 1783–1787 Digessit Olof Swartz, M.D.; Bibliopoliis Acad. M. Swederi: Holmiae, Upsaliæ & Aboæ, Sweden, 1788. [Google Scholar]
- Kitaura, M.J.; Marcelli, M.P.; Jungbluth, P.; Hora, B.R. Five supposedly well-known species of Leptogium section Mallotium. Mycosphere 2013, 4, 520–530. [Google Scholar] [CrossRef]
- Dodge, C.W. Some Lichens of Tropical Africa: IV: Dermatocarpaceae to Pertusariaceae. Beih. Nova Hedwig. 1964, 12, 1–282. [Google Scholar]
- Taylor, T. New lichens, principally from the Herbarium of Sir William, J. Hooker. Lond. J. Bot. 1847, 6, 148–197. [Google Scholar]
- Montagne, J.F.C. Sylloge Generum Specierumque Cryptogamarum, Quas in Variis Operibus Descriptas Iconibusque Illustratas, Nunc ad Diagnosim Reductas, Nonnullasque Novas Interjectas, Ordine Systematico Disposuit J.F. Cam. Montagne; J.-B. Baillière: Paris, France, 1856. [Google Scholar]
- Kitaura, M.J.; Koch, N.M.; Lucheta, F.; Käffer, M.I.; Schmitt, J.L.; Pedroso, J.; Martins, S.A.; Rodrigues, A.S.; Canêz, L.S. A new species and new records of Leptogium (Ach.) Gray (Collemataceae, Peltigerales) from Rio Grande do Sul State with an identification key for the genus. An. Acad. Bras. Cienc. 2019, 91, e20180313. [Google Scholar] [CrossRef]
- Nylander, W. Synopsis Methodica Lichenum Omnium Hucusque Cognitorum, Praemissa Introductione Lingua Gallica; Nabu Press: Charleston, SC, USA, 1858. [Google Scholar]
- Kitaura, M.J.; Bernardo, C.M.; Koch, N.M.; Rodrigues, A.S.; Torres, J.-M.; Barbosa, T.D.; Da CANÊZ, L.S.; Spielmann, A.A.; Honda, N.K.; Fleig, M.; et al. Leptogium (Collemataceae, Peltigerales) from Mato Grosso do Sul state, Brazil: Nine new records, three new taxa and a key for the species. Phytotaxa 2019, 399, 127. [Google Scholar] [CrossRef]
- Barker-Webb, P.; Berthelot, S. Histoire naturelle des Iles Canaries; Béthune: Paris, France, 1840. [Google Scholar]
- Bouly de Lesdain, M. Lichens du Mexique, recueillis par les frères G. Arsène et Amable Saint-Pierre. III Supplément. Ann. Crypt. Exot. 1933, 6, 99–130. [Google Scholar]
- Vainio, E.A. Additamenta ad lichenographiam Antillarum illustrandum. Ann. Acad. Sci. Fenn. 1915, 6, 1–226. [Google Scholar]
- Jørgensen, P.M. Proposals to reject the name Collema proboscidale and to conserve the name Collema phyllocarpum with a conserved type, thereby stabilizing nomenclature of some tropical Leptogium species (Collemataceae, Lecanorales). Taxon 2002, 51, 567–568. [Google Scholar] [CrossRef]
- Jørgensen, P.M.; James, P.W. Studies on Some Leptogium Species of Western Europe. Lichenologist 1983, 15, 109–125. [Google Scholar] [CrossRef]
- Muggia, L.; Pérez-Ortega, S.; Kopun, T.; Zellnig, G.; Grube, M. Photobiont selectivity leads to ecological tolerance and evolutionary divergence in a polymorphic complex of lichenized fungi. Ann. Bot. 2014, 114, 463–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lücking, R.; Dal-Forno, M.; Sikaroodi, M.; Gillevet, P.M.; Bungartz, F.; Moncada, B.; Yánez-Ayabaca, A.; Chaves, J.L.; Coca, L.F.; Lawrey, J.D. A single macrolichen constitutes hundreds of unrecognized species. Proc. Natl. Acad. Sci. USA 2014, 111, 11091–11096. [Google Scholar] [CrossRef] [Green Version]
- Magain, N.; Sérusiaux, E. Dismantling the treasured flagship lichen Sticta fuliginosa (Peltigerales) into four species in Western Europe. Mycol. Prog. 2015, 14, 125. [Google Scholar] [CrossRef]
- Moncada, B.; Lücking, R.; Suárez, A. Molecular phylogeny of the genus Sticta (lichenized Ascomycota: Lobariaceae) in Colombia. Fungal Divers. 2014, 64, 205–231. [Google Scholar] [CrossRef]
- Spribille, T. Relative symbiont input and the lichen symbiotic outcome. Curr. Opin. Plant Biol. 2018, 44, 57–63. [Google Scholar] [CrossRef]
- Spribille, T.; Tagirdzhanova, G.; Goyette, S.; Tuovinen, V.; Case, R.; Zandberg, W.F. 3D biofilms: In search of the polysaccharides holding together lichen symbioses. FEMS Microbiol. Lett. 2020, 367. [Google Scholar] [CrossRef] [Green Version]
- Rikkinen, J. Habitat shifts and morphological variation of Pseudevernia furfuracea along a topographical gradient. Symb. Bot. Ups. 1997, 32, 223–245. [Google Scholar]









| Leptogium Species | Distribution | Ecology | Comments | Refs | |
| L. austroamericanum | K | Lower-elevation woodland, common. | |||
| L. burnetiae | K, T | High-montane Podocarpus forest, rare. | [32,33,34,35] | ||
| L. caespitosum | K, T | Lower-montane forest/woodland, common. | [32] | ||
| L. ethiopicum | K, T 1 | Montane Podocarpus and Ocotea forest, common. | [64] | ||
| L. javanicum | K, T | Montane forest, rare. | [32,33] | ||
| L. juressianum | K, T 1 | Lower-montane and montane forest. | |||
| L. krogiae2 | K, T | Montane forest, common. | [32,33,36] | ||
| L. marginellum | K, T | Lower-montane forest/woodland, rare. | [32,33] | ||
| L. resupinans | K, T 1 | High-montane Erica forest. | [32,34] | ||
| Leptogium morphotypes | Putative species | Clade(s) | |||
| Adpressum | K, T | 6 | H, K, L | [32,34] | |
| Austroamericanum | K, T | ~2 | I, (K), P | Name species occurs in East Africa. | [32,33,35] |
| Azureum | K, T | ~14 | K, Q, R | [32,33,35] | |
| Brebissonii | K, T 1 | 3 | E | [32] | |
| Burgessii | K, T | 1–2 | D | Name species probably occurs in East Africa. | [32,35] |
| Cochleatum | K, T | 4–5 | K, N, O, Q | Name species probably does not occur in East Africa. | [32,33,35] |
| Coralloideum | K, T | ~1 | E, (H) | [32,33,34] | |
| Cyanescens | K, T | ~21 | F, G, J, K, M, R | [32,33,35] | |
| Juressianum | K, T | 2 | D | Name species occurs in East Africa. | [32] |
| Laceroides | K, T | 1 | D | Name species probably does not occur in East Africa. | [32,33,34] |
| Phyllocarpum | K, T | 4 | L | Name species probably does not occur in East Africa. | [32,33] |
| Sessile | K, T | ~1 | Q | Name species probably does not occur in East Africa. | [32,33] |
| Other groups | |||||
| Clade B | K | 1–2 | B | ||
| L. rivulare group | K 1, T | 6 | C | [33] | |
| Leptogium species previously reported from East Africa but not found in this study | |||||
| L. asiaticum | K, T | [32] | |||
| L. digitatum | K | [32] | |||
| L. furfuraceum | K | [32,35] | |||
| L. punctulatum | - | [32] | |||
| L. rivulare | T | [33] | |||
| L. vesiculosum | K, T | [32] | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaasalainen, U.; Tuovinen, V.; Kirika, P.M.; Mollel, N.P.; Hemp, A.; Rikkinen, J. Diversity of Leptogium (Collemataceae, Ascomycota) in East African Montane Ecosystems. Microorganisms 2021, 9, 314. https://doi.org/10.3390/microorganisms9020314
Kaasalainen U, Tuovinen V, Kirika PM, Mollel NP, Hemp A, Rikkinen J. Diversity of Leptogium (Collemataceae, Ascomycota) in East African Montane Ecosystems. Microorganisms. 2021; 9(2):314. https://doi.org/10.3390/microorganisms9020314
Chicago/Turabian StyleKaasalainen, Ulla, Veera Tuovinen, Paul M. Kirika, Neduvoto P. Mollel, Andreas Hemp, and Jouko Rikkinen. 2021. "Diversity of Leptogium (Collemataceae, Ascomycota) in East African Montane Ecosystems" Microorganisms 9, no. 2: 314. https://doi.org/10.3390/microorganisms9020314
APA StyleKaasalainen, U., Tuovinen, V., Kirika, P. M., Mollel, N. P., Hemp, A., & Rikkinen, J. (2021). Diversity of Leptogium (Collemataceae, Ascomycota) in East African Montane Ecosystems. Microorganisms, 9(2), 314. https://doi.org/10.3390/microorganisms9020314