Increasing Hepatitis E Virus Seroprevalence in Domestic Pigs and Wild Boar in Bulgaria
Abstract
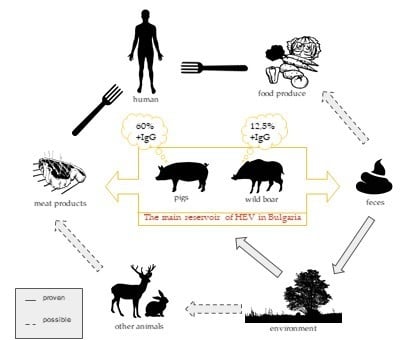
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Selection Criteria
2.2. Ethics Statement
2.3. Sample Processing
2.4. Testing Assay
2.5. Data and Statistical Analysis
3. Results
3.1. HEV Seroprevalence in Domestic Pigs
3.2. HEV Seroprevalence in Wild Boar
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kmush, B.L.; Nelson, K.E.; Labrique, A.B. Risk factors for hepatitis E virus infection and disease. Expert Rev. Anti Infect. Ther. 2015, 13, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Purdy, M.A.; Harrison, T.J.; Jameel, S.; Meng, X.-J.; Okamoto, H.; Van der Poel, W.H.M.; Smith, D.B. ICTV Virus Taxonomy Profile: Hepeviridae. J. Gen. Virol. 2017, 98, 2645–2646. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.B.; Simmonds, P.; Jameel, S.; Emerson, S.U.; Harrison, T.J.; Meng, X.J.; Okamoto, H.; Van der Poel, W.H.; Purdy, M.A.; International Committee on the Taxonomy of Viruses Hepeviridae Study Group. Consensus proposals for classification of the family Hepeviridae. J. Gen. Virol. 2014, 95, 2223–2232. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Izopet, J.; Pavio, N.; Aggarwal, R.; Labrique, A.; Wedemeyer, H.; Dalton, H.R. Hepatitis E virus infection. Nat. Rev. Dis. Primer 2017, 3, 17086. [Google Scholar] [CrossRef]
- Anheyer-Behmenburg, H.E.; Szabo, K.; Schotte, U.; Binder, A.; Klein, G.; Johne, R. Hepatitis E Virus in Wild Boars and Spillover Infection in Red and Roe Deer, Germany, 2013–2015. Emerg. Infect. Dis. 2017, 23, 130–133. [Google Scholar] [CrossRef] [Green Version]
- Colson, P.; Borentain, P.; Queyriaux, B.; Kaba, M.; Moal, V.; Gallian, P.; Heyries, L.; Raoult, D.; Gerolami, R. Pig liver sausage as a source of hepatitis E virus transmission to humans. J. Infect. Dis. 2010, 202, 825–834. [Google Scholar] [CrossRef] [Green Version]
- Riveiro-Barciela, M.; Mínguez, B.; Gironés, R.; Rodriguez-Frías, F.; Quer, J.; Buti, M. Phylogenetic demonstration of hepatitis E infection transmitted by pork meat ingestion. J. Clin. Gastroenterol. 2015, 49, 165–168. [Google Scholar] [CrossRef]
- Drobeniuc, J.; Greene-Montfort, T.; Le, N.-T.; Mixson-Hayden, T.R.; Ganova-Raeva, L.; Dong, C.; Novak, R.T.; Sharapov, U.M.; Tohme, R.A.; Teshale, E.; et al. Laboratory-based Surveillance for Hepatitis E Virus Infection, United States, 2005–2012. Emerg. Infect. Dis. 2013, 19, 218–222. [Google Scholar] [CrossRef]
- Woo, P.C.Y.; Lau, S.K.P.; Teng, J.L.L.; Cao, K.-Y.; Wernery, U.; Schountz, T.; Chiu, T.H.; Tsang, A.K.L.; Wong, P.-C.; Wong, E.Y.M.; et al. New Hepatitis E Virus Genotype in Bactrian Camels, Xinjiang, China, 2013. Emerg. Infect. Dis. 2016, 22, 2219–2221. [Google Scholar] [CrossRef]
- Arends, J.E.; Ghisetti, V.; Irving, W.; Dalton, H.R.; Izopet, J.; Hoepelman, A.I.M.; Salmon, D. Hepatitis E: An emerging infection in high income countries. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2014, 59, 81–88. [Google Scholar] [CrossRef]
- Salines, M.; Andraud, M.; Rose, N. From the epidemiology of hepatitis E virus (HEV) within the swine reservoir to public health risk mitigation strategies: A comprehensive review. Vet. Res. 2017, 48, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lange, H.; Øverbø, J.; Borgen, K.; Dudman, S.; Hoddevik, G.; Urdahl, A.M.; Vold, L.; Sjurseth, S.K. Hepatitis E in Norway: Seroprevalence in humans and swine. Epidemiol. Infect. 2017, 145, 181–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinelli, N.; Luppi, A.; Cordioli, P.; Lombardi, G.; Lavazza, A. Prevalence of hepatitis E virus antibodies in pigs in Northern Italy. Infect. Ecol. Epidemiol. 2011, 1, 7331. [Google Scholar] [CrossRef] [PubMed]
- Seminati, C.; Mateu, E.; Peralta, B.; de Deus, N.; Martin, M. Distribution of hepatitis E virus infection and its prevalence in pigs on commercial farms in Spain. Vet. J. 2008, 175, 130–132. [Google Scholar] [CrossRef]
- Breum, S.Ø.; Hjulsager, C.K.; de Deus, N.; Segalés, J.; Larsen, L.E. Hepatitis E virus is highly prevalent in the Danish pig population. Vet. Microbiol. 2010, 146, 144–149. [Google Scholar] [CrossRef]
- Ivanova, A.; Tefanova, V.; Reshetnjak, I.; Kuznetsova, T.; Geller, J.; Lundkvist, Å.; Janson, M.; Neare, K.; Velström, K.; Jokelainen, P.; et al. Hepatitis E Virus in Domestic Pigs, Wild Boars, Pig Farm Workers, and Hunters in Estonia. Food Environ. Virol. 2015, 7, 403–412. [Google Scholar] [CrossRef]
- Bouwknegt, M.; Frankena, K.; Rutjes, S.A.; Wellenberg, G.J.; de Husman, A.M.R.; van der Poel, W.H.M.; de Jong, M.C.M. Estimation of hepatitis E virus transmission among pigs due to contact-exposure. Vet. Res. 2008, 39, 1. [Google Scholar] [CrossRef] [Green Version]
- Casas, M.; Cortés, R.; Pina, S.; Peralta, B.; Allepuz, A.; Cortey, M.; Casal, J.; Martín, M. Longitudinal study of hepatitis E virus infection in Spanish farrow-to-finish swine herds. Vet. Microbiol. 2011, 148, 27–34. [Google Scholar] [CrossRef]
- de Deus, N.; Casas, M.; Peralta, B.; Nofrarías, M.; Pina, S.; Martín, M.; Segalés, J. Hepatitis E virus infection dynamics and organic distribution in naturally infected pigs in a farrow-to-finish farm. Vet. Microbiol. 2008, 132, 19–28. [Google Scholar] [CrossRef] [Green Version]
- Leblanc, D.; Ward, P.; Gagné, M.-J.; Poitras, E.; Müller, P.; Trottier, Y.-L.; Simard, C.; Houde, A. Presence of hepatitis E virus in a naturally infected swine herd from nursery to slaughter. Int. J. Food Microbiol. 2007, 117, 160–166. [Google Scholar] [CrossRef]
- Krog, J.S.; Larsen, L.E.; Breum, S.Ø. Tracing Hepatitis E Virus in Pigs From Birth to Slaughter. Front. Vet. Sci. 2019, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crossan, C.; Grierson, S.; Thomson, J.; Ward, A.; Nunez-Garcia, J.; Banks, M.; Scobie, L. Prevalence of hepatitis E virus in slaughter-age pigs in Scotland. Epidemiol. Infect. 2015, 143, 2237–2240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halbur, P.G.; Kasorndorkbua, C.; Gilbert, C.; Guenette, D.; Potters, M.B.; Purcell, R.H.; Emerson, S.U.; Toth, T.E.; Meng, X.J. Comparative Pathogenesis of Infection of Pigs with Hepatitis E Viruses Recovered from a Pig and a Human. J. Clin. Microbiol. 2001, 39, 918–923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinelli, N.; Pavoni, E.; Filogari, D.; Ferrari, N.; Chiari, M.; Canelli, E.; Lombardi, G. Hepatitis E Virus in Wild Boar in the Central Northern Part of Italy. Transbound. Emerg. Dis. 2015, 62, 217–222. [Google Scholar] [CrossRef]
- Porea, D.; Anita, A.; Demange, A.; Raileanu, C.; Oslobanu Ludu, L.; Anita, D.; Savuta, G.; Pavio, N. Molecular detection of hepatitis E virus in wild boar population in eastern Romania. Transbound. Emerg. Dis. 2018, 65, 527–533. [Google Scholar] [CrossRef]
- Rivero-Juarez, A.; Risalde, M.A.; Frias, M.; García-Bocanegra, I.; Lopez-Lopez, P.; Cano-Terriza, D.; Camacho, A.; Jimenez-Ruiz, S.; Gomez-Villamandos, J.C.; Rivero, A. Prevalence of hepatitis E virus infection in wild boars from Spain: A possible seasonal pattern? BMC Vet. Res. 2018, 14, 54. [Google Scholar] [CrossRef] [Green Version]
- Spancerniene, U.; Grigas, J.; Buitkuviene, J.; Zymantiene, J.; Juozaitiene, V.; Stankeviciute, M.; Razukevicius, D.; Zienius, D.; Stankevicius, A. Prevalence and phylogenetic analysis of hepatitis E virus in pigs, wild boars, roe deer, red deer and moose in Lithuania. Acta Vet. Scand. 2018, 60, 13. [Google Scholar] [CrossRef] [Green Version]
- Weigand, K.; Weigand, K.; Schemmerer, M.; Müller, M.; Wenzel, J.J. Hepatitis E Seroprevalence and Genotyping in a Cohort of Wild Boars in Southern Germany and Eastern Alsace. Food Environ. Virol. 2018, 10, 167–175. [Google Scholar] [CrossRef]
- Sonoda, H.; Abe, M.; Sugimoto, T.; Sato, Y.; Bando, M.; Fukui, E.; Mizuo, H.; Takahashi, M.; Nishizawa, T.; Okamoto, H. Prevalence of Hepatitis E Virus (HEV) Infection in Wild Boars and Deer and Genetic Identification of a Genotype 3 HEV from a Boar in Japan. J. Clin. Microbiol. 2004, 42, 5371–5374. [Google Scholar] [CrossRef] [Green Version]
- Dremsek, P.; Wenzel, J.J.; Johne, R.; Ziller, M.; Hofmann, J.; Groschup, M.H.; Werdermann, S.; Mohn, U.; Dorn, S.; Motz, M.; et al. Seroprevalence study in forestry workers from eastern Germany using novel genotype 3- and rat hepatitis E virus-specific immunoglobulin G ELISAs. Med. Microbiol. Immunol. 2012, 201, 189–200. [Google Scholar] [CrossRef]
- Bruni, R.; Villano, U.; Equestre, M.; Chionne, P.; Madonna, E.; Trandeva-Bankova, D.; Peleva-Pishmisheva, M.; Tenev, T.; Cella, E.; Ciccozzi, M.; et al. Hepatitis E virus genotypes and subgenotypes causing acute hepatitis, Bulgaria, 2013–2015. PLoS ONE 2018, 13, e0198045. [Google Scholar] [CrossRef] [PubMed]
- Mrzljak, A.; Dinjar-Kujundzic, P.; Jemersic, L.; Prpic, J.; Barbic, L.; Savic, V.; Stevanovic, V.; Vilibic-Cavlek, T. Epidemiology of hepatitis E in South-East Europe in the “One Health” concept. World J. Gastroenterol. 2019, 25, 3168–3182. [Google Scholar] [CrossRef] [PubMed]
- Mazalovska, M.; Varadinov, N.; Koynarski, T.; Minkov, I.; Teoharov, P.; Lomonossoff, G.P.; Zahmanova, G. Detection of Serum Antibodies to Hepatitis E Virus Based on HEV Genotype 3 ORF2 Capsid Protein Expressed in Nicotiana benthamiana. Ann. Lab. Med. 2017, 37, 313–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsachev, I.; Baymakova, M.; Ciccozzi, M.; Pepovich, R.; Kundurzhiev, T.; Marutsov, P.; Dimitrov, K.K.; Gospodinova, K.; Pishmisheva, M.; Pekova, L. Seroprevalence of Hepatitis E Virus Infection in Pigs from Southern Bulgaria. Vector Borne Zoonotic Dis. Larchmt. N 2019, 19, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Di Bartolo, I.; Angeloni, G.; Ponterio, E.; Ostanello, F.; Ruggeri, F.M. Detection of hepatitis E virus in pork liver sausages. Int. J. Food Microbiol. 2015, 193, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.J.; Halbur, P.G.; Haynes, J.S.; Tsareva, T.S.; Bruna, J.D.; Royer, R.L.; Purcell, R.H.; Emerson, S.U. Experimental infection of pigs with the newly identified swine hepatitis E virus (swine HEV), but not with human strains of HEV. Arch. Virol. 1998, 143, 1405–1415. [Google Scholar] [CrossRef] [PubMed]
- Kanai, Y.; Tsujikawa, M.; Yunoki, M.; Nishiyama, S.; Ikuta, K.; Hagiwara, K. Long-term shedding of hepatitis E virus in the feces of pigs infected naturally, born to sows with and without maternal antibodies. J. Med. Virol. 2010, 82, 69–76. [Google Scholar] [CrossRef]
- Salines, M.; Barnaud, E.; Andraud, M.; Eono, F.; Renson, P.; Bourry, O.; Pavio, N.; Rose, N. Hepatitis E virus chronic infection of swine co-infected with Porcine Reproductive and Respiratory Syndrome Virus. Vet. Res. 2015, 46, 55. [Google Scholar] [CrossRef] [Green Version]
- Rutjes, S.A.; Bouwknegt, M.; van der Giessen, J.W.; de Roda Husman, A.M.; Reusken, C.B.E.M. Seroprevalence of Hepatitis E Virus in Pigs from Different Farming Systems in The Netherlands. J. Food Prot. 2014, 77, 640–642. [Google Scholar] [CrossRef]
- Walachowski, S.; Dorenlor, V.; Lefevre, J.; Lunazzi, A.; Eono, F.; Merbah, T.; Eveno, E.; Pavio, N.; Rose, N. Risk factors associated with the presence of hepatitis E virus in livers and seroprevalence in slaughter-age pigs: A retrospective study of 90 swine farms in France. Epidemiol. Infect. 2014, 142, 1934–1944. [Google Scholar] [CrossRef]
- Pishmisheva, M.; Baymakova, M.; Golkocheva-Markova, E.; Kundurzhiev, T.; Pepovich, R.; Popov, G.; Tsachev, I. First Serological Study of Hepatitis E Virus Infection in Pigs in Bulgaria. Comptes Rendus Académie Bulg. Sci. Sci. Math. Nat. 2018, 71, 1001–1008. [Google Scholar] [CrossRef]
- Aniţă, A.; Gorgan, L.; Aniţă, D.; Oşlobanu, L.; Pavio, N.; Savuţa, G. Evidence of hepatitis E infection in swine and humans in the East Region of Romania. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2014, 29, 232–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savuţa, G.; Adriana, A.; Aniţă, D.; Luanda, L.; Pavio, N. Preliminary epidemiological investigations regarding hepatitis E virus infection in swine from the North-east of Romania. Bull. USAMV-CN 2007, 64, 356–358. [Google Scholar]
- Lupulovic, D.; Lazic, S.; Prodanov-Radulovic, J.; Oya, N.; Escribano-Romero, E.; Saiz, J.-C.; Petrovic, T. First Serological Study of Hepatitis E Virus Infection in Backyard Pigs from Serbia. Food Environ. Virol. 2010, 2, 110–113. [Google Scholar] [CrossRef]
- Burri, C.; Vial, F.; Ryser-Degiorgis, M.-P.; Schwermer, H.; Darling, K.; Reist, M.; Wu, N.; Beerli, O.; Schöning, J.; Cavassini, M.; et al. Seroprevalence of Hepatitis E Virus in Domestic Pigs and Wild Boars in Switzerland. Zoonoses Public Health 2014, 61, 537–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiner, M.; Tokarska-Rodak, M.; Plewik, D.; Pańczuk, A.; Szepeluk, A.; Krajewska, M. Preliminary study on the detection of hepatitis E virus (HEV) antibodies in pigs and wild boars in Poland. J. Vet. Res. 2016, 60, 385–389. [Google Scholar] [CrossRef] [Green Version]
- Porea, D.; Anita, A.; Paslaru, A.; Savuta, G. Serological evidence of wild boar hepatitis E infection in three counties from Eastern Romania. Lucr. Stiintifice Univ. Stiinte Agric. Banat. Timisoara Med. Vet. 2015, 48, 174–178. [Google Scholar]
- Porea, D.; Anita, A.; Paslaru, A.; Savuta, G. Wild Boar Hepatitis E Seroprevalence in Hunting Funds from Buzău and GalaÅ£i Counties from buzä‚ u and Galaèši Counties. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Vet. Med. 2016, 73, 44–48. [Google Scholar] [CrossRef] [Green Version]
- Carpentier, A.; Chaussade, H.; Rigaud, E.; Rodriguez, J.; Berthault, C.; Boué, F.; Tognon, M.; Touzé, A.; Garcia-Bonnet, N.; Choutet, P.; et al. High hepatitis E virus seroprevalence in forestry workers and in wild boars in France. J. Clin. Microbiol. 2012, 50, 2888–2893. [Google Scholar] [CrossRef] [Green Version]
- Rutjes, S.A.; Lodder-Verschoor, F.; Lodder, W.J.; van der Giessen, J.; Reesink, H.; Bouwknegt, M.; de Roda Husman, A.M. Seroprevalence and molecular detection of hepatitis E virus in wild boar and red deer in The Netherlands. J. Virol. Methods 2010, 168, 197–206. [Google Scholar] [CrossRef]
- Baymakova, M.; Sakem, B.; Plochev, K.; Popov, G.T.; Mihaylova-Garnizova, R.; Kovaleva, V.; Kundurdjiev, T. Epidemiological characteristics and clinical manifestations of hepatitis E virus infection in Bulgaria: A report on 20 patients. Srp. Arh. Celok. Lek. 2016, 144, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Stoykova, Z.; Ivanova, L.; Tsaneva-Damyanova, D.; Kostadinova, T. Hepatitis E virus infection in Northeastern Bulgaria. Meditsinski Pregl. Med. Rev. 2017, 53, 30–34. [Google Scholar]
- Teoharov, P.; Kevorkyan, A.; Raycheva, R.; Golkocheva-Markova, E.; Trandeva-Bankova, D.; Andonov, A. Data on the prevalence of hepatitis E virus in Bulgaria. Comptes Rendus L’Academie Bulg. Sci. 2014, 67, 1427–1432. [Google Scholar]
- Krumbholz, A.; Mohn, U.; Lange, J.; Motz, M.; Wenzel, J.J.; Jilg, W.; Walther, M.; Straube, E.; Wutzler, P.; Zell, R. Prevalence of hepatitis E virus-specific antibodies in humans with occupational exposure to pigs. Med. Microbiol. Immunol. 2012, 201, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.J.; Wiseman, B.; Elvinger, F.; Guenette, D.K.; Toth, T.E.; Engle, R.E.; Emerson, S.U.; Purcell, R.H. Prevalence of antibodies to hepatitis E virus in veterinarians working with swine and in normal blood donors in the United States and other countries. J. Clin. Microbiol. 2002, 40, 117–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zahmanova, G.G.; Mazalovska, M.; Takova, K.H.; Toneva, V.T.; Minkov, I.N.; Mardanova, E.S.; Ravin, N.V.; Lomonossoff, G.P. Rapid High-Yield Transient Expression of Swine Hepatitis E ORF2 Capsid Proteins in Nicotiana benthamiana Plants and Production of Chimeric Hepatitis E Virus-Like Particles Bearing the M2e Influenza Epitope. Plants 2020, 9, 29. [Google Scholar] [CrossRef] [Green Version]
- Ponterio, E.; Di Bartolo, I.; Orrù, G.; Liciardi, M.; Ostanello, F.; Ruggeri, F.M. Detection of serum antibodies to hepatitis E virus in domestic pigs in Italy using a recombinant swine HEV capsid protein. BMC Vet. Res. 2014, 10, 133. [Google Scholar] [CrossRef] [Green Version]
- Jiménez de Oya, N.; Escribano-Romero, E.; Blázquez, A.-B.; Lorenzo, M.; Martín-Acebes, M.A.; Blasco, R.; Saiz, J.-C. Characterization of Hepatitis E Virus Recombinant ORF2 Proteins Expressed by Vaccinia Viruses. J. Virol. 2012, 86, 7880–7886. [Google Scholar] [CrossRef] [Green Version]



| Year | Dobrich | Pazardzhik | Plovdiv | Razgrad | Ruse | Varna | Shumen | Silistra | Stara Zagora | Yambol | Total | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2017 | IgG+ | n | 34 | 34 | |||||||||
| % | 45.33 | 45.33 | |||||||||||
| Total | N | 75 | 75 | ||||||||||
| 2018 | IgG+ | n | 6 | 9 | 7 | 8 | 30 | ||||||
| % | 33.33 | 30 | 46.67 | 53.33 | 38.46 | ||||||||
| Total | N | 18 | 30 | 15 | 15 | 78 | |||||||
| 2019 | IgG+ | n | 19 | 20 | 20 | 18 | 20 | 20 | 20 | 20 | 20 | 19 | 196 |
| % | 95 | 100 | 100 | 90 | 100 | 100 | 100 | 100 | 100 | 100 | 98 | ||
| Total | N | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 200 | |
| Total | IgG+ | n | 25 | 20 | 20 | 18 | 29 | 27 | 28 | 20 | 54 | 19 | 260 |
| % | 65.79 | 100 | 100 | 90 | 58 | 77.14 | 80 | 100 | 56.84 | 95 | 73.65 | ||
| Total | N | 38 | 20 | 20 | 20 | 50 | 35 | 35 | 20 | 95 | 20 | 353 | |
| Variable | N | HEV Positive | HEV Negative | OR (95% CI) | p |
|---|---|---|---|---|---|
| 2017 | 75 | 34 (55.67) [8.43] | 41 (19.33) [24.28] | 1.3 (95% CI: 0.69–2.5) | 0.3894 |
| 2018 | 78 | 30 (57.89) [13.44] | 48 (20.11) [38.69] | 0.75 (95% CI: 0.39–1.43) | 0.3894 |
| 2019 | 200 | 198 (148.44) [16.55] | 2 (51.56) [47.64] | 1.8984 (95% CI: 1.22–2.94) | 0.0044 |
| Region | Positive HEV IgG | Negative HEV IgG | Totals | p |
|---|---|---|---|---|
| Southern Bulgaria | 113 (110.88) [0.04] | 32 (34.12) [0.13] | 145 | 0.58 |
| Northern Bulgaria | 147 (149.12) [0.03] | 48 (45.88) [0.1] | 195 | 0.58 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takova, K.; Koynarski, T.; Minkov, I.; Ivanova, Z.; Toneva, V.; Zahmanova, G. Increasing Hepatitis E Virus Seroprevalence in Domestic Pigs and Wild Boar in Bulgaria. Animals 2020, 10, 1521. https://doi.org/10.3390/ani10091521
Takova K, Koynarski T, Minkov I, Ivanova Z, Toneva V, Zahmanova G. Increasing Hepatitis E Virus Seroprevalence in Domestic Pigs and Wild Boar in Bulgaria. Animals. 2020; 10(9):1521. https://doi.org/10.3390/ani10091521
Chicago/Turabian StyleTakova, Katerina, Tsvetoslav Koynarski, Ivan Minkov, Zdravka Ivanova, Valentina Toneva, and Gergana Zahmanova. 2020. "Increasing Hepatitis E Virus Seroprevalence in Domestic Pigs and Wild Boar in Bulgaria" Animals 10, no. 9: 1521. https://doi.org/10.3390/ani10091521
APA StyleTakova, K., Koynarski, T., Minkov, I., Ivanova, Z., Toneva, V., & Zahmanova, G. (2020). Increasing Hepatitis E Virus Seroprevalence in Domestic Pigs and Wild Boar in Bulgaria. Animals, 10(9), 1521. https://doi.org/10.3390/ani10091521