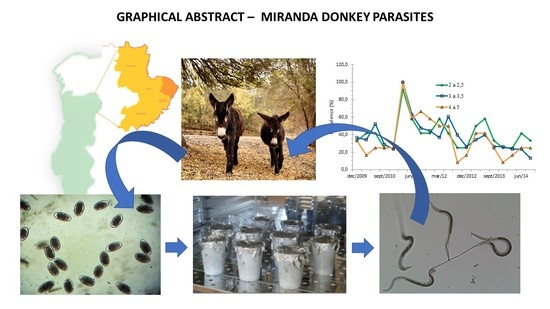
Gastrointestinal Parasitism in Miranda Donkeys: Epidemiology and Selective Control of Strongyles Infection in the Northeast of Portugal
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Statistical Analysis
3. Results
3.1. Climate
3.2. Donkey Parameters
3.3. Prevalence of Parasitism
3.4. Parasitic Levels
3.5. Level of Parasite Infection (LPI)
3.6. Biodiversity of Parasites
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT—Food and Agriculture Organization Corporate Statistical Database (2015)—Food and Agriculture Organization of the United Nations Statistics Division. Available online: faostat3.fao.org/browse/Q/QA/E (accessed on 7 November 2015).
- Arsenos, G.; Gelasakis, A.I.; Papadopoulos, E. The status of donkeys (Equus asinus) in Greece. J. Hell. Vet. Med. Soc. 2010, 61, 212–219. [Google Scholar] [CrossRef] [Green Version]
- Kugler, W.; Grunenfelder, H.; Broxham, E. Donkey Breeds in Europe Inventory, Description, Need for Action, Conservation; Report 2007/2008; Werner Stamm Foundation for the Support of Rare Equines: Oberwil, Switzerland; Margarethe & Rudolf Gsell Foundation: Basel, Switzerland; Bristol Foundation: Zurich, Switzerland, 2007; p. 62. [Google Scholar]
- Blakeway, S. The multi-dimensional donkey in landscapes of donkey-human interaction. Relat. Beyond Anthr. 2014, 2, 59–77. [Google Scholar] [CrossRef] [Green Version]
- FAO—Food and Agriculture Organization of the United Nations—Draught animal power... An Overview. Agricultural Engineering Branch. Agricultural Support Systems Division. Available online: www.fao.org/ag/ags/agse/chapterps1/chapterps1-e.htm (accessed on 7 December 2015).
- Barbosa, J.C. O gado asinino em Trás-os-Montes. Contribuição para o conhecimento da sua importância socio-económica. In Proceedings of the V Colóquio Hispano-Português de Estudos Rurais, Bragança, Portugal, 23–24 October 2003; p. 12. (In Portuguese). [Google Scholar]
- Bacelar, A.C. A utilização do Burro (Equus asinus) como recurso ecoturístico. In II Curso de Pós-Graduação de Turismo de Natureza; Faculdade de Ciências, Universidade do Porto: Porto, Portugal, 2006. (In Portuguese) [Google Scholar]
- AEPGA—Associação para o Estudo e Protecção do Gado Asinino. Raça Asinina de Miranda. Available online: www.aepga.pt (accessed on 25 June 2015). (In Portuguese).
- Hosseini, S.H.; Meshgi, B.; Eslami, A.; Bokai, S.; Sobhani, M.; Ebrahimi, R.A. Prevalence and biodiversity of helminth parasites in donkeys (Equus asinus) in Iran. Int. J. Vet. Res. 2009, 3, 95–99. [Google Scholar]
- Chitra, R.; Rajedran, S.; Prasanna, D.; Kirubakaran, A. Influences of age on the prevalence of parasitic infections among donkeys in Erode district, Tamilnandu, India. Vet. World 2011, 4, 258–259. [Google Scholar] [CrossRef]
- Swann, W.J. Improving the welfare of working equine animals in developing countries. Appl. Anim. Behav. Sci. 2006, 100, 148–151. [Google Scholar] [CrossRef]
- Waqas, M.; Nawaz, M.S.; Sajid, M.; Ahmad, Z.; Mushtaq, A.; Jabbar, A.; Zubair, M. Strongylosis (red worms infestation); a potential threat to donkey’s health and performance. Glob. Vet. 2015, 14, 345–350. [Google Scholar]
- Yilma, J.M.; Feseha, G.; Svendsen, E.D.; Mohammed, A. Health problems of working donkeys in Debre Zeit and Menagesha Regions of Ethiopia. In Donkeys, Mules and Horses in Tropical Agricultural Development; Fielding, D., Pearson, R.A., Eds.; University of Edinburgh: Edinburgh, UK, 1991; p. 336. [Google Scholar]
- Bucknell, D.G.; Hoste, H.; Gasser, R.B.; Beveridge, I. The structure of the community of strongyloid nematodes of domestic equids. J. Helminthol. 1996, 70, 185–192. [Google Scholar] [CrossRef]
- Lyons, E.T.; Drudge, J.H.; Tolliver, S.C. Larval Cyathostomiasis. Vet. Clin. N. Am. Equine Pract. 2000, 16, 501–513. [Google Scholar] [CrossRef]
- Ayele, G.; Feseha, G.; Bojia, E.; Joe, A. Prevalence of gastro-intestinal parasites of donkeys in Dugda Bora District, Ethiopia. Livest. Res. Rural Dev. 2006, 18, 136. [Google Scholar]
- Waqas, M.; Khan, M.S.; Durrani, A.Z.; Khan, M.A.; Avais, M.; Khan, S.A.; Rehman, S.U.; Hussain, A.; Nasir, A.; Hussain, A.; et al. Prevalence of gastrointestinal parasites, chemotherapy and haematology of strongylosis in donkeys of district Lahore, Pakistan. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 198–207. [Google Scholar]
- Medica, D.L.; Sukhdeo, M.V.K. Estimating transmission potential in gastrointestinal nematodes (Order: Strongylida). J. Parasitol. 2001, 87, 442–445. [Google Scholar] [CrossRef]
- Morris, C.; Trawford, A.; Svendsen, E. Donkey: Hero or villain of the parasite world? Past, present and future. In Veterinary Parasitology; Morris, C., Trawford, A., Svendsen, E., Eds.; The Donkey Sanctuary: Sidmouth, UK, 2004; Volume 125, pp. 43–58. [Google Scholar]
- Javed, K.; Ijaz, M.; Ali, M.M.; Khan, I.; Mehmood, K.; Ali, S. Prevalence and hematology of tick borne hemoparasitic diseases in equines in and around Lahore. Pak. J. Zool. 2014, 46, 401–408. [Google Scholar]
- Curran, M.; Feseha, G.; Smith, D. The impact of access to animal health services on donkey health and livelihoods in Ethiopia. J. Trop. Anim. Health Prod. 2005, 37, 47–65. [Google Scholar] [CrossRef] [PubMed]
- Krecek, R.C.; Waller, P.J. Towards the implementation of the “basket of options” approach to helminth parasite control of livestock: Emphasis on the tropics/subtropics. Vet. Parasitol. 2006, 139, 270–282. [Google Scholar] [CrossRef]
- Krecek, R.C. Donkey parasites: Detect, diagnose, delete. In Proceedings of the Second Donkey Welfare Symposium; Lecture Series 2; UC Davis: Davis, CA, USA, 2014. [Google Scholar]
- Asefa, Z.; Kumsa, B.; Endebu, B.; Gizachew, A.; Merga, T.; Debrla, E. Endoparasites of donkeys in Sululta and Gefersa Districts of Central Oromia, Ethiopia. J. Anim. Vet. Adv. 2011, 10, 1850–1854. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, M.K. Sustainable equine parasite control: Perspectives and research needs. Vet. Parasitol. 2012, 185, 32–44. [Google Scholar] [CrossRef]
- Madeira de Carvalho, L.M. Estrongilidose dos equídeos-biologia, patologia, epidemiologia e controlo. In Memoriam Prof. Ignacio Navarrete López-Cózar; Tovar, J., Reina, D., Eds.; Universidad de Extremadura: Cáceres, España, 2006; pp. 277–326. (In Portuguese) [Google Scholar]
- Couto, M.; Santos, A.S.; Laborda, J.; Nóvoa, M.; Ferreira, L.M.; Madeira de Carvalho, L.M. Grazing behaviour of Miranda Donkeys in a natural mountain pasture and parasitic level changes. Livest. Sci. 2016, 186, 16–21. [Google Scholar] [CrossRef]
- Sousa, S.; Rodriques, J.; Silva, A.; Pimentel, M.; Madeira de Carvalho, L.M. Infecção parasitária dos asininos da raça de Miranda em 2005 e 2008. In Proceedings of the IV Congresso da Sociedade Portuguesa de Ciências Veterinárias & I Congresso Ibérico de Epidemiologia, Santarém, Portugal, 27–29 November 2008; p. 117. (In Portuguese). [Google Scholar]
- Sousa, S.; Madeira de Carvalho, L.M. Parasitismo intestinal numa população de asininos com desparasitação regular. Acta Parasitol. Port. 2009, 16, 220–221. (In Portuguese) [Google Scholar]
- Nielsen, M.K. Restrictions of anthelmintic usage: Perspectives and potential consequences. Parasites Vectors 2009, 2 (Suppl. 2), S7. [Google Scholar] [CrossRef] [Green Version]
- Madeira de Carvalho, L.M. Epidemiology and Control of Strongylosis in Different Horse Production Systems in Portugal. Ph.D. Thesis, Faculty of Veterinary Medicine, Technical University of Lisbon, Lisbon, Portugal, 2002. [Google Scholar]
- Smith, D.; Wood, S. Donkey nutrition. In The Professional Handbook of the Donkey, 4th ed.; Duncan, J., Hadrill, D., Eds.; Compiled for Donkey Sanctuary by Elisabeth D. Svendsen: Wales, UK, 2008; pp. 10–27. [Google Scholar]
- Meirinhos, L.M.P. A evolução da Terra de Miranda: Um Estudo com Base nos Sistemas de Informação Geográfica. Master’s Thesis, University of Porto, Porto, Portugal, 2014. (In Portuguese). [Google Scholar]
- Thienpont, D.; Rochette, F.; Vanparijs, O.F.J. Diagnóstico de las Helminthiasis por Medio de Examen Coprológico, 2nd ed.; Janssen Research Foundation: Beerse, Bélgica, 1986; p. 205. (In Portuguese) [Google Scholar]
- Lopes, A.S.; Melo-Franco, B.; Nunes, T.; Sousa, S.; Fabrica, P.; São-Braz, B.; Madeira de Carvalho, L.M. Distribution, Major Epidemiological Features and Control of Equid Gastrointestinal Parasites in Mainland Portugal. In Advances in Animal Health, Medicine and Production; Freitas Duarte, A., Lopes da Costa, L., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Madeira de Carvalho, L.M.; Pereira da Fonseca, I.M.; Afonso-Roque, M.M.; Marcos, M.V.M.; Carvalho-Varela, M. Estudo das parasitoses dos equídeos do Ribatejo: Parasitoses gastrintestinais e pulmonares. Acta Parasitol. Port. 1993, 1, 59. (In Portuguese) [Google Scholar]
- Roberts, F.H.S.; O’Sullivan, P.J. Methods for egg counts and larval cultures for strongyles infesting the gastro-intestinal tract of cattle. Aust. J. Agric. Res. 1950, 1, 99–102. [Google Scholar] [CrossRef]
- Ueno, H.; Gutierres, V.C. Manual para Diagnóstico das Helmintoses de Ruminantes, 2nd ed.; Japan International Cooperation Agency: Tóquio, Japão, 1983; p. 176. (In Portuguese) [Google Scholar]
- Madeira de Carvalho, L.M.; Fazendeiro, M.I.; Afonso-Roque, M.M. Estudo morfométrico das larvas infectantes (L3) dos estrongilídeos (Nematoda: Strongylidae) dos equídeos. 1. Género Cyathostomum, sensu lato. Acta Parasitol. Port. 2004, 11, 21–32. (In Portuguese) [Google Scholar]
- Madeira de Carvalho, L.M.; Gillespie, A.T.; Serra, P.M.; Bernardo, F.A.; Farrim, A.P.; Fazendeiro, I. Eficácia do fungo nematófago Duddingtonia flagrans no controlo biológico da estrongilidose equina no Ribatejo. Rev. Port. Ciênc. Vet. 2007, 102, 233–247. (In Portuguese) [Google Scholar]
- Cernea, M.; Madeira de Carvalho, L.M.; Cozma, V.; Cernea, L.; Raileanu, S.; Silberg, R.; Gut, A. Atlas of Diagnosis of Equine Strongylidosis, 1st ed.; Academic Pres–Universitatea de Stiinte Agricole si Medicina Veterinara Cluj-Napoca: Cluj, Romania, 2008; p. 120. ISBN 978-973-744-127-0. [Google Scholar]
- Madeira de Carvalho, L.M.; Cernea, M.S.; Martins, S.; Sousa, S.; Gersão, S.; Cernea, L.C. Comparative study of cyathostomin horse infection in Portugal and Romania based in L3 subpopulations of Cyathostomum sensu latum. Rev. Sci. Parasitol. 2008, 9, 48–56. [Google Scholar]
- Santos, D.W.; Madeira de Carvalho, L.M.; Molento, M.B. Identification of third stage larval types of cyathostomins of equids: An improved perspective. Vet. Parasitol. 2018, 260, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Madeira de Carvalho, L.M.; Fazendeiro, M.I.; Afonso-Roque, M.M. Estudo morfométrico das larvas infectantes (L3) dos estrongilídeos (Nematoda: Strongylidae) dos equídeos. 3. Conclusões, perspectivas futuras e proposta de chave de identificação de alguns nemátodes gastrintestinais mais comuns dos equídeos em Portugal. Acta Parasitol. Port. 2008, 15, 57–63. (In Portuguese) [Google Scholar]
- Nielsen, M.K.; Haaning, N.; Olsen, S.N. Strongyle egg shedding consistency in horses on farms using selective therapy in Denmark. Vet. Parasitol. 2006, 135, 333–335. [Google Scholar] [CrossRef]
- Becher, A.M.; Mahling, M.; Nielsen, M.K.; Pfister, K. Selective anthelmintic therapy of horses in the Federal states of Bavaria (Germany) and Salzburg (Austria): An investigation into strongyle egg shedding consistency. Vet. Parasitol. 2010, 171, 116–122. [Google Scholar] [CrossRef] [Green Version]
- Francisco, R.; Paz-Silva, A.; Francisco, I.; Cortiñas, F.J.; Miguélez, S.; Suárez, J.L.; Cazapal-Monteiro, C.F.; Arias, M.S.; Sánchez-Andrade, R. Preliminary analysis of the results of selective therapy against strongyles in pasturing horses. J. Equine Vet. Sci. 2012, 32, 274–280. [Google Scholar] [CrossRef]
- Trawford, A.F.; Burden, F.A. Drug resistant cyathostomins in donkey herds; lessons in management for all equids. J. Equine Vet. Sci. 2012, 32, S43. [Google Scholar] [CrossRef]
- Nielsen, M.K.; Pfister, K.; Von Samson-Himmelstjerna, G. Selective therapy in equine parasite control-application and limitations. Vet. Parasitol. 2014, 202, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Warnick, L.D. Daily variability of equine fecal strongyle egg counts. Cornell Vet. 1992, 82, 453–463. [Google Scholar] [PubMed]
- Molnar, V.; Kassai, T. The distribution of nematode egg counts and larval counts in grazing sheep and their implications for parasite control. Vet. Parasitol. 1994, 24, 103–108. [Google Scholar]
- Greite, L. Untersuchungen zur Verbreitung von Strongylus Vulgaris im Rahmen der Selektiven Entwurmung bei Pferden in Süddeutschland. Ph.D. Thesis, Ludwig-Maximilians-Universität, München, Germany, 2013. (In German). [Google Scholar]
- Madeira de Carvalho, L.M.; Sousa, S.; Cernea, M.; Cernea, L.C.; Arias, M.; Paz-Silva, A. Strongyles shed in faeces as a means of monitoring the parasite scenario in horse stud farms. In Horses: Breeding, Health Disorders and Effects on Performance & Behavior; Paz Silva, A., Arias, M.S., Sanchéz-Andrade, R., Eds.; Nova Science Publishers: New York, NY, USA, 2014; pp. 93–125. [Google Scholar]
- Monteiro, A.; Amaro, D. Parque Natural do Douro Internacional: Uma área protegida nas Arribas do Douro e Águeda. In Arribes del Duero: El hogar del Águla Perdicera y de la Cigueña Nega; González, J., Alberti, J., Fernández, A., Alonso, M., Eds.; Consejeria de Meio Ambiente Desarrollo del proyeto LIFE-NAT-B4/3200/97/253: Junta de Castilla y Leon, España, 2000; Volume 190, pp. 159–186. (In Portuguese) [Google Scholar]
- Moreira, N.; Aguiar, C.; Pires, J.M. Lameiros e Outros Prados e Pastagens de Elevado Valor Florístico. Pastagens de Montanha; Direcção Geral de Desenvolvimento Rural: Lisbon, Portugal, 2001; p. 47. (In Portuguese) [Google Scholar]
- Pôças, I.M.V.A. Os Lameiros no Contexto das Paisagens de Montanha. Monitorização por Detecção Remota em Diferentes Escalas Espácio-Temporais. Ph.D. Thesis, University of Porto, Porto, Portugal, 2014. (In Portuguese). [Google Scholar]
- Méndez, G.S.; Aguiar, C. Ecología de los “lameiros” en una aldea del Planalto de Miranda, Trás-os-Montes, Portugal. In Pastos: Fuente Natural de Energía. In Proceedings of the 4th Reunião Iberica de Pastagens e Forragens, Zamora-Miranda do Douro, Portugal, 3–6 May 2010; pp. 75–80. (In Portuguese). [Google Scholar]
- Relf, V.E.; Morgan, E.R.; Hodgkinson, J.E.; Matthews, J.B. Helminth egg excretion with regard to age, gender and management practices on UK Thoroughbred studs. Parasitology 2013, 140, 641–652. [Google Scholar] [CrossRef]
- Kuzmina, T.A. Strongylids (Nematoda: Strongylidae) of domestic horses in Ukraine: Modern state of Fauna and structure of the parasite community. Parazitologia 2012, 46, 127–138. [Google Scholar]
- Matthews, J.B.; Burden, F.A. Common helminth infections of donkeys and their control in temperate regions. Equine Vet. Educ. 2013, 25, 461–467. [Google Scholar] [CrossRef]
- Matthews, J.B. An update on cyathostomins: Anthelmintic resistance and worm control. Equine Vet. Educ. 2008, 20, 552–560. [Google Scholar] [CrossRef]
- Matthews, J.B. Anthelmintic resistance in equine nematodes. Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 310–315. [Google Scholar] [CrossRef] [Green Version]
- Schneider, S.; Pfister, K.; Becher, A.M.; Scheuerle, M.C. Strongyle infections and parasitic control strategies in German horses-a risk assessment. Vet. Res. 2014, 10, 262. [Google Scholar] [CrossRef] [Green Version]
- AEPGA—Associação para o Estudo e Protecção do Gado Asinino. O Burro de Miranda na Gestão de Vegetação Arbustiva-Baixa e de Ecossistemas de Elevado Valor Conservacionista; Final Report of EDP Biodiversidade 2010; AEPGA: Miranda do Douro, Portugal, 2015; p. 48. (In Portuguese) [Google Scholar]
- Wannas, H.Y.; Dawood, K.H.A.; Gassem, G.H.A. Prevalence of gastro-intestinal parasites in horses and donkeys in Al-Diwaniyah Governorate. Al Qadisiya J. Vet. Med. Sci. 2012, 11, 148–155. [Google Scholar] [CrossRef] [Green Version]
- Getachew, M.A. Endoparasites of Working Donkeys in Ethiopia: Epidemiological Study and Mathematical Modelling. Ph.D. Thesis, University of Glasgow, Glasgow, UK, 2006. [Google Scholar]
- Bell, N.J.; Morriss, C.J.; Trawford, A.F.; Reid, S.W.J. The effect of age and sex on the faecal egg count (FEC) in the donkey. In Proceedings of the 18th International Conference of the World Association for the Advancement of Veterinary Parasitology (WAAVP), Stresa, Italy, 26–30 August 2001. [Google Scholar]
- Tesfu, N.; Asrade, B.; Abebe, R.; Kasaye, S. Prevalence and risk factors of gastrointestinal nematode parasites of horse and donkeys in Hawassa Town, Ethiopia. Vet. Sci. Technol. 2014, 5, 210. [Google Scholar]
- Mezgebu, T.; Tafess, K.; Tamiru, F. Prevalence of gastrointestinal parasites of horses and donkeys in and around Gondar Town, Ethiopia. Open J. Vet. Med. 2013, 3, 267–272. [Google Scholar] [CrossRef] [Green Version]
- Sheferaw, D.; Alemu, M. Epidemiological study of gastrointestinal helminths of equines in Damot-Gale district, Wolaita zone, Ethiopia. J. Parasit. Dis. 2015, 39, 315–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsegaye, B.; Chala, A. Prevalence of endoparasitic helminths of donkeys in and around Haramaya district, Eastern Ethiopia. J. Vet. Med. Anim. Health 2015, 7, 221–224. [Google Scholar]
- Vázquez, I.F. Epidemiología y Control de los Principales Parasitismos del Caballo en Galicia; Universidad de Santiago de Compostela: Santiago, Spain, 2010; p. 225. [Google Scholar]
- Ramsey, Y.H.; Christley, R.M.; Matthews, J.B.; Hodgkinson, J.E.; McGoldrick, J.; Love, S. Seasonal development of Cyathostominae larvae on pasture in a northern temperate region of the United Kingdom. Vet. Parasitol. 2004, 119, 307–318. [Google Scholar] [CrossRef]
- Lloyd, S.; Martin, S.A. Controlled trial on the effects of radionic healing and anthelmintics on faecal egg counts in horses. Vet. Rec. 2006, 158, 734–737. [Google Scholar] [CrossRef]
- Kuzmina, T.A.; Kuzmin, Y.I.; Kharchenko, V.A. Field study on the survival, migration and overwintering of infective larvae of horse strongyles on pasture in central Ukraine. Vet. Parasitol. 2006, 141, 264–272. [Google Scholar] [CrossRef]
- Baudena, M.A.; Chapman, M.R.; French, D.D.; Klei, T.R. Seasonal development and survival of equine cyathostome larvae on pasture in south Louisiana. Vet. Parasitol. 2000, 88, 51–60. [Google Scholar] [CrossRef]
- Elsener, J.; Villeneuve, A. Comparative long-term efficacy of ivermectin and moxidectin over winter in Canadian horses treated at removal from pastures for winter housing. Can. Vet. J. 2009, 50, 486–490. [Google Scholar]
- Giles, C.J.; Urquhart, K.A.; Longstaffe, J.A. Larval Cyathostomiasis (immature trichonema-induced enteropathy): A report of 15 clinical cases. Equine Vet. J. 1985, 17, 196–201. [Google Scholar] [CrossRef]
- Reinemeyer, C.R. Small strongyles. Recent advances. Vet. Clin. N. Am. Equine Pract. 1986, 2, 281–312. [Google Scholar] [CrossRef]
- Preto, E.; Sá, A. Património Geológico nos Parques Naturais do Douro Internacional e Arribas del Duero (Ramo Norte); Porto Editora: Porto, Portugal, 2007; p. 28. (In Portuguese) [Google Scholar]
- Mattioli, R.C.; Zinsstag, J.; Pfister, K. Frequency of trypanosomosis and gastrointestinal parasites in draught donkeys in The Gambia in relation to animal husbandry. Trop. Anim. Health Prod. 1994, 26, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Wells, D.; Krecek, R.C.; Wells, M.; Guthrie, A.J.; Lourens, J.C. Helminth levels of working donkeys kept under different management systems in the Moretele 1 district of the North-West Province, South Africa. Vet. Parasitol. 1998, 77, 163–177. [Google Scholar] [CrossRef]
- Gebread, F. Diseases and health problems of donkeys abroad. In The Professional Handbook of the Donkey, 3rd ed.; Svendsen, E.D., Ed.; Whittet Books: London, UK, 1997; p. 400. [Google Scholar]
- Collobert-Laugier, A.; Hoste, H.; Sevin, C.; Dorchies, P. Prevalence, abundance and site distribution of equine small strongyles in Normandy, France. Vet. Parasitol. 2002, 110, 77–83. [Google Scholar] [CrossRef]
- Matthee, S.; Dreyer, F.H.; Hoffmann, W.A.; Niekirk, F.E. An introductory survey of helminth control practices in South Africa and anthelmintic resistance on Thoroughbred stud farms in the Western Cape Province. J. S. Afr. Vet. Assoc. 2002, 73, 195–200. [Google Scholar] [CrossRef] [Green Version]
- Kuzmina, T.; Kharchenko, V.; Zvegintsova, N. Comparative study of the intestinal strongylid communities of equidae in the Askania-Nova biosphere reserve, Ukraine. J. Helminthol. 2007, 44, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Kuzmina, T.A.; Kuzmin, Y.I. The community of strongylids (Nematoda, Strongylida) of working donkeys (Equus asinus) in Ukraine. Vestn. Zool. 2008, 42, 18–23. [Google Scholar] [CrossRef] [Green Version]
- Getachew, M.; Trawford, A.; Feseha, G.; Reid, S.W. Gastrointestinal parasites of working donkeys of Ethiopia. Trop. Anim. Health Prod. 2010, 42, 27–33. [Google Scholar] [CrossRef]
- Ferreira, G.M.S.; Dutra, F.A.F.; Filho, E.F.A.; Santos, A.C.G. Parasitismo gastrintestinal e hematologia em equinos e asininos da mesorregião da aglomeração urbana, São Luís, Maranhão. Arch. Vet. Sci. 2014, 19, 22–30. (In Portuguese) [Google Scholar] [CrossRef]
- Menzel, M.A. Selektive Entwurmung der Pferde in Einer Oberbayrischen Pferdepraxis: Einführung Sowie Wissenschaftliche und Betriebswirtschaftliche Analyse. Ph.D. Thesis, Ludwig-Maximilians-Universität, München, Germany, 2013. (In German). [Google Scholar]
- Matthee, S.; Krecek, R.C.; Milne, S.A.; Boshoff, M.; Guthrie, A.J. Impact of management interventions on helminth levels, and body and blood measurements in working donkeys in South Africa. Vet. Parasitol. 2002, 107, 103–113. [Google Scholar] [CrossRef]
- Lind, E.O.; Eysker, M.; Nilsson, O.; Uggla, A.; Höglund, J. Expulsion of small strongyle nematodes (cyathostomin spp) following deworming of horses on a stud farm in Sweden. Vet. Parasitol. 2003, 115, 289–299. [Google Scholar] [CrossRef]
- Peregrine, A.S.; McEwen, B.; Bienzle, D.; Koch, T.G.; Weese, J.S. Larval cyathostominosis in horses in Ontario: An emerging disease? Can. Vet. Med. 2006, 47, 80–82. [Google Scholar]
- Love, S. Treatment and prevention of intestinal parasite-associated disease. Vet. Clin. N. Am. Equine Pract. 2003, 19, 791–806. [Google Scholar] [CrossRef] [PubMed]
- Becher, A.M.; Pfister, K. The efficacy of anthelmintic drugs against horse strongyles in the area of Salzburg and preliminary results of selective anthelmintic treatment. Wien. Klin. Wochenschr. 2010, 122, 71–75. [Google Scholar] [CrossRef]
- Madeira de Carvalho, L.M.; Gomes, L.; Cernea, M.; Cernea, C.; Santos, C.A.; Bernardes, N.; Rosário, M.A.; Soares, M.J.; Fazendeiro, M.I. Parasitismo gastrintestinal e seu controlo em asininos e híbridos estabulados. Rev. Port. Ciênc. Vet. 2007, 102, 225–231. (In Portuguese) [Google Scholar]
- Ibrahim, N.; Berhanu, T.; Deressa, B.; Tolosa, T. Survey of prevalence of helminth parasites of donkeys in and around Hawassa Town, Southern Ethiopia. Glob. Vet. 2011, 6, 223–227. [Google Scholar]
- Filho, E.F.A.; Santos, A.C.G.; Dutra, F.A.F.; Ferreira, G.M.S.; Guerra, R.M.C. Avaliação do parasitismo gastrintestinal de asininos da ilha de São Luís, Estado do Maranhão. Encicl. Biosf. Cent. Cient. Conhecer 2013, 9, 393–399. (In Portuguese) [Google Scholar]
- Matto, T.N.; Bharkad, G.P.; Bhat, S.A. Prevalence of gastrointestinal helminth parasites of equids from organized farms of Mumbai and Pune. J. Parasit. Dis. 2015, 39, 179–185. [Google Scholar] [CrossRef]
- Borji, H.; Moosavi, Z.; Ahmadi, F. Cranial Mesenteric Arterial Obstruction Due to Strongylus vulgaris Larvae in a Donkey (Equus asinus). Iran. J. Parasitol. 2014, 9, 441–444. [Google Scholar]
- Pandey, V.S.; Eysker, M. Parasites of the stomach in donkeys of the highveld of Zimbabwe. Vet. Q. 1988, 10, 246–248. [Google Scholar] [CrossRef]
- Khallaayoune, K. Benefit of a strategic deworming programme in working donkeys in Marocco. In Donkeys, Mules and Horses in Tropical Agricultural Development; Fielding, D., Pearson, R.A., Eds.; Centre for Tropical Veterinary Medicine, University of Edinburgh: Edinburgh, UK, 1991; pp. 174–180. [Google Scholar]
- Pilo, C.; Altea, A.; Pirino, S.; Nicolussi, P.; Varcasia, A.; Genchi, M.; Scala, A. Strongylus vulgaris (Looss, 1900) in horses in Italy: Is it still a problem? Vet. Parasitol. 2012, 184, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Studzińska, M.B.; Tomczuk, K.; Demkowska-Kutrzepa, M.; Szczepaniak, K. The Strongylidae belonging to Strongylus genus in horses from southeastern Poland. Parasitol. Res. 2012, 111, 1417–1421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hertzberg, H.; Schwarzwald, C.C.; Grimm, F.; Frey, C.F.; Gottstein, B.; Gerber, V. Helminth control in the adult horse: The need for a re-orientation. Schweiz. Arch. Tierheilkd. 2014, 156, 61–70. [Google Scholar] [CrossRef]
- Getachew, A.M.; Innocent, G.T.; Trawford, A.F.; Feseha, G.; Reid, S.J.W.; Love, S. Equine parascarosis under the tropical weather conditions of Ethiopia: A coprological and postmortem study. Vet. Rec. 2008, 162, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Trawford, A.; Mulugeta, G. Parasites. In The Professional Handbook of the Donkey, 4th ed.; Duncan, J., Hadrill, D., Eds.; Compiled for Donkey Sanctuary by Elisabeth D. Svendsen: Wales, UK, 2008; pp. 82–110. [Google Scholar]





| Temperature (Mean) (°C) | Maximum Temperature (Mean) (°C) | Minimum Temperature (Mean) (°C) | Rainfall (Mean) (mm) | |
|---|---|---|---|---|
| January | 5.1 | 9.1 | 1.2 | 112.6 |
| February | 5.4 | 10.8 | 0.0 | 102.2 |
| March | 9.2 | 14.2 | 3.8 | 72.6 |
| April | 11.9 | 17.7 | 6.1 | 75.8 |
| May | 14.5 | 21.1 | 8.0 | 49.2 |
| June | 18.4 | 25.6 | 11.2 | 30.6 |
| July | 22.0 | 30.0 | 14.0 | 14.6 |
| August | 21.9 | 30.1 | 13.6 | 14.6 |
| September | 19.4 | 26.8 | 11.9 | 45.4 |
| October | 14.1 | 20.1 | 8.1 | 112.4 |
| November | 8.3 | 12.9 | 3.7 | 95.4 |
| December | 4.9 | 12.9 | 0.5 | 139.3 |
| Females | Geldings | |
|---|---|---|
| Age (years) | 6.8 (SD = 5.2; Max = 20; Min = 2) | 14.5 (SD = 6.3; Max = 20; Min = 4) |
| Body Condition Index | 3.2 (SD = 0.7; Max = 5; Min = 2) | 3.2 (SD = 0.5; Max = 4; Min = 2.5) |
| Thoracic Perimeter (cm) | 141 (SD = 11.5; Max = 168; Min = 115) | 142 (SD = 14.4; Max = 163; Min = 120) |
| Categories | Females (n) | Geldings (n) | Breeding Male (n) | |
|---|---|---|---|---|
| Body Condition Index (groups) | 2–2.5 | 9 | 3 | - |
| 3–3.5 | 25 | 12 | 1 | |
| 4–5 | 8 | 14 | - | |
| Thoracic Perimeter (groups) | 115–129 | 7 | 5 | - |
| 130–149 | 25 | 7 | 1 | |
| 150–168 | 10 | 7 | - |
| Prevalence (%) | Confidence Interval 95% (%) | ||
|---|---|---|---|
| Age group | Younger than 4 years | 46.7 | 16.7–78.9 |
| 4 to 10 years | 38.2 | 21.8–57.6 | |
| Older than 10 years | 35.6 | 17.8–60.0 | |
| Sex | Female | 39.5 | 27.1–55.5 |
| Geldings | 37.1 | 19.2–59.0 | |
| Body Condition Index | 2–2.5 | 40.8 | 16.0–70.7 |
| 3–3.5 | 38.9 | 25.6–58.3 | |
| 4–5 | 35.8 | 12.8–66.7 | |
| Thoracic Perimeter | 115–129 | 40.0 | 19.3–68.1 |
| 130–149 | 40.0 | 24.7–56.3 | |
| 150–168 | 37.6 | 17.3–58.7 |
| December | March | June | September | Total (year) | Average | Standard Deviation | |
|---|---|---|---|---|---|---|---|
| 1st year | 2200 | 2000 | 2700 | 1600 | 8500 | 2125.0 | 457.3 |
| 2nd year | 1400 | 6026 | 3695 | 3100 | 14,221 | 3555.3 | 1912.8 |
| 3rd year | 2803 | 2710 | 3400 | 1900 | 10,813 | 2703.3 | 616.6 |
| 4th year | 1500 | 2200 | 2700 | 1700 | 8100 | 2025.0 | 537.7 |
| 5th year | 1400 | 1401 | 1700 | 1200 | 5701 | 1425.3 | 206.1 |
| Total (month) | 9303 | 14,337 | 14,195 | 9500 | 47,335 | n.a. | n.a. |
| Average | 1860.6 | 2867.4 | 2839.0 | 1900.0 | 9467.0 | n.a. | n.a. |
| Standard Deviation | 624.0 | 1826.9 | 771.9 | 717.6 | 3217.1 | n.a. | n.a. |
| L3 n (%) | Cyathostominae % | Strongylinae % | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Genus Cyathostomum Morphotypes | |||||||||||
| A | B | C | D | E | F | G | H | S. vulgaris | Oesophagodontus | ||
| December | 9303 (19.7) | 91.22 | 0.08 | 1.34 | 7.14 | 0.01 | 0.01 | 0.20 | 0.00 | 0.00 | 0.00 |
| March | 14,337 (30.3) | 91.50 | 0.13 | 1.40 | 6.03 | 0.03 | 0.04 | 0.38 | 0.00 | 0.04 | 0.01 |
| June | 14,195 (30.0) | 90.98 | 0.09 | 1.20 | 7.09 | 0.02 | 0.03 | 0.22 | 0.01 | 0.02 | 0.00 |
| September | 9500 (20.0) | 93.56 | 0.08 | 1.11 | 5.04 | 0.02 | 0.02 | 0.15 | 0.00 | 0.02 | 0.00 |
| Total | 47,335 | 91.7 | 0.1 | 1.3 | 6.4 | 0.02 | 0.03 | 0.3 | 0.002 | 0.02 | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramalho Sousa, S.; Anastácio, S.; Nóvoa, M.; Paz-Silva, A.; Madeira de Carvalho, L.M. Gastrointestinal Parasitism in Miranda Donkeys: Epidemiology and Selective Control of Strongyles Infection in the Northeast of Portugal. Animals 2021, 11, 155. https://doi.org/10.3390/ani11010155
Ramalho Sousa S, Anastácio S, Nóvoa M, Paz-Silva A, Madeira de Carvalho LM. Gastrointestinal Parasitism in Miranda Donkeys: Epidemiology and Selective Control of Strongyles Infection in the Northeast of Portugal. Animals. 2021; 11(1):155. https://doi.org/10.3390/ani11010155
Chicago/Turabian StyleRamalho Sousa, Sérgio, Sofia Anastácio, Miguel Nóvoa, Adolfo Paz-Silva, and Luís Manuel Madeira de Carvalho. 2021. "Gastrointestinal Parasitism in Miranda Donkeys: Epidemiology and Selective Control of Strongyles Infection in the Northeast of Portugal" Animals 11, no. 1: 155. https://doi.org/10.3390/ani11010155
APA StyleRamalho Sousa, S., Anastácio, S., Nóvoa, M., Paz-Silva, A., & Madeira de Carvalho, L. M. (2021). Gastrointestinal Parasitism in Miranda Donkeys: Epidemiology and Selective Control of Strongyles Infection in the Northeast of Portugal. Animals, 11(1), 155. https://doi.org/10.3390/ani11010155