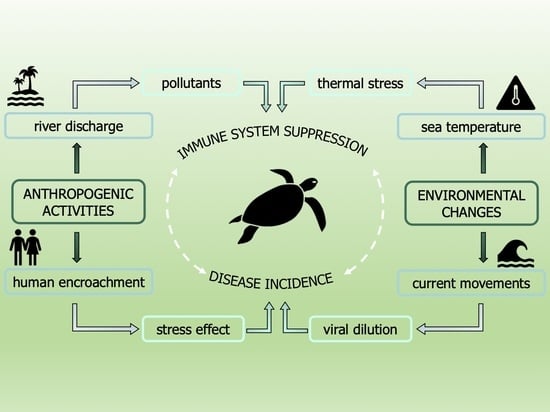
Occurrence of Fibropapillomatosis in Green Turtles (Chelonia mydas) in Relation to Environmental Changes in Coastal Ecosystems in Texas and Florida: A Retrospective Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.1.1. FP Prevalence Data
2.1.2. Coastal Water Quality Data
2.1.3. Demographic Data
2.1.4. Riverine Water Quality Data
2.1.5. Red Tide Data
2.2. Statistical Analyses
2.2.1. Spatial and Timeframes Selected for Analyses
2.2.2. Analysis of Coastal and Demographic Datasets
2.2.3. Analysis of Riverine Water Quality Dataset
2.2.4. Analysis of Red Tide Dataset
3. Results
3.1. Multiple Linear Regression of FP Prevalence and Environmental and Demographic Factors
3.2. Multiple Linear Regression Analysis of FP Prevalence and Riverine Water Quality
3.3. Multiple Linear Regression of FP Prevalence and Karenia brevis Algal Blooms in Florida
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, K.; Ariel, E.; Burgess, G.; Read, M. A review of fibropapillomatosis in Green turtles (Chelonia mydas). Vet. J. 2016, 212, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Duffy, D.J.; Schnitzler, C.; Karpinski, L.; Thomas, R.; Whilde, J.; Eastman, C.; Yang, C.; Krstic, A.; Rollinson, D.; Zirkelbach, B.; et al. Sea turtle fibropapilloma tumors share genomic drivers and therapeutic vulnerabilities with human cancers. Commun. Biol. 2018, 1, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.M.; Coates, C.W. Fibro-epithelial growths of the skin in large marine turtles, Chelonia mydas (Linnaeus). Zoologica 1938, 23, 93–96. [Google Scholar] [CrossRef]
- Hirama, S.; Ehrhart, L.M. Description, prevalence and severity of green turtle fibropapillomatosis in three developmental habitats on the east coast of Florida. Florida Sci. 2007, 70, 435–448. [Google Scholar]
- Lawrance, M.F.; Mans, K.L.; Sutton, E.; Savage, A.E. Molecular evolution of fi bropapilloma-associated herpesviruses infecting juvenile green and loggerhead sea turtles. Virology 2018, 521, 190–197. [Google Scholar] [CrossRef]
- Chabot, R.M.; Welsh, R.C.; Mott, C.R.; Guertin, J.R.; Shamblin, B.M.; Witherington, B.E. A sea turtle population assessment for Florida’s Big Bend, Northeastern Gulf of Mexico. Gulf Caribb. Res. 2021, 32, 19–33. [Google Scholar] [CrossRef]
- Work, T.M.; Balazs, G.H.; Rameyer, R.A.; Morris, R.A. Retrospective pathology survey of green turtles Chelonia mydas with fibropapillomatosis in the Hawaiian Islands, 1993–2003. Dis. Aquat. Organ. 2004, 62, 163–176. [Google Scholar] [CrossRef] [Green Version]
- Herbst, L.H.; Jacobson, E.R.; Moretti, R.; Brown, T.; Sundberg, J.P.; Klein, P.A. Experimental transmission of green turtle fibropapillomatosis using cell-free tumor extracts. Dis. Aquat. Organ. 1995, 22, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Herbst, L.; Ene, A.; Su, M.; Desalle, R.; Lenz, J. Tumor outbreaks in marine turtles are not due to recent herpesvirus mutations. Curr. Biol. 2004, 14, 697–699. [Google Scholar] [CrossRef] [Green Version]
- Whitmore, L.; Yetsko, K.; Farrell, J.A.; Page-Karjian, A.; Daniel, W.; Shaver, D.J.; Frandsen, H.R.; Walker, J.S.; Crowder, W.; Bovery, C.; et al. Evolutionary genomic comparisons of chelonid herpesvirus 5 ( ChHV5 ) from fibropapillomatosis-afflicted green (Chelonia mydas), olive ridley (Lepidochelys olivacea), and Kemp’s Ridley (Lepidochelys kempii) sea turtles. Animals 2021, 11, 2489. [Google Scholar] [CrossRef]
- Hazel, J.; Lawler, I.R.; Hamann, M. Diving at the shallow end: Green turtle behaviour in near-shore foraging habitat. J. Exp. Mar. Bio. Ecol. 2009, 371, 84–92. [Google Scholar] [CrossRef]
- Domiciano, I.G.; Domit, C.; Bracarense, A.P.F.R.L. The green turtle Chelonia mydas as a marine and coastal environmental sentinels: Anthropogenic activities and diseases. Semin. Agrar. 2017, 38, 3417–3434. [Google Scholar] [CrossRef] [Green Version]
- Godoy, D.A.; Stockin, K.A. Anthropogenic impacts on green turtles Chelonia mydas in New Zealand. Endanger. Species Res. 2018, 37, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Shaver, D.J.; Walker, J.S.; Backof, T.F. Fibropapillomatosis prevalence and distribution in green turtles Chelonia mydas in Texas (USA). Dis. Aquat. Organ. 2019, 136, 175–182. [Google Scholar] [CrossRef] [Green Version]
- dos Santos, R.G.; Martins, A.S.; Torezani, E.; Baptistotte, C.; Farias, J.d.; Horta, P.A.; Work, T.M.; Balazs, G.H. Relationship between fibropapillomatosis and environmental quality: A case study with Chelonia mydas off Brazil. Dis. Aquat. Organ. 2010, 89, 87–95. [Google Scholar] [CrossRef] [Green Version]
- Sposato, P.; Keating, P.; Lutz, P.L.; Milton, S.L. Evaluation of immune function in two populations of green sea urtles (Chelonia mydas) in a degraded versus a nondegraded habitat. J. Wildl. Dis. 2021, 57, 761–772. [Google Scholar] [CrossRef]
- Rossi, S.; Sánchez-Sarmiento, A.M.; dos Santos, R.G.; Zamana, R.R.; Prioste, F.E.S.; Gattamorta, M.A.; Ochoa, P.F.C.; Grisi-Filho, J.H.H.; Matushima, E.R. Monitoring green sea turtles in Brazilian feeding areas: Relating body condition index to fibropapillomatosis prevalence. J. Mar. Biol. Assoc. UK 2019, 99, 1879–1887. [Google Scholar] [CrossRef]
- Duffy, D.J.; Martindale, M.Q. Perspectives on the expansion of human precision oncology and genomic approaches to sea turtle fibropapillomatosis. Commun. Biol. 2019, 2, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Herbst, L.H.; Klein, P.A. Green turtle fibropapillomatosis: Challenges to assessing the role of environmental cofactors. Environ. Health Perspect. 1995, 103 (Suppl. S4), 27–30. [Google Scholar] [CrossRef] [Green Version]
- Foley, A.M.; Schroeder, B.A.; Redlow, A.E.; Fick-Child, K.J.; Teas, W.G. Fibropapillomatosis in stranded green turtles (Chelonia mydas) from the eastern United States (1980-98): Trends and associations with environmental factors. J. Wildl. Dis. 2005, 41, 29–41. [Google Scholar] [CrossRef] [Green Version]
- Mansfield, K.L.; Wyneken, J.; Porter, W.P.; Luo, J. First satellite tracks of neonate sea turtles redefine the ‘lost years’ oceanic niche. Proc. R. Soc. B Biol. Sci. 2014, 281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farrell, J.; Thomas, R.; Martindale, M.Q.; David, J. Characterisation of fibropapillomatosis tumour growth profiles in green sea turtles (Chelonia mydas). Testudo 2018, 8, 12–29. [Google Scholar]
- Haines, H.; Kleese, W. Effect of water temperature on performance. Infect. Immun. 1977, 15, 2012–2013. [Google Scholar]
- Gilad, O.; Yun, S.; Adkison, M.A.; Way, K.; Willits, N.H.; Bercovier, H.; Hedrick, R.P. Molecular comparison of isolates of an emerging fish pathogen, koi herpesvirus, and the effect of water temperature on mortality of experimentally infected koi. J. Gen. Virol. 2003, 84, 2661–2668. [Google Scholar] [CrossRef]
- Lafferty, K.D.; Porter, J.W.; Ford, S.E. Are diseases increasing in the ocean? Annu. Rev. Ecol. Evol. Syst. 2004, 35, 31–54. [Google Scholar] [CrossRef] [Green Version]
- Landsberg, J.H.; Balazs, G.H.; Steidinger, K.A.; Baden, D.G.; Work, T.M.; Russell, D.J. The potential role of natural tumor promoters in marine turtle fibropapillomatosis. J. Aquat. Anim. Health 1999, 11, 199–210. [Google Scholar] [CrossRef]
- Pierce, R.H.; Henry, M.S. Harmful algal toxins of the Florida red tide (Karenia brevis): Natural chemical stressors in South Florida coastal ecosystems. Ecotoxicology 2009, 17, 623–631. [Google Scholar] [CrossRef]
- Greenblatt, R.J.; Work, T.M.; Dutton, P.; Sutton, C.A.; Spraker, T.R.; Casey, R.N.; Diez, C.E.; Parker, D.; Leger, J.S.; Balazs, G.H.; et al. Geographic variation in marine turtle fibropapillomatosis. J. Zoo Wildl. Med. 2005, 36, 527–530. [Google Scholar] [CrossRef]
- Keller, J.M.; Balazs, G.H.; Nilsen, F.; Rice, M.; Work, T.M.; Jensen, B.A. Investigating the potential role of persistent organic pollutants in Hawaiian green sea turtle fibropapillomatosis. Environ. Sci. Technol. 2014, 48, 7807–7816. [Google Scholar] [CrossRef]
- Villa, C.A.; Flint, M.; Bell, I.; Hof, C.; Limpus, C.J.; Gaus, C. Trace element reference intervals in the blood of healthy green sea turtles to evaluate exposure of coastal populations. Environ. Pollut. 2017, 220, 1465–1476. [Google Scholar] [CrossRef] [Green Version]
- Patrício, A.R.; Diez, C.E.; van Dam, R.P.; Godley, B.J. Novel insights into the dynamics of green turtle fibropapillomatosis. Mar. Ecol. Prog. Ser. 2016, 547, 247–255. [Google Scholar] [CrossRef]
- Hing, S.; Narayan, E.J.; Thompson, R.C.A.; Godfrey, S.S. The relationship between physiological stress and wildlife disease: Consequences for health and conservation. Wildl. Res. 2016, 43, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Wetz, J.J.; Lipp, E.K.; Griffin, D.W.; Lukasik, J.; Wait, D.; Sobsey, M.D.; Scott, T.M.; Rose, J.B. Presence, infectivity, and stability of enteric viruses in seawater: Relationship to marine water quality in the Florida Keys. Mar. Pollut. Bull. 2004, 48, 698–704. [Google Scholar] [CrossRef] [PubMed]
- van Houtan, K.S.; Smith, C.M.; Dailer, M.L.; Kawachi, M. Eutrophication and the dietary promotion of sea turtle tumors. PeerJ 2014, 2014, 1–17. [Google Scholar] [CrossRef] [Green Version]
- da Silva, C.C.; Klein, R.D.; Barcarolli, I.F.; Bianchini, A. Metal contamination as a possible etiology of fibropapillomatosis in juvenile female green sea turtles Chelonia mydas from the southern Atlantic Ocean. Aquat. Toxicol. 2016, 170, 42–51. [Google Scholar] [CrossRef]
- Yaghmour, F.; Samara, F.; Alam, I. Analysis of polychlorinated biphenyls, polycyclic aromatic hydrocarbons and organochlorine pesticides in the tissues of green sea turtles, Chelonia mydas, (Linnaeus, 1758) from the eastern coast of the United Arab Emirates. Mar. Pollut. Bull. 2020, 160, 111574. [Google Scholar] [CrossRef]
- Andreani, G.; Santoro, M.; Cottignoli, S.; Fabbri, M.; Carpenè, E.; Isani, G. Metal distribution and metallothionein in loggerhead (Caretta caretta) and green (Chelonia mydas) sea turtles. Sci. Total Environ. 2008, 390, 287–294. [Google Scholar] [CrossRef]
- Godley, B.J.; Thompson, D.R.; Furness, R.W. Do heavy metal concentrations pose a threat to marine turtles from the Mediterranean Sea? Mar. Pollut. Bull. 1999, 38, 497–502. [Google Scholar] [CrossRef]
- Rahmstorf, S. Ocean circulation and climate during the past 120,000 years. Nature 2002, 419, 207–214. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, Y.; Shi, J. Relationship between sea surface salinity and ocean circulation and climate change. Sci. China Earth Sci. 2019, 62, 771–782. [Google Scholar] [CrossRef]
- Weisberg, R.H.; Liu, Y.; Lembke, C.; Hu, C.; Hubbard, K.; Garrett, M. The coastal ocean circulation influence on the 2018 West Florida shelf K. brevis red tide bloom. J. Geophys. Res. Ocean 2019, 124, 2501–2512. [Google Scholar] [CrossRef] [Green Version]
- Page-karjian, A.; Serrano, M.E.; Cartzendafner, J.; Morgan, A.; Ritchie, B.W.; Gregory, C.R.; McNeill, J.B.; Perrault, J.R.; Christiansen, E.F.; Harms, C.A. Molecular assessment of Chelonid Alphaherpesvirus 5 infection in tumor-free green (Chelonia mydas) and loggerhead (Caretta caretta) sea turtles in North Carolina, USA, 2015–2019. Animals 2020, 10, 1964. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.; Burgess, G.; Budd, A.M.; Huerlimann, R.; Mashkour, N.; Ariel, E. Molecular evidence for horizontal transmission of chelonid alphaherpesvirus 5 at green turtle (Chelonia mydas) foraging grounds in Queensland, Australia. PLoS ONE 2020, 15, e227268. [Google Scholar] [CrossRef]
- Formia, A.; Deem, S.; Billes, A.; Ngouessono, S.; Parnell, R.; Collins, T.; Sounguet, G.-P.; Gibudi, A.; Villarubia, A.; Balazs, G.H.; et al. Fibropapillomatosis confirmed in Chelonia mydas in the Gulf of Guinea, West Africa. Mar. Turt. Newsl. 2007, 116, 20–22. [Google Scholar]
- Herbst, L.; Greiner, E.; Ehrhart, M.; Bagley, A.; Klein, P.A. Serological turtles association infection, from between and in Fibropapillomatosis. J. Wildl. Dis. 1998, 34, 496–507. [Google Scholar] [CrossRef] [Green Version]
- Herbst, L.; Moretti, R.; Brown, T.; Klein, P. Sensitivity of the transmissible green turtle fibropapillomatosis agent to chloroform and ultracentrifugation conditions. Dis. Aquat. Organ. 1996, 25, 225–228. [Google Scholar] [CrossRef]
- Work, T.M.; Dagenais, J.; Balazs, G.H.; Schettle, N.; Ackermann, M. Dynamics of virus shedding and in situ confirmation of Chelonid Herpesvirus 5 in Hawaiian green turtles with Fibropapillomatosis. Vet. Pathol. 2015, 52, 1195–1201. [Google Scholar] [CrossRef]
- McLean, D.M.; Brown, J.R. Marine and freshwater virus dispersal. Acad. Manag. Rev. 1968, 31, 386–408. [Google Scholar]
- McLean, D.M.; Brown, J.R.; Laak, R. Virus dispersal by water. Am. Water Work. Assoc. 1966, 6, 157–165. [Google Scholar] [CrossRef]
- Hirama, S.; Ehrhart, L.M.; Rea, L.D.; Kiltie, R.A. Relating fibropapilloma tumor severity to blood parameters in green turtles Chelonia mydas. Dis. Aquat. Organ. 2014, 111, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Borrowman, K. Prevalence and Severity of Fibropapillomatosis in Juvenile Green Turtles (Chelonia mydas) in Three Habitats on Florida’s East Coast. Masters Thesis, University of Central Florida, Orlando, FL, USA, 2008. [Google Scholar]
- Bleck, R. An oceanic general circulation model in pressure coordinates. Adv. Atmos. Sci. 2001, 18, 18–21. [Google Scholar] [CrossRef]
- Halliwell, G.R. Evaluation of vertical coordinate and vertical mixing algorithms in the HYbrid-Coordinate Ocean Model (HYCOM). Ocean Model. 2004, 7, 285–322. [Google Scholar] [CrossRef]
- Southwood, A.L.; Reina, R.D.; Jones, V.S.; Jones, D.R. Seasonal diving patterns and body temperatures of juvenile green turtles at Heron Island, Australia. Can. J. Zool. 2003, 81, 1014–1024. [Google Scholar] [CrossRef]
- Seminoff, J.A.; Resendiz, A.; Nichols, W.J. Home range of green turtles Chelonia mydas at a coastal foraging area in the Gulf of California, Mexico. Mar. Ecol. Prog. Ser. 2002, 242, 253–265. [Google Scholar] [CrossRef] [Green Version]
- Esteban, N.; van Dam, R.P.; Harrison, E.; Herrera, A.; Berkel, J. Green and hawksbill turtles in the Lesser Antilles demonstrate behavioural plasticity in inter-nesting behaviour and post-nesting migration. Mar. Biol. 2015, 162, 1153–1163. [Google Scholar] [CrossRef] [Green Version]
- Sloan, K.A.; Addison, D.S.; Glinsky, A.T.; Benscoter, A.M.; Hart, K.M. Inter-nesting movements, migratory pathways, and resident foraging areas of green sea turtles (Chelonia mydas) satellite-tagged in Southwest Florida. Front. Mar. Sci. 2022, 8, 1–14. [Google Scholar] [CrossRef]
- Bourgeois, T.; Orr, J.C.; Resplandy, L.; Terhaar, J.; Ethe, C.; Gehlen, M.; Bopp, L. Coastal-ocean uptake of anthropogenic carbon. Biogeosciences 2016, 13, 4167–4185. [Google Scholar] [CrossRef] [Green Version]
- Gray, M.W.; Pinton, D.; Canestrelli, A.; Dix, N.; Marcum, P.; Kimbro, D.; Grizzle, R. Beyond residence time: Quantifying factors that drive the spatially explicit filtration services of an abundant native oyster population. Estuaries Coasts 2021, 44, 1–18. [Google Scholar] [CrossRef]
- Page-Karjian, A.; Norton, T.M.; Krimer, P.; Groner, M.; Nelson, S.E.; Gottdenker, N.L. Factors influencing survivorship of rehabilitating green sea turtles (Chelonia mydas) with fibropapillomatosis. J. Zoo Wildl. Med. 2014, 45, 507–519. [Google Scholar] [CrossRef]
- Penick, D.N.; Paladino, F.V.; Steyermark, A.C.; Spotila, J.R. Thermal dependence of tissue metabolism in the green turtle, Chelonia mydas. Comp. Biochem. Physiol. A Physiol. 1996, 113, 293–296. [Google Scholar] [CrossRef]
- Williard, A.S.; Hall, A.G.; Fujisaki, I.; McNeill, J.B. Oceanic overwintering in juvenile green turtles Chelonia mydas from a temperate latitude foraging ground. Mar. Ecol. Prog. Ser. 2017, 564, 235–240. [Google Scholar] [CrossRef] [Green Version]
- Abraham, J.P.; Baringer, M.; Bindoff, N.L.; Boyer, T.; Cheng, L.J.; Church, J.A.; Conroy, J.L.; Domingues, C.M.; Fasullo, J.T.; Gilson, J.; et al. A review of global ocean temperature observations: Implications for ocean heat content estimates and climate change. Rev. Geophys. 2013, 51, 450–483. [Google Scholar] [CrossRef]
- Aguirre, A.A.; Lutz, P. Marine turtles as sentinels of ecosystem health: Is Fibropapillomatosis an indicator? Ecohealth 2004, 1, 275–283. [Google Scholar] [CrossRef]
- Kelley, J.R.; Kelley, K.L.; Savage, A.E.; Mansfield, K.L. Novel disease state model finds most juvenile green turtles develop and recover from fibropapillomatosis. Ecosphere 2022, 13, 1–13. [Google Scholar] [CrossRef]
- McGarrity, M.E. Stress protein expression and green turtle fibropapillomatosis. Master’s Thesis, Florida Atlantic University, Boca Raton, FL, USA, December 2005; p. 62. [Google Scholar]
- Fuhrmann, M.; Delisle, L.; Petton, B.; Corporeau, C.; Pernet, F. Metabolism of the Pacific oyster, Crassostrea gigas, is influenced by salinity and modulates survival to the Ostreid herpesvirus OsHV-1. Biol. Open 2018, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Fuhrmann, M.; Petton, B.; Quillien, V.; Faury, N.; Morga, B.; Pernet, F. Salinity influences disease-induced mortality of the oyster Crassostrea gigas and infectivity of the ostreid herpesvirus 1 (OsHV-1). Aquac. Environ. Interact. 2016, 8, 543–552. [Google Scholar] [CrossRef] [Green Version]
- Reina, R.D.; Jones, T.T.; Spotila, J.R. Salt and water regulation by the leatherback sea turtle Dermochelys coriacea. J. Exp. Biol. 2002, 205, 1853–1860. [Google Scholar] [CrossRef]
- Reina, R.D.; Cooper, P.D. Control of salt gland activity in the hatchling green sea turtle, Chelonia mydas. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2000, 170, 27–35. [Google Scholar] [CrossRef]
- Carr, A. The navigation of the green turtle. Sci. Am. 1965, 212, 78–87. [Google Scholar] [CrossRef]
- Campo, C.E.C.; Santos, R.C.d.; Araùjo, A.S.; Paes, N.N.G. First record of an immature green turtle Chelonia mydas (Linnaeus, 1758) (Testudines: Cheloniidae) on a fluvial island, Reserva Biológica do Parazinho, Amazonas river, Brazil. Check List 2013, 9, 434–435. [Google Scholar] [CrossRef] [Green Version]
- Espinoza-Rodríguez, N.; Barrios-Garrido, H.A. Southernmost record of an immature green turtle Chelonia mydas in the Maracaibo lake system, Venezuela. Mar. Turt. Newsl. 2021, 163, 18–20. [Google Scholar]
- Vuorinen, I.; Hänninen, J.; Viitasalo, M.; Helminen, U.; Kuosa, H. Proportion of copepod biomass declines with decreasing salinity in the Baltic Sea. ICES J. Mar. Sci. 1998, 55, 767–774. [Google Scholar] [CrossRef]
- Kelble, C.R.; Johns, E.M.; Nuttle, W.K.; Lee, T.N.; Smith, R.H.; Ortner, P.B. Salinity patterns of Florida Bay. Estuar. Coast. Shelf Sci. 2007, 71, 318–334. [Google Scholar] [CrossRef]
- Aguirre, A.A. Green turtle fibropapilloma: An epidemiologic perspective. In Research Plan for Marine Turtle Fibropapilloma; Balazs, G.H., Pooley, S.G., Eds.; Tech Memo NMFS-SWFSC-156; US Department of Commerce, NOAA: Honolulu, HI, USA, 1991; pp. 107–113. [Google Scholar]
- Zavala-Hidalgo, J.; Gallegos-García, A.; Martínez-López, B.; Morey, S.L.; O’Brien, J.J. Seasonal upwelling on the Western and Southern Shelves of the Gulf of Mexico. Ocean Dyn. 2006, 56, 333–338. [Google Scholar] [CrossRef]
- Thyng, K.M.; Hetland, R.D.; Ogle, M.T.; Zhang, X.; Chen, F.; Campbell, L. Origins of Karenia brevis harmful algal blooms along the Texas coast. Limnol. Oceanogr. Fluids Environ. 2013, 3, 269–278. [Google Scholar] [CrossRef]
- Tominack, S.A.; Coffey, K.Z.; Yoskowitz, D.; Sutton, G.; Wetz, M.S. An assessment of trends in the frequency and duration of Karenia brevis red tide blooms on the South Texas coast (western Gulf of Mexico). PLoS ONE 2020, 15, e239309. [Google Scholar] [CrossRef]
- Hermanussen, S.; Limpus, C.; Papke, O.; Blanshard, W.; Connell, D.; Gaus, C. Evaluating Spatial Patterns of Dioxins in Sediments to Aid Determination of Potential Implications for Marine Reptiles. In Proceedings of the Dioxin 2004: 24. International Symposium on Halogenated Environmental Organic Pollutants and POPs, Berlin, Germany, 6–10 September 2004; Volume 66, pp. 1861–1867. Available online: http://espace.library.uq.edu.au/view/UQ:101210 (accessed on 19 March 2022).
- Herren, R.M.; Bagley, D.A.; Bresette, M.J.; Holloway-Adkins, K.G.; Clark, D.; Witherington, B.E. Sea turtle abundance and demographic measurements in a marine protected areas in the Florida Keys (USA). Herpetol. Conserv. Biol. 2018, 26, 224–239. [Google Scholar]
- Fuentes, M.M.P.B.; Lawler, I.R.; Gyuris, E. Dietary preferences of juvenile green turtles (Chelonia mydas) on a tropical reef flat. Wildl. Res. 2006, 33, 671–678. [Google Scholar] [CrossRef]
- López-Mendoza, P.G.; Ruiz-Fernández, A.C.; Sanchez-Cabeza, J.A.; van Tussenbroek, B.I.; Cuellar-Martinez, T.; Pérez-Bernal, L.H. Temporal trends of organic carbon accumulation in seagrass meadows from the northern Mexican Caribbean. Catena 2020, 194, 104645. [Google Scholar] [CrossRef]
- Ravaglioli, C.; Bulleri, F.; Rühl, S.; McCoy, S.J.; Findlay, H.S.; Widdicombe, S.; Queirós, A.M. Ocean acidification and hypoxia alter organic carbon fluxes in marine soft sediments. Glob. Chang. Biol. 2019, 25, 4165–4178. [Google Scholar] [CrossRef]
- Swanson, K.M.; Flewelling, L.J.; Byrd, M.; Nunez, A.; Villareal, T.A. The 2008 Texas Dinophysis ovum bloom: Distribution and toxicity. Harmful Algae 2010, 9, 190–199. [Google Scholar] [CrossRef]
- Fire, S.E.; Wang, Z.; Byrd, M.; Whitehead, H.R.; Paternoster, J.; Morton, S.L. Co-occurrence of multiple classes of harmful algal toxins in bottlenose dolphins (Tursiops truncatus) stranding during an unusual mortality event in Texas, USA. Harmful Algae 2011, 10, 330–336. [Google Scholar] [CrossRef]
- Camacho-Muñoz, D.; Praptiwi, R.A.; Lawton, L.A.; Edwards, C. High value phycotoxins from the dinoflagellate Prorocentrum. Front. Mar. Sci. 2021, 8, 1–20. [Google Scholar] [CrossRef]
- Aguirre, A.A.; Balazs, G.H. Blood biochemistry values of green turtles, Chelonia mydas, with and without fibropapillomatosis. Comp. Haematol. Int. 2000, 10, 132–137. [Google Scholar] [CrossRef]
- Solis-Weiss, V.; Aleffi, F.; Bettoso, N.; Rossin, P.; Orel, G.; Fonda-Umani, S. Effects of industrial and urban pollution on the benthic macrofauna in the Bay of Muggia (industrial port of Trieste, Italy). Sci. Total Environ. 2004, 328, 247–263. [Google Scholar] [CrossRef]
- Perrault, J.R.; Perkins, C.R.; Ajemian, M.J.; Bresette, M.J.; Mott, C.R.; Page-Karjian, A. Harmful algal and cyanobacterial toxins in foraging green turtles (Chelonia mydas) in Florida’s Big Bend. Toxicon X 2020, 5, 100020. [Google Scholar] [CrossRef] [PubMed]
- Dela-Cruz, J.; Middleton, J.H.; Suthers, I.M. The influence of upwelling, coastal currents and water temperature on the distribution of the red tide dinoflagellate, Noctiluca scintillans, along the east coast of Australia. Hydrobiologia 2008, 598, 59–75. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, J.; Zheng, B.; Cai, W.; Lin, K.; Tang, J. Temporal and spatial distribution of red tide outbreaks in the Yangtze River Estuary and adjacent waters, China. Mar. Pollut. Bull. 2013, 72, 213–221. [Google Scholar] [CrossRef]
- Medina, M.; Kaplan, D.; Milbrandt, E.C.; Tomasko, D.; Huffaker, R.; Angelini, C. Nitrogen-enriched discharges from a highly managed watershed intensify red tide (Karenia brevis) blooms in southwest Florida. Sci. Total Environ. 2022, 827, 154149. [Google Scholar] [CrossRef]










| Predictor Variables | Source | U.M. |
|---|---|---|
| Sea surface water temperature | HYCOM | [°C] |
| Sea surface water salinity | HYCOM | [ppt] |
| Water current based residence time | HYCOM | [days] |
| Karenia brevis concentration | FWC-FWRI | [cells I−1] |
| Red Tide occurrence | FWC-FWRI | [days] |
| Watershed population density | US Census Bureau | [individuals km−2] |
| River discharge | USGS | [m3·s−1] |
| Water pH | USGS | [–] |
| Ammonia (NH3 + NH4+) | USGS | [mg L−1] |
| Chlorophyll | USGS | [mg L−1] |
| Nitrite (NO2) | USGS | [mg L−1] |
| Nitrate (NO3) | USGS | [mg L−1] |
| Organic nitrogen | USGS | [mg L−1] |
| Total nitrogen (nitrate + nitrite + ammonia + organic nitrogen) | USGS | [mg L−1] |
| Phosphorus | USGS | [mg L−1] |
| Orthophosphate (PO4) | USGS | [mg L−1] |
| Organic Carbon | USGS | [mg L−1] |
| Suspended Solids | USGS | [mg L−1] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manes, C.; Pinton, D.; Canestrelli, A.; Capua, I. Occurrence of Fibropapillomatosis in Green Turtles (Chelonia mydas) in Relation to Environmental Changes in Coastal Ecosystems in Texas and Florida: A Retrospective Study. Animals 2022, 12, 1236. https://doi.org/10.3390/ani12101236
Manes C, Pinton D, Canestrelli A, Capua I. Occurrence of Fibropapillomatosis in Green Turtles (Chelonia mydas) in Relation to Environmental Changes in Coastal Ecosystems in Texas and Florida: A Retrospective Study. Animals. 2022; 12(10):1236. https://doi.org/10.3390/ani12101236
Chicago/Turabian StyleManes, Costanza, Daniele Pinton, Alberto Canestrelli, and Ilaria Capua. 2022. "Occurrence of Fibropapillomatosis in Green Turtles (Chelonia mydas) in Relation to Environmental Changes in Coastal Ecosystems in Texas and Florida: A Retrospective Study" Animals 12, no. 10: 1236. https://doi.org/10.3390/ani12101236
APA StyleManes, C., Pinton, D., Canestrelli, A., & Capua, I. (2022). Occurrence of Fibropapillomatosis in Green Turtles (Chelonia mydas) in Relation to Environmental Changes in Coastal Ecosystems in Texas and Florida: A Retrospective Study. Animals, 12(10), 1236. https://doi.org/10.3390/ani12101236