Masculinization of Red Tilapia (Oreochromis spp.) Using 17α-Methyltestosterone-Loaded Alkyl Polyglucosides Integrated into Nanostructured Lipid Carriers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
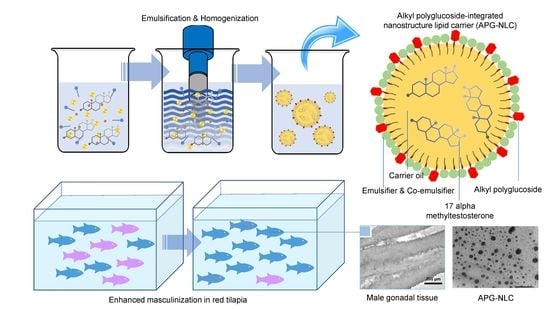
2.2. Formulation and Fabrication of Lipid-Based Nanoparticles
2.3. Characterization of the Lipid-Based Nanoparticles
2.3.1. Dynamic Light Scattering
2.3.2. Transmission Electron Microscopy
2.3.3. Encapsulation Efficiency
2.3.4. Release Kinetics of Testosterone in Lipid-Based Nanoparticles
2.4. Masculinization of Red Tilapia
2.4.1. Ethical Statement
2.4.2. Fish Production, Rearing and Experimental Protocol
2.4.3. Length–Weight Relationship
2.4.4. Relative Condition Factor
2.4.5. Sex Reversal Ratio Determined by the Gonad Squashing Technique
2.5. Statistical Analysis
3. Results
3.1. Characterization of the Lipid-Based Nanoparticles
3.2. Encapsulation Efficiency and Study of the In Vitro Release of MT from LBN
3.3. Red Tilapia Masculinization Using Testosterone Loaded in Lipid-Based Nanoparticles
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Omasaki, S.; Charo-Karisa, H.; Kahi, A.; Komen, H. Genotype by environment interaction for harvest weight, growth rate and shape between monosex and mixed sex Nile tilapia (Oreochromis niloticus). Aquaculture 2016, 458, 75–81. [Google Scholar] [CrossRef]
- Saha, P.; Hossain, M.E.; Prodhan, M.M.H.; Rahman, M.T.; Nielsen, M.; Khan, M.A. Profit and loss dynamics of aquaculture farming. Aquaculture 2022, 561, 738619. [Google Scholar] [CrossRef]
- El-Sayed, A.-F.M.; Abdel-Aziz, E.-S.H.; Abdel-Ghani, H.M. Effects of phytoestrogens on sex reversal of Nile tilapia (Oreochromis niloticus) larvae fed diets treated with 17α-Methyltestosterone. Aquaculture 2012, 360, 58–63. [Google Scholar] [CrossRef]
- Karaket, T.; Reungkhajorn, A.; Ponza, P. The optimum dose and period of 17α-methyltestosterone immersion on masculinization of red tilapia (Oreochromis spp.). Aquac. Fish. 2023, 8, 174–179. [Google Scholar] [CrossRef]
- Snake, M.; Maluwa, A.; Zidana, H.; Chigwechokha, P.; Simwaka, M. Production of a predominantly male tilapia progeny using two Malawian tilapias, Oreochromis shiranus and Oreochromis karongae. Aquac. Rep. 2020, 16, 100274. [Google Scholar] [CrossRef]
- Celik, I.; Guner, Y.; Celİk, P. Effect of orally-administered 17α-Methyltestosterone at different doses on the sex reversal of the Nile tilapia (Oreochromis niloticus, Linneaus 1758). J. Anim. Vet. Adv. 2011, 10, 853–857. [Google Scholar] [CrossRef]
- El-Greisy, Z.; El-Gamal, A. Monosex production of tilapia, Oreochromis niloticus using different doses of 17α-methyltestosterone with respect to the degree of sex stability after one year of treatment. Egypt. J. Aquat. Res. 2012, 38, 59–66. [Google Scholar] [CrossRef]
- Arriesgado, D.M.; Vicente, H.J.; Vicente, D.A. Production of Male Oreochromis niloticus GET Excel Tilapia by Egg Immersion in Methyl Testosterone Hormone. J. Environ. Aquat. Resour. 2011, 2, 21–33. [Google Scholar] [CrossRef]
- Ahmad, A.; Abdullah, S.R.S.; Hasan, H.A.; Othman, A.R.; Ismail, N.I. Aquaculture industry: Supply and demand, best practices, effluent and its current issues and treatment technology. J. Environ. Manag. 2021, 287, 112271. [Google Scholar] [CrossRef]
- Ding, J.; Sun, H.; Liang, A.; Liu, J.; Song, L.; Lv, M.; Zhu, D. Testosterone amendment alters metabolite profiles of the soil microbial community. Environ. Pollut. 2021, 272, 115928. [Google Scholar] [CrossRef]
- Azar, F.A.N.; Pezeshki, A.; Ghanbarzadeh, B.; Hamishehkar, H.; Mohammadi, M. Nanostructured lipid carriers: Promising delivery systems for encapsulation of food ingredients. J. Agric. Food Res. 2020, 2, 100084. [Google Scholar]
- Beloqui, A.; del Pozo-Rodríguez, A.; Isla, A.; Rodríguez-Gascón, A.; Solinís, M.Á. Nanostructured lipid carriers as oral delivery systems for poorly soluble drugs. J. Drug Deliv. Sci. Technol. 2017, 42, 144–154. [Google Scholar] [CrossRef]
- Müller, R.; Petersen, R.; Hommoss, A.; Pardeike, J. Nanostructured lipid carriers (NLC) in cosmetic dermal products. Adv. Drug Deliv. Rev. 2007, 59, 522–530. [Google Scholar] [CrossRef]
- Müller, R.; Radtke, M.; Wissing, S. Nanostructured lipid matrices for improved microencapsulation of drugs. Int. J. Pharm. 2002, 242, 121–128. [Google Scholar] [CrossRef]
- Fang, J.-Y.; Fang, C.-L.; Liu, C.-H.; Su, Y.-H. Lipid nanoparticles as vehicles for topical psoralen delivery: Solid lipid nanoparticles (SLN) versus nanostructured lipid carriers (NLC). Eur. J. Pharm. Biopharm. 2008, 70, 633–640. [Google Scholar] [CrossRef]
- Mu, H.; Holm, R.; Müllertz, A. Lipid-based formulations for oral administration of poorly water-soluble drugs. Int. J. Pharm. 2013, 453, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Nasirizadeh, S.; Malaekeh-Nikouei, B. Solid lipid nanoparticles and nanostructured lipid carriers in oral cancer drug delivery. J. Drug Deliv. Sci. Technol. 2020, 55, 101458. [Google Scholar] [CrossRef]
- Padhye, S.; Nagarsenker, M.S. Simvastatin solid lipid nanoparticles for oral delivery: Formulation development and in vivo evaluation. Indian J. Pharm. Sci. 2013, 75, 591–598. [Google Scholar]
- Salah, E.; Abouelfetouh, M.M.; Pan, Y.; Chen, D.; Xie, S. Solid lipid nanoparticles for enhanced oral absorption: A review. Colloids Surf. B Biointerfaces 2020, 196, 111305. [Google Scholar] [CrossRef]
- Silva, A.C.; Kumar, A.; Wild, W.; Ferreira, D.; Santos, D.; Forbes, B. Long-term stability, biocompatibility and oral delivery potential of risperidone-loaded solid lipid nanoparticles. Int. J. Pharm. 2012, 436, 798–805. [Google Scholar] [CrossRef]
- Gomaa, E.; Fathi, H.A.; Eissa, N.G.; Elsabahy, M. Methods for preparation of nanostructured lipid carriers. Methods 2022, 199, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Beloqui, A.; Solinís, M.Á.; des Rieux, A.; Préat, V.; Rodríguez-Gascón, A. Dextran–protamine coated nanostructured lipid carriers as mucus-penetrating nanoparticles for lipophilic drugs. Int. J. Pharm. 2014, 468, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, M.; Ribeiro, R.; Vieira, A.; Andrade, F.; Reis, S. Design of a nanostructured lipid carrier intended to improve the treatment of tuberculosis. Drug Des. Dev. Ther. 2016, 10, 2467. [Google Scholar] [CrossRef] [PubMed]
- Shimamoto, A.; Liu, J.; Kozawa, S.; Fujimiya, T. Determination of endogenous testosterone in rat tissues following fetal alcohol exposure using HPLC with UV detection. J. Chromatogr. B 2006, 836, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Pauly, D. Some Simple Methods for the Assessment of Tropical Fish Stocks; Food & Agriculture Organization: Rome, Italy, 1983. [Google Scholar]
- Froese, R. Cube law, condition factor and weight–length relationships: History, meta-analysis and recommendations. J. Appl. Ichthyol. 2006, 22, 241–253. [Google Scholar] [CrossRef]
- Le Cren, E.D. The length-weight relationship and seasonal cycle in gonad weight and condition in the perch (Perca fluviatilis). J. Anim. Ecol. 1951, 20, 201–219. [Google Scholar] [CrossRef]
- Guerrero III, R.D.; Shelton, W.L. An aceto-carmine squash method for sexing juvenile fishes. Progress. Fish-Cult. 1974, 36, 56. [Google Scholar] [CrossRef]
- Aziz, M.A.; Mostary, M.M.; Sume, I.J.; Uddin, M.H.; Khan, M.G.Q.; Alam, M.S.; Islam, M.S. The efficacy of using pine (Pinus massoniana) pollen as an alternative to synthetic steroids in producing monosex male Nile tilapia (Oreochromis niloticus, L.). Aquac. Fish Fish. 2022, 2, 375–383. [Google Scholar] [CrossRef]
- Rivero-Wendt, C.L.G.; Miranda-Vilela, A.L.; Domingues, I.; Oliveira, R.; Monteiro, M.S.; Moura-Mello, M.A.; Matias, R.; Soares, A.M.V.M.; Grisolia, C.K. Steroid androgen 17 alpha methyltestosterone used in fish farming induces biochemical alterations in zebrafish adults. J. Environ. Sci. Health Part A 2020, 55, 1321–1332. [Google Scholar] [CrossRef]
- Hasheesh, W.S.; Marie, M.-A.S.; Abbas, H.H.; Eshak, M.G.; Zahran, E.A. An evaluation of the effect of 17α-Methyltestosterone hormone on some biochemical, molecular and histological changes in the liver of Nile Tilapia, Oreochromis niloticus. Life Sci. J. 2011, 8, 343–358. [Google Scholar]
- Sudtida, P.T.; Chheang, L.; Math, C. Ecological risk of 17α-methyltestosterone contaminated water discharged from a full water recirculating earthen masculinization pond. Hum. Ecol. Risk Assess. Int. J. 2021, 27, 1696–1714. [Google Scholar]
- Megbowon, I. Tilapia Production in Nigeria. Fishnetwork 2011, 4, 18–22. [Google Scholar]
- Megbowon, I.; Mojekwu, T. Tilapia sex reversal using methyl testosterone (MT) and its effect on fish, man and environment. Biotechnology 2013, 13, 213–216. [Google Scholar] [CrossRef]
- Günther, C.; Kecskes, A.; Staks, T.; Täuber, U. Percutaneous absorption of methylprednisolone aceponate following topical application of Advantan® lotion on intact, inflamed and stripped skin of male volunteers. Skin Pharmacol. Physiol. 1998, 11, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Kelidari, H.R.; Saeedi, M.; Hajheydari, Z.; Akbari, J.; Morteza-Semnani, K.; Akhtari, J.; Valizadeh, H.; Asare-Addo, K.; Nokhodchi, A. Spironolactone loaded nanostructured lipid carrier gel for effective treatment of mild and moderate acne vulgaris: A randomized, double-blind, prospective trial. Colloids Surf. B Biointerfaces 2016, 146, 47–53. [Google Scholar] [CrossRef]
- Singh, A.; Neupane, Y.R.; Mangla, B.; Kohli, K. Nanostructured lipid carriers for oral bioavailability enhancement of exemestane: Formulation design, in vitro, ex vivo, and in vivo studies. J. Pharm. Sci. 2019, 108, 3382–3395. [Google Scholar] [CrossRef]
- Rather, M.A.; Sharma, R.; Gupta, S.; Ferosekhan, S.; Ramya, V.; Jadhao, S.B. Chitosan-nanoconjugated hormone nanoparticles for sustained surge of gonadotropins and enhanced reproductive output in female fish. PLoS ONE 2013, 8, e57094. [Google Scholar] [CrossRef]
- Rabelo, R.S.; Oliveira, I.F.; da Silva, V.M.; Prata, A.S.; Hubinger, M.D. Chitosan coated nanostructured lipid carriers (NLCs) for loading Vitamin D: A physical stability study. Int. J. Biol. Macromol. 2018, 119, 902–912. [Google Scholar] [CrossRef]
- Keck, C.M.; Kovačević, A.; Müller, R.H.; Savić, S.; Vuleta, G.; Milić, J. Formulation of solid lipid nanoparticles (SLN): The value of different alkyl polyglucoside surfactants. Int. J. Pharm. 2014, 474, 33–41. [Google Scholar] [CrossRef]
- Von Rybinski, W.; Hill, K. Alkyl polyglycosides—Properties and applications of a new class of surfactants. Angew. Chem. Int. Ed. 1998, 37, 1328–1345. [Google Scholar] [CrossRef]
- Qushawy, M. Effect of the surfactant and liquid lipid type in the physico-chemical characteristics of beeswax-based nanostructured lipid carrier (NLC) of metformin. Pharm. Nanotechnol. 2021, 9, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; McClements, D.J. Formation and stability of solid lipid nanoparticles fabricated using phase inversion temperature method. Colloids Surf. A Physicochem. Eng. Asp. 2016, 499, 79–87. [Google Scholar] [CrossRef]
- Jagdevappa, P.; Prashant, G.; Ravindra, K.; Sachin, J.; Satish, M.; Meghanath, S. Applications of solid lipid nanoparticle in novel drug delivery system. Br. Biomed. Bull. 2013, 1, 103–118. [Google Scholar]
- Jaiswal, P.; Gidwani, B.; Vyas, A. Nanostructured lipid carriers and their current application in targeted drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Dilbaghi, N.; Saharan, R.; Bhanjana, G. Nanotechnology as emerging tool for enhancing solubility of poorly water-soluble drugs. Bionanoscience 2012, 2, 227–250. [Google Scholar] [CrossRef]
- Cipolla, D.; Shekunov, B.; Blanchard, J.; Hickey, A. Lipid-based carriers for pulmonary products: Preclinical development and case studies in humans. Adv. Drug Deliv. Rev. 2014, 75, 53–80. [Google Scholar] [CrossRef]
- Sarathchandiran, I. A review on nanotechnology in solid lipid nanoparticles. Int. J. Pharm. Dev. Technol. 2012, 2, 45–61. [Google Scholar]
- Pajić, N.B.; Ilić, T.; Nikolić, I.; Dobričić, V.; Pantelić, I.; Savić, S. Alkyl polyglucoside-based adapalene-loaded microemulsions for targeted dermal delivery: Structure, stability and comparative biopharmaceutical characterization with a conventional dosage form. J. Drug Deliv. Sci. Technol. 2019, 54, 101245. [Google Scholar] [CrossRef]
- Phunpee, S.; Saesoo, S.; Sramala, I.; Jarussophon, S.; Sajomsang, W.; Puttipipatkhajorn, S.; Soottitantawat, A.; Ruktanonchai, U.R. A comparison of eugenol and menthol on encapsulation characteristics with water-soluble quaternized β-cyclodextrin grafted chitosan. Int. J. Biol. Macromol. 2016, 84, 472–480. [Google Scholar] [CrossRef]
- Singh, A.K. Introduction of modern endocrine techniques for the production of monosex population of fishes. Gen. Comp. Endocrinol. 2013, 181, 146–155. [Google Scholar] [CrossRef]
- Kobayashi, T.; Kajiura-Kobayashi, H.; Guan, G.; Nagahama, Y. Sexual dimorphic expression of DMRT1 and Sox9a during gonadal differentiation and hormone-induced sex reversal in the teleost fish Nile tilapia (Oreochromis niloticus). Dev. Dyn. 2008, 237, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, R.K.; Nakamura, M.; Kobayashi, T.; Nagahama, Y. Suppression of steroidogenic enzyme expression during androgen-induced sex reversal in Nile tilapia (Oreochromis niloticus). Gen. Comp. Endocrinol. 2006, 145, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Piferrer, F. Hormonal control of reproduction and growth: Endocrine control of sex differentiation in fish. In Encyclopedia of Fish Physiology; Farrell, A.P., Ed.; Academic Press: San Diego, CA, USA, 2011; pp. 1490–1499. [Google Scholar]
- Vinarukwong, N.; Lukkana, M.; Wongtavatchai, J. Decreasing duration of androgenic hormone feeding supplement for production of male monosex in tilapia (Oreochromis spp.) fry. Thai J. Vet. Med. 2018, 48, 375–383. [Google Scholar]
- Karaket, T.; Seel-audom, M.; Areechon, N. Analysis and Synthesis of Knowledge from Nile Tilapia Philosophers for Sustainable Culture in the Northern Thailand. Thai Sci. Technol. J. 2021, 29, 454–468. [Google Scholar]
- Amer, M.; Zaki, F.M.; Tahoun, A.; Said, M. Effects of 17 α-Methyltestosterone and Tribulus terrestris extract on sex ratio and gonads histology of Red tilapia hybrid. Egypt. J. Nutr. Feed. 2021, 24, 189–198. [Google Scholar] [CrossRef]
- Alcántar-Vázquez, J.P. Sex proportion in Nile tilapia Oreochromis niloticus fed estrogen mixtures: A case of paradoxical masculinization. Lat. Am. J. Aquat. Res. 2018, 46, 337–345. [Google Scholar] [CrossRef]
- Rinchard, J.; Dabrowski, K.; Garcia–Abiado, M.A.; Ottobre, J. Uptake and depletion of plasma 17α-methyltestosterone during induction of masculinization in muskellunge, Esox masquinongy: Effect on plasma steroids and sex reversal. Steroids 1999, 64, 518–525. [Google Scholar] [CrossRef]
- Marín-Ramírez, J.; Alcántar-Vázquez, J.; Antonio-Estrada, C.; Moreno-de la Torre, R.; Calzada-Ruiz, D. Feminization of nile tilapia Oreochromis niloticus (L.) by diethylstilbestrol growth and gonadosomatic index. Ecosist. Recur. Agropecu. 2016, 3, 51–61. [Google Scholar]
- Juárez-Juárez, V.; Alcántar-Vázquez, J.; Antonio-Estrada, C.; Marín-Ramírez, J.; Moreno-De La Torre, R. Feminization by 17α-ethinylestradiol of the progeny of XY-female Nile tilapia (Oreochromis niloticus). Effects on growth, condition factor and gonadosomatic index. Turk. J. Fish. Aquat. Sci. 2017, 17, 599–607. [Google Scholar] [CrossRef]
- Rima, N.; Rahman, M.; Sarker, M. Optimization of 17-alpha methyltestosterone (MT) hormone dose during masculinization of Nile tilapia (Oreochromis niloticus) fry. J. Noakhali Sci. Technol. Univ. 2017, 1, 35–41. [Google Scholar]
- Kamble, M.T.; Chavan, B.R.; Ataguba, G.; Azpeitia, T.; Medhe, S.; Jain, S.; Jadhav, R. Application of Moringa oleifera for development of Sustainable and Biosecure Aquaculture. Aquac. Indones. 2015, 15, 64–73. [Google Scholar] [CrossRef]
- Malik, A.; Abbas, G.; Soomro, M.; Shah, S.; Asadullah, A.; Bhutto, A.; Jamali, G.; Roonjho, Z. Length-weight relationship and condition factor of red tilapia (Hybrid) reared in cemented tanks of Sun-bright Red Tilapia and ornamental hatchery-Karachi, Sindh-Pakistan. Sindh Univ. Res. J. (Sci. Ser.) 2017, 49, 159–162. [Google Scholar]
- Cishahayo, L.; Yongo, E.; Mutethya, E.; Waithaka, E.; Ndayishimiye, R. Length–weight relationship, condition factor, sex ratio and size at first maturity of the blue-spotted tilapia (Oreochromis leucostictus) in Lake Naivasha, Kenya. Lakes Reserv. Res. Manag. 2022, 27, e12417. [Google Scholar] [CrossRef]
- Komba, E.A.; Munubi, R.N.; Chenyambuga, S.W. Comparison of body length-weight relationship and condition factor for Nile tilapia (Oreochromis niloticus) cultured in two different climatic conditions in Tanzania. Int. J. Fish. Aquat. Stud. 2020, 8, 44–48. [Google Scholar]





| Formulations | ||||
|---|---|---|---|---|
| Composition | SLN | APG-SLN | NLC | APG-NLC |
| 17 α-methyltestosterone | 0.2 | 0.2 | 0.2 | 0.2 |
| Medium chain triglyceride (MCT) | 0.0 | 0.0 | 5.0 | 5.0 |
| Alkyl-polyglucoside | 0.0 | 2.0 | 0.0 | 2.0 |
| Span 80 | 3.0 | 3.0 | 3.0 | 3.0 |
| Ethoxydiglycol | 4.0 | 4.0 | 3.0 | 3.0 |
| Tween 20 | 3.0 | 3.0 | 3.0 | 3.0 |
| Synperonic PE/F68 | 2.0 | 2.0 | 2.0 | 2.0 |
| Glycerol | 2.5 | 2.5 | 2.5 | 2.5 |
| DI water | 85.3 | 83.3 | 81.3 | 79.3 |
| Total | 100.0 | 100.0 | 100.0 | 100.0 |
| Formulation | Particle Size (nm) | Surface Charge (mV) | Polydispersity Index |
|---|---|---|---|
| SLN | 125.30 ± 1.34 | −30.4 ± 2.43 | 0.211 ± 0.002 |
| APG-SLN | 122.23 ± 1.50 | −34.0 ± 1.47 | 0.228 ± 0.020 |
| NLC | 94.56 ± 0.17 | −28.5 ± 3.50 | 0.148 ± 0.010 |
| APG-NLC | 84.21 ± 0.60 | −30.9 ± 3.48 | 0.123 ± 0.010 |
| MT-ET | ND | ND | ND |
| Sample | k (10−1/h) | n | R2 |
|---|---|---|---|
| MT-ET (0–2 h) | 0.012 | 0.3563 | 0.9521 |
| MT-ET (3–8 h) | 0.000 | 0.0416 | 0.894 |
| MT-NLC | 0.587 | 0.9481 | 0.9981 |
| MT-APG-NLC | 0.637 | 1.1246 | 0.994 |
| Treatments | Male (%) | No. of Fish | Male: Female (1:1) | p-Value | b ± SE | Exponentiation of b | Wald X2 | Survival Rate (%) | Cost (THB/Fish) | Compared with T4 (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Control 1 | 50.3 ± 3.2 ab | 340 | 1:0.99 | 0.914 | −0.012 ± 0.108 | 0.988 | 0.012 | 91.5 ± 2.1 | - | - |
| T1 | 43.5 ± 3.8 a | 340 | 1:1.29 | 0.017 | 0.260 ± 0.109 | 1.297 | 5.662 | 91.0 ± 4.1 | 0.0011 | −80.49 |
| T2 | 57.1 ± 4.5 b | 340 | 1:0.76 | 0.009 | −0.284 ± 0.110 | 0.753 | 6.731 | 89.5 ± 2.9 | 0.0029 | −50.00 |
| T3 | 55.0 ± 2.1 b | 340 | 1:0.82 | 0.066 | −0.201 ± 0.109 | 0.818 | 3.389 | 88.8 ± 3.9 | 0.0023 | −60.98 |
| T4 | 89.7 ± 3.6 c | 340 | 1:0.11 | 0.000 | −2.165 ± 0.178 | 0.115 | 147.160 | 88.5 ± 3.7 | 0.0059 | 0.00 |
| Control 2 | 45.9 ± 2.0 a | 340 | 1:1.19 | 0.129 | 0.165 ± 0.109 | 1.179 | 2.301 | 90.3 ± 3.7 | - | - |
| T5 | 78.5 ± 4.8 c | 340 | 1:0.27 | 0.000 | −1.297 ± 0.132 | 0.273 | 96.404 | 91.3 ± 2.1 | 0.0015 | −73.85 |
| T6 | 98.5 ± 1.4 d | 340 | 1:0.02 | 0.000 | −4.205 ± 0.451 | 0.015 | 87.097 | 92.0 ± 2.9 | 0.0039 | −32.98 |
| T7 | 50.6 ± 2.8 a | 340 | 1:0.98 | 0.828 | −0.024 ± 0.108 | 0.977 | 0.047 | 87.3 ± 4.4 | 0.0031 | −47.70 |
| T8 | 66.8 ± 1.7 b | 340 | 1:0.50 | 0.000 | −0.698 ± 0.115 | 0.498 | 36.711 | 85.5 ± 1.7 | 0.0079 | +34.03 |
| Treatments | Control | MT-ET (30 ppm) | MT-ET (60 ppm) | MT-APG-NLC (30 ppm) | MT-APG-NLC (60 ppm) |
|---|---|---|---|---|---|
| N | 50 | 50 | 50 | 50 | 50 |
| Lmin-max (cm) | 2.2–8.8 | 2.9–7.9 | 2.9–8.7 | 4.3–8.3 | 2.0–7.0 |
| Wmin-max (g) | 1–7.3 | 1.9–6.8 | 1.6–7.4 | 2.9–6.5 | 1.1–5.2 |
| a | −0.372 | −0.258 | −0.351 | −0.307 | −0.363 |
| b | 1.3423 | 1.196 | 1.286 | 1.225 | 1.289 |
| SE (b) | 0.031 | 0.038 | 0.028 | 0.018 | 0.020 |
| CI (b) | 1.280–1.405 | 1.119–1.273 | 1.230–1.341 | 1.189–1.260 | 1.249–1.330 |
| r2 | 0.975 | 0.953 | 0.978 | 0.990 | 0.988 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| t-test sig | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Growth behavior | Negative allometry | Negative allometry | Negative allometry | Negative allometry | Negative allometry |
| Kn | 1.003 | 1.002 | 1.001 | 1.000 | 1.001 |
| Min-Max | 0.818–1.445 | 0.830–1.148 | 0.876–1.102 | 0.967–1.067 | 0.926–1.072 |
| SE | 0.011 | 0.009 | 0.006 | 0.003 | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yostawonkul, J.; Kitiyodom, S.; Supchukun, K.; Thumrongsiri, N.; Saengkrit, N.; Pinpimai, K.; Hajitou, A.; Thompson, K.D.; Rattanapinyopituk, K.; Maita, M.; et al. Masculinization of Red Tilapia (Oreochromis spp.) Using 17α-Methyltestosterone-Loaded Alkyl Polyglucosides Integrated into Nanostructured Lipid Carriers. Animals 2023, 13, 1364. https://doi.org/10.3390/ani13081364
Yostawonkul J, Kitiyodom S, Supchukun K, Thumrongsiri N, Saengkrit N, Pinpimai K, Hajitou A, Thompson KD, Rattanapinyopituk K, Maita M, et al. Masculinization of Red Tilapia (Oreochromis spp.) Using 17α-Methyltestosterone-Loaded Alkyl Polyglucosides Integrated into Nanostructured Lipid Carriers. Animals. 2023; 13(8):1364. https://doi.org/10.3390/ani13081364
Chicago/Turabian StyleYostawonkul, Jakarwan, Sirikorn Kitiyodom, Kittipat Supchukun, Nutthanit Thumrongsiri, Nattika Saengkrit, Komkiew Pinpimai, Amin Hajitou, Kim D. Thompson, Kasem Rattanapinyopituk, Masashi Maita, and et al. 2023. "Masculinization of Red Tilapia (Oreochromis spp.) Using 17α-Methyltestosterone-Loaded Alkyl Polyglucosides Integrated into Nanostructured Lipid Carriers" Animals 13, no. 8: 1364. https://doi.org/10.3390/ani13081364
APA StyleYostawonkul, J., Kitiyodom, S., Supchukun, K., Thumrongsiri, N., Saengkrit, N., Pinpimai, K., Hajitou, A., Thompson, K. D., Rattanapinyopituk, K., Maita, M., Kamble, M. T., Yata, T., & Pirarat, N. (2023). Masculinization of Red Tilapia (Oreochromis spp.) Using 17α-Methyltestosterone-Loaded Alkyl Polyglucosides Integrated into Nanostructured Lipid Carriers. Animals, 13(8), 1364. https://doi.org/10.3390/ani13081364