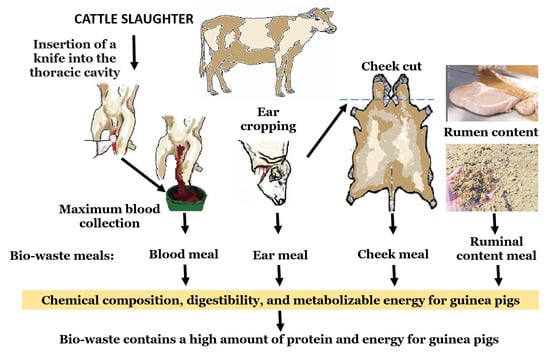
The Nutritional Value of Biowaste Bovine Slaughterhouse Meals for Monogastric Species Feeding: The Guinea Pig as an Animal Model
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Ethics Statement
2.2. Study Location
2.3. Experimental Animals
2.4. Process of Obtaining Bovine Slaughterhouse Biowaste Meals
2.5. Laboratory Analysis
2.6. Digestibility and Voluntary Consumption Trials of Biowaste Meals
- T0: RD diet: 100% barley meal;
- T1: RCM diet: 80% RD + 20% RCM;
- T2: EaM diet: 80% RD + 20% EaM;
- T3: BM diet: 80% RD + 20% BM;
- T4: CM diet: 80% RD + 20% CM.
2.7. Digestibility Coefficient Calculations and Total Digestible Nutrients
2.8. Statistical Analysis
3. Results
3.1. Chemical Composition of Biowaste Meals from Cattle Slaughter
3.2. Apparent Digestibility Coefficients of Slaughter Biowaste Meals
3.3. Digestible Components of Slaughter Biowaste Meals and Energy Contribution
3.4. Live Weights of Experimental Animals
3.5. Voluntary Consumption of Bovine Slaughter Biowaste Meals
4. Discussion
4.1. Approximate Composition of Bovine Slaughter Biowaste Meals
4.2. Apparent Digestibility of Bovine Slaughter Biowaste Meals
4.3. Total Digestible Nutrients of Bovine Slaughter Biowaste Meals
4.4. Live Weight and Weight Gain per Guinea Pig per Treatment
4.5. Voluntary Consumption of Biowaste Meals from Bovine Slaughter
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mustapa, M.A.; Kallas, Z. Towards more sustainable animal-feed alternatives: A survey on Spanish consumers’ willingness to consume animal products fed with insects. Sustain. Prod. Consum. 2023, 41, 9–20. [Google Scholar] [CrossRef]
- Lammers, P.J.; Carlson, S.L.; Zdorkowski, G.A.; Honeyman, M.S. Reducing food insecurity in developing countries through meat production: The potential of the guinea pig (Cavia porcellus). Renew. Agric. Food Syst. 2009, 24, 155–162. [Google Scholar] [CrossRef]
- Cawthorn, D.M.; Hoffman, L.C. Controversial cuisine: A global account of the demand, supply and acceptance of “unconventional” and “exotic” meats. Meat Sci. 2016, 120, 19–36. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Macías, D.; Barba-Maggi, L.; Morales-delaNuez, M.; Palmay-Paredes, J. Guinea pig for meat production: A systematic review of factors affecting the production, carcass and meat quality. Meat Sci. 2018, 143, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Dalle Zotte, A.; Cullere, M. Carcass Traits and Meat Quality of Rabbit, Hare, Guinea Pig and Capybara. In More than Beef, Pork and Chicken—The Production, Processing, and Quality Traits of Other Sources of Meat for Human Diet; Springer International Publishing: Cham, Switzerland, 2019; pp. 167–210. [Google Scholar] [CrossRef]
- Ngoula, F.; Guemdjo Tekam, M.; Kenfack, A.; Tadondjou Tchingo, C.; Nouboudem, S.; Ngoumtsop, H.; Tsafack, B.; Teguia, A.; Kamtchouing, P.; Galeotti, M.; et al. Effects of heat stress on some reproductive parameters of male cavie (Cavia porcellus) and mitigation strategies using guava (Psidium guajava) leaves essential oil. J. Ther. Biol. 2017, 64, 67–72. [Google Scholar] [CrossRef] [PubMed]
- MINAGRI. Potencial del Mercado Internacional para la Carne de Cuy 2019. Agencia Peruana de Noticias Andina, viewed 22 December 2021. 2019. Available online: https://cdn.www.gob.pe/uploads/document/file/419810/potencial-mercado-intern-carne-cuy.pdf (accessed on 12 November 2023).
- Camino, M.; Hidalgo, V. Evaluación de dos genotipos de cuyes (Cavia porcellus) alimentados con concentrado y exclusión de forraje verde. Rev. Investig. Vet. Perú 2014, 25, 190–197. [Google Scholar] [CrossRef]
- Sarria, B.J.; Vergara, R.V.; Cantaro, S.J.; Rojas, P.A. Assessment of digestible energy levels in two feeding systems in the productive and reproduction performance of guinea pigs (Cavia porcellus). RIVEP 2019, 30, 1515–1526. [Google Scholar] [CrossRef]
- Rothacher, M.; Hatt, J.M.; Clauss, M. A comparison of commercially available feeds for rabbits, guinea pigs, chinchillas and degus with evidence of their diet and feeding behaviour in natural habitats. Schweiz Arch. Tierheilkd. 2023, 165, 726–736. [Google Scholar] [CrossRef]
- Mozhiarasi, V.; Natarajan, T.S. Slaughterhouse and poultry wastes: Management practices, feedstocks for renewable energy production, and recovery of value-added products. Biomass Convers. Biorefin. 2022, 1–24. [Google Scholar] [CrossRef]
- Toldrá, F.; Reig, M.; Mora, L. Management of meat by- and co-products for an improved meat processing sustainability. Meat Sci. 2021, 181, 108608. [Google Scholar] [CrossRef]
- Alonge, D.O. Textbook of Meat and Milk Hygiene; Farmcoe Press: Ibadan, Nigeria, 2005; pp. 77–86. [Google Scholar]
- Adhikari, B.B.; Chae, M.; Bressler, D.C. Utilization of slaughterhouse waste in value-added applications: Recent advances in the development of wood adhesives. Polymers 2018, 10, 176. [Google Scholar] [CrossRef] [PubMed]
- Meeker, D.L. North American rendering-processing high quality protein and fats for feed. R. Bras. Zootech. 2009, 38, 432–440. [Google Scholar] [CrossRef]
- Mmereki, D.; Li, B.; Meng, L. Hazardous and toxic waste management in Botswana: Practices and challenges. Waste Manag. Res. 2014, 32, 1158–1168. [Google Scholar] [CrossRef]
- Tsai, F.M.; Bui, T.D.; Tseng, M.L.; Wu, K.J. A causal municipal solid waste management model for sustainable cities in Vietnam under uncertainty: A comparison. Resour. Conserv. Recycl. 2020, 154, 104599. [Google Scholar] [CrossRef]
- Varjani, S.; Upasani, V.N. Bioaugmentation of Pseudomonas aeruginosa NCIM 5514—A novel oily waste degrader for treatment of petroleum hydrocarbons. Bioresour. Technol. 2021, 319, 124240. [Google Scholar] [CrossRef]
- Salas, G.; Condorhuamán, C. Tratamiento de las aguas residuales de un centro de beneficio o matadero de ganado. Rev. Peru. Quím. Ing. Quím. 2008, 11, 29–35. Available online: https://revistasinvestigacion.unmsm.edu.pe/index.php/quim/article/view/4885 (accessed on 13 October 2023).
- Rahman, U.U.; Sahar, A.; Khan, M.A. Recovery and utilization of effluents from meat processing industries. Food Res. Int. 2014, 65, 322–328. [Google Scholar] [CrossRef]
- Agbabiaka, L.A.; Anukam, K.U.; Nwachukwu, U.N. Nutritive value of dried rumen digesta as replacement for soybean in diets of Nile Tilapia (Oreochromis niloticus) fingerlings. Pak. J. Nutr. 2011, 10, 568–571. [Google Scholar] [CrossRef]
- Castro-Bedriñana, J.; Chirinos-Peinado, D.; Lizárraga-Velásquez, F.; Ríos-Ríos, E.; Quispe-Ramos, R. Nutritional quality of bovine cheeks meal from tannery industry as a protein source for monogastric animals. Res. Square 2022. [Google Scholar] [CrossRef]
- Onu, P.N.; Otuma, M.O.; Odukwe, C.A.; Aniebo, A.O. Effects of different levels of bovine blood/rumen content mixture on productive performance, carcass characteristics and economics of production of finisher broilers. Int. J. Food Agric. Vet. Sci. 2011, 1, 10–16. Available online: http://www.cibtech.org/jfav.htm (accessed on 15 May 2023).
- Adeniji, A.A.; Jimoh, A. Effects of replacing maize with enzyme- supplemented bovine rumen content in the diets of pullet chicks. Int. J. Poult. Sci. 2007, 6, 814–817. [Google Scholar] [CrossRef]
- Togun, V.A.; Farinu, G.O.; Ojebiyi, O.O.; Awotunde, A.I. Effect of replacing maize with a mixture of rumen content and blood meal on the performance of growing rabbits: Initial study with mash feed. World Rabbit. Sci. 2009, 17, 21–26. [Google Scholar] [CrossRef]
- Castro-Bedriñana, J.; Chirinos-Peinado, D. Nutritional value of some raw materials for guinea pigs (Cavia porcellus) feeding. Transl. Anim. Sci. 2021, 5, txab019. [Google Scholar] [CrossRef] [PubMed]
- Castro-Bedriñana, J.; Chirinos-Peinado, D.; Quijada-Caro, E. Digestible and metabolizable energy prediction models in guinea pig feedstuffs. J. Appl. Anim. Res. 2022, 50, 355–362. [Google Scholar] [CrossRef]
- Uicab-Brito, L.A.; Sandoval-Castro, C.A. Uso del contenido Ruminal y algunos residuos de la industria cárnica en la elaboración de composta. Trop. Subtrop. Agroecosyst. 2003, 2, 45–63. Available online: http://redalyc.uaemex.mx/src/inicio/ArtPdfRed.jsp?iCve=93912118001 (accessed on 15 May 2023).
- ARRIVE. The ARRIVE Guidelines (Animal Research: Reporting of In Vivo Experiments). 2022. Available online: https://arriveguidelines.org/ (accessed on 15 May 2023).
- Arnfield, A.J.; Köppen Climate Classification. Encyclopedia Britannica 2020. Available online: https://www.britannica.com/science/Koppen-climate-classification (accessed on 15 May 2023).
- Schofield, J.; Noonan, D.; Chen, Y.; Penson, P. Chapter 12—Laboratory Animals Regulations and Recommendations for Global Collaborative Research: Australia and New Zealand. In Laboratory Animals; Guillén, J., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 333–376. [Google Scholar] [CrossRef]
- AOAC International. Official Method of Analysis of AOAC International, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2007. [Google Scholar]
- Kong, C.; Adeola, O. Evaluation of amino acid and energy utilization in feedstuff for swine and poultry diets. Asian-Aust. J. Anim. Sci. 2014, 27, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Kassa, A. The Different Methods of Measuring Feed Digestibility: A Review. EC Nutr. 2019, 14, 68–74. Available online: https://www.ecronicon.com/ecnu/pdf/ECNU-14-00542.pdf (accessed on 17 September 2023).
- Castro-Bedriñana, J.; Chirinos-Peinado, D.; Sosa-Blas, H. Digestibility, digestible and metabolizable energy of earthworm meal (Eisenia foetida) included in two levels in guinea pigs (Cavia porcellus). Adv. Sci. Technol. Eng. Syst. J. 2020, 5, 171–177. [Google Scholar] [CrossRef]
- McDonald, I.W. Nutritional aspects of protein metabolism in ruminants. Aust. Vet. J. 1968, 44, 145. [Google Scholar] [CrossRef]
- McDonald, P.; Edwards, R.A.; Greenhalgh, J.F.D.; Morgan, C.A.; Sinclair, L.A.; Wilkinson, R.G. Animal Nutrition, 7th ed.; PEARSON: London, UK, 2010; p. 692. [Google Scholar]
- Church, D.C.; Pond, W.G.; Pond, K.R. Fundamentos de Nutrición y Alimentación de Animales, 2nd ed.; Limusa SA: Ciudad de Mexico, Mexico, 2018; p. 636. ISBN 978-968-18-5299-3. [Google Scholar]
- Weiss, W.; Tebbe, A. Estimating digestible energy values of feeds and diets and integrating those values into net energy systems. Transl. Anim. Sci. 2019, 3, 953–961. [Google Scholar] [CrossRef]
- Hales, K.E. Relationships between digestible energy and metabolizable energy in current feedlot diets. Transl. Anim. Sci. 2019, 3, 945–952. [Google Scholar] [CrossRef]
- Mishra, J.; Abraham, R.J.; Rao, V.A.; Asha Rajini, R.; Mishra, B.P.; Sarangi, N.R. Chemical composition of solar dried blood and the ruminal content and its effect on performance of Japanese quails. Vet. World 2015, 8, 82–87. [Google Scholar] [CrossRef]
- Esonu, B.O.; Azubuike, J.C.; Udedibie, A.B.I.; Emenalom, O.O.; Iwuji, T.C.; Odoemenam, V. Evaluation of the nutritive value of mixture of fermented bovine blood and rumen digesta for broiler finisher. J. Nat. Sci. Res. 2011, 1, 2224–3186. Available online: https://api.semanticscholar.org/CorpusID:56322242 (accessed on 18 October 2023).
- Okpanachi, U.; Aribido, S.O.; Daikwo, I.S. Growth and haematological response of growing rabbits to diets containing graded levels of sun-dried bovine rumen content. Afr. J. Food Agric. Nutr. Dev. 2010, 10, 4444–4457. [Google Scholar] [CrossRef]
- Makinde, O.; Sonaiya, B.; Adeyeye, S. Conversion of abattoir wastes into livestock feed: Chemical composition of sun-dried rumen content blood meal and its effect on performance of broiler chickens. Conference on international research on food security, natural meal. Int. J. Poult. Sci. 2008, 6, 875–882. [Google Scholar]
- Núñez-Torres, O.P.; Aragadvay-Yungan, R.G.; Guerrero-López, J.R.; Villacís-Aldaz, L.A. Comportamiento productivo en cuyes (Cavia porcellus) utilizando contenidos ruminales. J. Selva And. Anim. Sci. 2016, 3, 87–97. Available online: http://www.scielo.org.bo/scielo.php?script=sci_arttext&pid=S2311-25812016000200003&lng=es&tlng=es (accessed on 18 October 2023). [CrossRef]
- Bracho, H. Valoración del Contenido Ruminal de Bovinos Beneficiados en el Municipio Piritu Estado Falcon-Venezuela, Como Recurso Alimenticio. 2017. Available online: https://www.engormix.com/ganaderia-leche/articulos/valoracion-contenido-ruminal-bovinos-t40610.htm (accessed on 15 May 2023).
- Capelo, M. Efecto en los Parámetros Productivos e Indicadores Organolépticos de la Inclusión del Contenido Ruminal Deshidratado en el Balanceado de Pollos. Doctoral Dissertation, Universidad de Machala, Machala, Ecuador, 2018. Available online: http://repositorio.utmachala.edu.ec (accessed on 15 May 2023).
- Chinachi, L. Evaluación de Tres Niveles de Contenido Ruminal en Alimentación de Cuyes en la Etapa de Engorde”. Doctoral Dissertation, Universidad Técnica de Ambato, Ambato, Ecuador, 2014. Available online: https://repositorio.uta.edu.ec (accessed on 15 May 2023).
- Laines Canepa, J.R.; Sosa Olivie, J.A.; Hernández Hernandez, L.; Sandoval Arreola, M.M. Aprovechamiento del Contenido Gástrico Ruminal Vacuno Generado en un Rastro Municipal Pequeño; Aidisnet: São Paulo, Brazil, 2006; Available online: https://aidisnet.org/wp-content/uploads/2019/07/224-Mexico-oral.pdf (accessed on 15 May 2023).
- Fisgativa, H.; Marcilhac, C.; Girault, R.; Daumer, M.L.; Trémier, A.; Dabert, P.; Béline, F. Physico-chemical, biochemical, and nutritional characterization of 42 organic wastes and residues from France. Data Brief 2018, 19, 1953–1962. [Google Scholar] [CrossRef]
- Zamora, S.; Callacná, M. Parámetros productivos de cuyes (Cavia porcellus) suplementados con harina de sangre bovina. Rev. RICBA 2017, 1, 47–52. [Google Scholar] [CrossRef]
- Cuenca, C.; Álvarez, C.; Ortiz-Galindo, J.; Guerrero-Zárate, R.; Perera-García, M.; Hernández-Gómez, R.; Nolasco-Soria, H. Digestibilidad in vitro de ingredientes proteínicos en la mojarra castarrica Cichlasoma urophthalmus. Univ. Cienc. Tróp. Húmed. 2013, 29, 263–275. Available online: https://www.scielo.org.mx/pdf/uc/v29n3/v29n3a5.pdf (accessed on 18 October 2023).
- Muñoz, A.; Segovia, E.; Futagawa, M.; Marchant, C.; Flores, F. Coeficientes de digestibilidad total y de proteínas en alimentos experimentales para juveniles de Oplegnathus insignis (Kner, 1867) (Perciformes, Oplegnathidae). Lat. Am. J. Aquat. Res. 2015, 43, 304–308. [Google Scholar] [CrossRef]
- Villarreal-Cavazos, D.; Ricque-Marie, D.; Peña-Rodríguez, A.; Nieto-López, M.; Tapia-Salazar, M.; Lemme, A.; Gamboa-Delgado, J.; Cruz-Suárez, L. Apparent digestibility of dry matter, crude protein, and amino acids of six rendered by-products in juvenile Litopenaeus vannamei. Cienc. Mar. 2014, 40, 163–172. [Google Scholar] [CrossRef]
- Vásquez-Torres, W.; Yossa, M.I.; Gutiérrez-Espinosa, M.C. Digestibilidad aparente de ingredientes de origen vegetal y animal en la cachama. Pesqui. Agropecu. Bras. 2013, 48, 920–927. [Google Scholar] [CrossRef]
- Wilson, J.M.; Castro, L.F.C. Morphological diversity of the gastrointestinal tract in fishes. In Fish Physiology; Grosell, M., Farrell, A.P., Brauner, C.J., Eds.; Academic Press: Cambridge, MA, USA, 2010; Volume 30, pp. 1–55. ISSN 1546-5098. ISBN 9780123749826. [Google Scholar] [CrossRef]
- German, D.P. Do herbivorous minnows have “plug-flow reactor” guts? Evidence from digestive enzyme activities, gastrointestinal fermentation, and luminal nutrient concentrations. J. Comp. Physiol. 2009, 179, 759–771. [Google Scholar] [CrossRef]
- Castrillo, C.; Vicente, F.; Guada, J.A. The effect of crude fiber on apparent digestibility and digestible energy content of extruded dog foods. J. Anim. Physiol. Anim. Nutr. 2001, 85, 231–236. [Google Scholar] [CrossRef]
- Rosero, P.M.; Barreros, C.A. Evaluación de Tres Niveles de Proteína de Harina de Sangre Como Dieta Suplementaria en la Etapa de Crecimiento-Engorde en Cuyes (Cavia porcellus) de la Granja Producuy. Bachelor’s Thesis, Universidad Técnica de Ambato, Ambato, Ecuador, 2017. Available online: https://repositorio.uta.edu.ec/jspui/handle/123456789/26401 (accessed on 25 June 2023).
- Bindelle, J.; Ilunga, Y.; Delacollette, M.; Muland Kayij, M.; Umba di M’Balu, J.; Kindele, E.; Buldgen, A. Voluntary intake, chemical composition and in vitro digestibility of fresh forages fed to Guinea pigs in periurban rearing systems of Kinshasa (Democratic Republic of Congo). Trop. Anim. Health Prod. 2007, 39, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.M. Voluntary Food Intake and Diet Selection in Farm Animals; CABI Publishing: Wallingford, UK, 2017; p. 453. ISBN 978-1-84593-279-4. [Google Scholar]
- Paredes, A.; Goicochea, M.; Emilio, P. Efecto de cinco dietas con diferentes proporciones de fibra detergente neutro y almidón en el rendimiento productivo, comportamiento ingestivo y peso de órganos digestivos del cuy (Cavia porcellus). RIVEP 2021, 32, e19495. [Google Scholar] [CrossRef]

| Biowaste | Collection | Reception | Pretreatment | Processing | Storage and Packaging |
|---|---|---|---|---|---|
| Ruminal content meal (RCM) | After slaughter and removal of the rumen reticulum from the digestive tract | Polyethylene buckets Drained on a 0.1 mm sieve | Pre-drying under shade (3 days), with dough movements 2 times/day | Drying by forced convection (Binder: FED20) 80 °C/4 h. Grinding (2 mm screen) | Polyethylene bags |
| Ear meal (EaM) | Cutting, washing, and rinsing the ears | Hair removal. Immerse in water at 70 °C/2 min. Peeling with blade | Cooking 90 °C/10 min. Chopped (2 cm2) | Drying in a forced convection oven at 80 °C/8 h. Grinding (2 mm screen) | Polyethylene bags |
| Blood meal (BM) | During the benefit after the jugular cut | Polyethylene buckets | Coagulation by cooking in a steel container (90 °C/10 min) with constant stirring and cutting (2 cm2) | Drying in a forced convection oven at 80 °C/8 h. Grinding (2 mm screen) | Polyethylene bags |
| Cheek meal (CM) | Cutting, lacquering, and rinsing cheeks | Hair removal. Immerse in water at 70 °C/2 min. | Cooking 90 °C/10 min | Cut into pieces (2 cm2) and dried in a forced convection oven at 80 °C/8 h. Grinding (2 mm screen) | Polyethylene bags |
| Reference Diet (RD) | RD + 80% RCM | RD + 80% EaM | RD + 80% BM | RD + 80% CM | |
|---|---|---|---|---|---|
| T0 | 100 | 80 | 80 | 80 | 80 |
| T1 | 0 | 20 | 0 | 0 | 0 |
| T2 | 0 | 0 | 20 | 0 | 0 |
| T3 | 0 | 0 | 0 | 20 | 0 |
| T4 | 0 | 0 | 0 | 0 | 20 |
| Total, % | 100 | 100 | 100 | 100 | 100 |
| DM, % | 91 | 89.80 | 90.58 | 91.20 | 91.15 |
| CP, % | 12 | 12.60 | 22.13 | 24.08 | 26.44 |
| EE, % | 4 | 4.30 | 7.68 | 3.30 | 4.58 |
| CF, % | 5.8 | 12.24 | 4.64 | 4.64 | 4.64 |
| ME, kcal/kg | 2884 | 2877.80 | 2972.20 | 2823.80 | 2984.40 |
| Biowaste Meals | DM (%) | CP (%) | EE (%) | CF (%) | NFE (%) | Ash (%) |
|---|---|---|---|---|---|---|
| RCM | 85.00 ± 0.33 | 15.00 ± 0.30 | 5.50 ± 0.30 | 38.00 ± 1.37 | 30.50 ± 1.84 | 11.00 ± 1.07 |
| EaM | 88.91 ± 1.32 | 62.64 ± 0.61 | 22.4 ± 0.52 | † | † | 14.86 ± 0.13 |
| BM | 92.00 ± 0.30 | 72.40 ± 1.19 | 0.50 ± 0.06 | † | † | 4.00 ± 0.16 |
| CM | 91.76 ± 0.02 | 84.19 ± 0.02 | 6.91 ± 0.03 | † | † | 8.90 ± 0.01 |
| Biowaste Meals | DM (%) | CP (%) | EE (%) | CF (%) | NFE (%) |
|---|---|---|---|---|---|
| RCM | 83.05 ± 5.51 a | 67.65 ± 4.69 b | 82.76 ± 11.35 b | 91.52 ± 4.73 | 77.91 ± 9.21 |
| EaM | 88.28 ± 2.38 a | 71.10 ± 7.95 b | 94.11 ± 3.02 a | - | - |
| BM | 88.53 ± 3.20 a | 97.63 ± 0.74 a | 65.25 ± 20.77 c | - | - |
| CM | 76.34 ± 1.53 b | 96.03 ± 0.39 a | 82.37 ± 1.28 b | - | - |
| Biowaste Meals | MS (%) | PB (%) | EE (%) | CF (%) | ELN (%) | NDT (%) | ED (Kcal/kg) | ME (Kcal/kg) |
|---|---|---|---|---|---|---|---|---|
| RCM | 70.60 ± 4.69 | 10.15 ± 0.70 d | 4.55 ± 0.62 | 34.78 ± 1.80 | 23.76 ± 2.81 | 78.93 ± 5.36 | 3480 ± 236 | 2853 ± 194 b |
| EaM | 78.49 ± 2.12 | 44.54 ± 4.98 c | 21.08 ± 0.68 | - | - | 91.97 ± 6.02 | 4055 ± 265 | 3325 ± 217 a |
| BM | 81.45 ± 2.95 | 70.68 ± 0.54 b | 0.35 ± 0.09 | - | - | 71.46 ± 0.56 | 3151 ± 24.8 | 2583 ± 20 c |
| CM | 70.05 ± 1.40 | 80.84 ± 0.33 a | 5.69 ± 0.09 | - | - | 93.65 ± 0.53 | 4129 ± 23.26 | 3386 ± 19 a |
| Parameter | RCM | EaM | BM | CM |
|---|---|---|---|---|
| Initial weight means, g | 807.78 ± 68.75 a | 834.44 ± 53.35 a | 840.33 ± 67.17 a | 806.11 ± 58.28 a |
| Final weight means, g | 836.33 ± 65.82 a | 878.11 ± 53.17 a | 888.44 ± 63.6 a | 855.22 ± 55.51 a |
| Total weight gain means, g | 28.56 ± 5.48 b | 43.67 ± 8.14 a | 48.11 ± 8.93 a | 49.11 ± 7.22 a |
| Daily weight gain means, g | 4.08 ± 0.78 b | 6.24 ± 1.16 a | 6.87 ± 1.27 a | 7.02 ± 1.03 a |
| Biowaste Meals * | Voluntary Dry Matter Consumption | |
|---|---|---|
| Live Weight Percentage | g/Kg W0.75 | |
| RCM | 0.65 ± 0.23 b | 12.41 ± 2.03 b |
| EaM | 0.45 ± 0.17 b | 10.15 ± 1.76 b |
| BM | 0.58 ± 0.22 b | 11.55 ± 2.19 b |
| CM | 0.79 ± 0.07 a | 39.97 ± 2.75 a |
| Rumen Content Meal: | Humidity | Crude Protein | Crude Fiber | Ethereal Extract | Ash | NFE | |
|---|---|---|---|---|---|---|---|
| Our study | Ruminal content | 15.00 | 15.00 | 38.00 | 5.50 | 11.00 | 30.50 |
| [41] 2015 | Ruminal content | 7.36 | 18.26 | 24.99 | 3.60 | 14.47 | ud |
| [42] 2011 | Fermented bovine blood and rumen digesta | 7.20 | 29.86 | 21.90 | 23.50 | 7.40 | 12.14 |
| [43] 2010 | Sun-dried ruminal content | 7.95 | 13.56 | 31.90 | 0.75 | 16.20 | 25.70 |
| [44] 2008 | Sun-dried ruminal content | 71.15 | 12.85 | 9.45 | 3.35 | 8.36 | 37.14 |
| [45] 2016 | Ruminal content | 87.40 | 9.00 | 34.1 | 0.098 | 13.70 | 30.50 |
| [46] 2017 | Ruminal content | 8.80 | 6.77 | 21.99 | 0.00 | 17.11 | ud |
| [47] 2018 | Ruminal content | 12.00 | 13.00 | 27.00 | 2.00 | ud | ud |
| [48] 2014 | Ruminal content | 12.60 | 9.00 | 27.00 | 0.10 | 13.70 | 30.50 |
| [49] 2006 | Fresh ruminal content | 87.85 | ud | ud | ud | 14.40 | ud |
| Blood meal: | |||||||
| Our study | Blood meal | 8.00 | 72.40 | 0.00 | 0.50 | 4.00 | 0.00 |
| [50] 2018 | Blood meal | 92.50 | 0.00 | 7.50 | 0.00 | ||
| [41] 2015 | Blood meal | 10.13 | 84.87 | 0.38 | 0.52 | 4.66 | |
| [51] 2017 | Blood meal | 75.76 | 1.03 | 4.87 | |||
| [51] 2017 | Blood meal | 72.14 | 1.96 | 7.38 | |||
| [52] 2013 | Blood meal | 4.40 | 72.00 | <1.00 | |||
| [26] 2021 | Blood meal | 11.85 | 92.25 | 0.00 | 0.56 | 7.19 | |
| Ear meal: | |||||||
| Our study | Ear meal | 11.09 | 62.64 | 0.00 | 22.40 | 14.86 | 0.00 |
| There are no reports | |||||||
| Cheek meal: | |||||||
| Our study | Cheek meal | 8.24 | 84.19 | 0.00 | 6.91 | 8.90 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chirinos-Peinado, D.; Castro-Bedriñana, J.; Álvaro-Ordoñez, P.; Quispe-Ramos, R.; García-Olarte, E.; Ríos-Ríos, E. The Nutritional Value of Biowaste Bovine Slaughterhouse Meals for Monogastric Species Feeding: The Guinea Pig as an Animal Model. Animals 2024, 14, 1129. https://doi.org/10.3390/ani14071129
Chirinos-Peinado D, Castro-Bedriñana J, Álvaro-Ordoñez P, Quispe-Ramos R, García-Olarte E, Ríos-Ríos E. The Nutritional Value of Biowaste Bovine Slaughterhouse Meals for Monogastric Species Feeding: The Guinea Pig as an Animal Model. Animals. 2024; 14(7):1129. https://doi.org/10.3390/ani14071129
Chicago/Turabian StyleChirinos-Peinado, Doris, Jorge Castro-Bedriñana, Patricia Álvaro-Ordoñez, Rolando Quispe-Ramos, Edgar García-Olarte, and Elva Ríos-Ríos. 2024. "The Nutritional Value of Biowaste Bovine Slaughterhouse Meals for Monogastric Species Feeding: The Guinea Pig as an Animal Model" Animals 14, no. 7: 1129. https://doi.org/10.3390/ani14071129
APA StyleChirinos-Peinado, D., Castro-Bedriñana, J., Álvaro-Ordoñez, P., Quispe-Ramos, R., García-Olarte, E., & Ríos-Ríos, E. (2024). The Nutritional Value of Biowaste Bovine Slaughterhouse Meals for Monogastric Species Feeding: The Guinea Pig as an Animal Model. Animals, 14(7), 1129. https://doi.org/10.3390/ani14071129