Revealing the True Morphological Structure of Macroporous Soft Hydrogels for Tissue Engineering
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Porogen Particles
2.3. Preparation of Hydrogels
2.4. Methacryloylation of Fluorescein
2.5. Scanning Electron Microscopy (SEM)
2.6. Laser Scanning Confocal Microscopy (LSCM)
2.7. Three-Dimensional Computer Reconstructions
2.8. Swelling of Hydrogels
2.9. Gravimetric Determination of Pore Volume in Swollen Gel
2.10. Cell Growth on Hydrogel Scaffolds
3. Results and Discussion
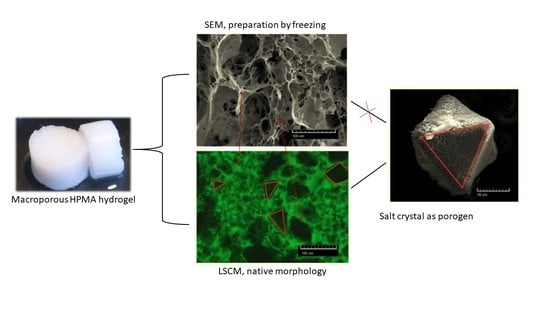
3.1. Porous pHPMA Hydrogel Preparation and Morphology
3.2. Salt Porogen Features
3.3. Pore Distribution Analysis
3.4. Swelling of Macroporous Hydrogels
3.5. 3D Reconstruction of the Swollen Hydrogel Volume
3.6. Cell Cultivation on Hydrogels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C. 2015. [Google Scholar] [CrossRef] [PubMed]
- Šprincl, L.; Vacík, J.; Kopeček, J.; Lím, D. Biological tolerance of poly(N-substituted methacrylamides). J. Biomed. Mater. Res. 1971. [Google Scholar] [CrossRef] [PubMed]
- Rape, A.D.; Guo, W.H.; Wang, Y.L. Responses of cells to adhesion-mediated signals: A universal mechanism, in: Mechanobiol. Cell-Cell Cell-Matrix Interact. 2011. [Google Scholar] [CrossRef]
- Trimaille, T.; Pertici, V.; Gigmes, D. Hydrogels à base de polymères synthétiques pour la réparation médullaire. Comptes Rendus Chim. 2016. [Google Scholar] [CrossRef]
- Silva, N.A.; Sousa, N.; Reis, R.L.; Salgado, A.J. From basics to clinical: A comprehensive review on spinal cord injury. Prog. Neurobiol. 2014. [Google Scholar] [CrossRef]
- Lien, S.M.; Ko, L.Y.; Huang, T.J. Effect of pore size on ECM secretion and cell growth in gelatin scaffold for articular cartilage tissue engineering. Acta Biomater. 2009. [Google Scholar] [CrossRef]
- Brauker, J.H.; Carr-Brendel, V.E.; Martinson, L.A.; Crudele, J.; Johnston, W.D.; Johnson, R.C. Neovascularization of synthetic membranes directed by membrane microarchitecture. J. Biomed. Mater. Res. 1995. [Google Scholar] [CrossRef]
- Klawitter, J.J.; Hulbert, S.F. Application of porous ceramics for the attachment of load bearing internal orthopedic applications. J. Biomed. Mater. Res. 1971. [Google Scholar] [CrossRef]
- Yang, S.; Leong, K.F.; Du, Z.; Chua, C.K. The design of scaffolds for use in tissue engineering. Tissue Eng. 2001. [Google Scholar] [CrossRef] [Green Version]
- Whang, K.; Healy, K.E.; Elenz, D.R.; Nam, E.K.; Tsai, D.C.; Thomas, C.H.; Nuber, G.W.; Glorieux, F.H.; Travers, R.; Sprague, S.M. Engineering bone regeneration with bioabsorbable scaffolds with novel microarchitecture. Tissue Eng. 1999. [Google Scholar] [CrossRef] [PubMed]
- Přádný, M.; Lesný, P.; Fiala, J.; Vacík, J.; Šlouf, M.; Michálek, J.; Syková, E. Macroporous hydrogels based on 2-hydroxyethyl methacrylate. Part 1. Copolymers of 2-hydroxyethyl methacrylate with methacrylic acid. Collect. Czechoslov. Chem. Commun. 2003. [Google Scholar] [CrossRef] [Green Version]
- Macková, H.; Plichta, Z.; Hlídková, H.; Sedláček, O.; Konefal, R.; Sadakbayeva, Z.; Dušková-Smrčková, M.; Horák, D.; Kubinová, Š. Reductively Degradable Poly(2-hydroxyethyl methacrylate) Hydrogels with Oriented Porosity for Tissue Engineering Applications. ACS Appl. Mater. Interfaces. 2017. [Google Scholar] [CrossRef] [PubMed]
- Texter, J. Templating hydrogels. Colloid Polym. Sci. 2009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Annabi, N.; Nichol, J.W.; Zhong, X.; Ji, C.; Koshy, S.; Khademhosseini, A.; Dehghani, F. Controlling the porosity and microarchitecture of hydrogels for tissue engineering. Tissue Eng. Part B Rev. 2010. [Google Scholar] [CrossRef] [PubMed]
- Lozinsky, V. Cryostructuring of Polymeric Systems. 50.† Cryogels and Cryotropic Gel-Formation: Terms and Definitions. Gels 2018, 4, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okay, O.; Lozinsky, V.I. Synthesis and structure–property relationships of cryogels. Adv. Polym. Sci. 2014. [Google Scholar] [CrossRef]
- de France, K.J.; Xu, F.; Hoare, T. Structured Macroporous Hydrogels: Progress, Challenges, and Opportunities. Adv. Healthc. Mater. 2018. [Google Scholar] [CrossRef]
- Macková, H.; Plichta, Z.; Proks, V.; Kotelnikov, I.; Kučka, J.; Hlídková, H.; Horák, D.; Kubinová, Š.; Jiráková, K. RGDS- and SIKVAVS-Modified Superporous Poly(2-hydroxyethyl methacrylate) Scaffolds for Tissue Engineering Applications. Macromol. Biosci. 2016. [Google Scholar] [CrossRef]
- Liu, X.; Smith, L.A.; Hu, J.; Ma, P.X. Biomimetic nanofibrous gelatin/apatite composite scaffolds for bone tissue engineering. Biomaterials 2009. [Google Scholar] [CrossRef] [Green Version]
- Baule, A.; Makse, H.A. Fundamental challenges in packing problems: From spherical to non-spherical particles. Soft Matter. 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, W.; Zhu, Z.; Zhang, D. Continuum percolation-based tortuosity and thermal conductivity of soft superball systems: Shape dependence from octahedra: Via spheres to cubes. Soft Matter. 2018. [Google Scholar] [CrossRef]
- Liu, S.; Jin, M.; Chen, Y.; Gao, H.; Shi, X.; Cheng, W.; Ren, L.; Wang, Y. High internal phase emulsions stabilised by supramolecular cellulose nanocrystals and their application as cell-adhesive macroporous hydrogel monoliths. J. Mater. Chem. B. 2017. [Google Scholar] [CrossRef]
- Coukouma, A.E.; Asher, S.A. Increased volume responsiveness of macroporous hydrogels. Sens. Actuators B Chem. 2018. [Google Scholar] [CrossRef]
- Aston, R.; Sewell, K.; Klein, T.; Lawrie, G.; Grøndahl, L. Evaluation of the impact of freezing preparation techniques on the characterisation of alginate hydrogels by cryo-SEM. Eur. Polym. J. 2016. [Google Scholar] [CrossRef] [Green Version]
- Paterson, S.M.; Casadio, Y.S.; Brown, D.H.; Shaw, J.A.; Chirila, T.V.; Baker, M.V. Laser scanning confocal microscopy versus scanning electron microscopy for characterization of polymer morphology: Sample preparation drastically distorts morphologies of poly(2-hydroxyethyl methacrylate)-based hydrogels. J. Appl. Polym. Sci. 2013. [Google Scholar] [CrossRef]
- Spiller, K.L.; Laurencin, S.J.; Charlton, D.; Maher, S.A.; Lowman, A.M. Superporous hydrogels for cartilage repair: Evaluation of the morphological and mechanical properties. Acta Biomater. 2008. [Google Scholar] [CrossRef]
- Parmenter, C.D.J.; Fay, M.W.; Hartfield, C.; Eltaher, H.M. Making the practically impossible “Merely difficult”-Cryogenic FIB lift-out for “Damage free” soft matter imaging. Microsc. Res. Tech. 2016. [Google Scholar] [CrossRef] [PubMed]
- Barnett, H.H.; Heimbuck, A.M.; Pursell, I.; Hegab, R.A.; Sawyer, B.J.; Newman, J.J.; Caldorera-Moore, M.E. Poly (ethylene glycol) hydrogel scaffolds with multiscale porosity for culture of human adipose-derived stem cells. J. Biomater. Sci. Polym. Ed. 2019. [Google Scholar] [CrossRef] [PubMed]
- di Staso, S.; Agnifili, L.; Ciancaglini, M.; Murano, G.; Borrelli, E.; Mastropasqua, L. In vivo scanning laser confocal microscopy of conjunctival goblet cells in medically-controlled glaucoma. In Vivo (Brooklyn) 2018. [Google Scholar] [CrossRef] [Green Version]
- Přádný, M.; Dušková-Smrčková, M.; Dušek, K.; Janoušková, O.; Sadakbayeva, Z.; Šlouf, M.; Michálek, J. Macroporous 2-hydroxyethyl methacrylate hydrogels of dual porosity for cell cultivation: Morphology, swelling, permeability, and mechanical behavior. J. Polym. Res. 2014. [Google Scholar] [CrossRef]
- Janoušková, O.; Přádný, M.; Vetrík, M.; Krumbholcová, E.C.; Michálek, J.; Smrčková, M.D. Biomimetic modification of dual porosity poly(2-hydroxyethyl methacrylate) hydrogel scaffolds-porosity and stem cell growth evaluation. Biomed. Mater. 2019. [Google Scholar] [CrossRef]
- Chytil, P.; Etrych, T.; Kříž, J.; Šubr, V.; Ulbrich, K. N-(2-Hydroxypropyl)methacrylamide-based polymer conjugates with pH-controlled activation of doxorubicin for cell-specific or passive tumour targeting. Synthesis by RAFT polymerisation and physicochemical characterization. Eur. J. Pharm. Sci. 2010. [Google Scholar] [CrossRef] [PubMed]
- Vetrík, M.; Přádný, M.; Hrubý, M.; Michálek, J. Hydrazone-based hydrogel hydrolytically degradable in acidic environment. Polym. Degrad. Stab. 2011. [Google Scholar] [CrossRef]
- Grenier, J.; Duval, H.; Barou, F.; Lv, P.; David, B.; Letourneur, D. Mechanisms of pore formation in hydrogel scaffolds textured by freeze-drying. Acta Biomater. 2019. [Google Scholar] [CrossRef] [PubMed]
- Arsiccio, A.; Barresi, A.A.; Pisano, R. Prediction of Ice Crystal Size Distribution after Freezing of Pharmaceutical Solutions. Cryst. Growth Des. 2017. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, H.W. Emerging properties of hydrogels in tissue engineering. J. Tissue Eng. 2017. [Google Scholar] [CrossRef] [Green Version]







| Groups/Sets | (a) | (b) | (c) | (d) |
|---|---|---|---|---|
| −23 °C | −78 °C | −195 °C | No Freezing | |
| 1 (0–30 µm) | 1a | 1b | 1c | 1d |
| 2 (30–50 µm) | 2a | 2b | 2c | 2d |
| 3 (50–90 µm) | 3a | 3b | 3c | 3d |
| Pore Size | 1 (0–30 µm) | 2 (30–50 µm) | 3 (50–90 µm) | |||
|---|---|---|---|---|---|---|
| Min | Max | Min | Max | Min | Max | |
| (a) −23 °C | 8 | 76 | 6 | 107 | 11 | 134 |
| (b) −78 °C | 8 | 48 | 9 | 68 | 6 | 97 |
| (c) −195 °C | 9 | 41 | 10 | 70 | 6 | 75 |
| (d) No Freezing | 8 | 35 | 8 | 53 | 8 | 90 |
| Porogen Particle Range (µm) | Mean Size of Particle Fraction (a) (µm) | Volume Fraction of Particles of Given Mean Size (b) (Pore vol. -%) |
|---|---|---|
| 0–30 | 30±2.5(c) | 46 |
| 15 ± 2.5 | 32 | |
| 30–50 | 45±2.5 | 40 |
| 35 ± 2.5 | 14 | |
| 50–90 | 67±5.5 | 40 |
| 92 ± 2.5 | 37 |
| Sets of Hydrogels | Swelling Degree SD [wt.-%] (a) | Pore Volume Fraction in Swollen Hydrogel | ||
|---|---|---|---|---|
| Gravimetric Experiment | Computed Reconstruction | ΦPT(b) | ΦPL(c) | |
| pHPMA Matrix Neat | 92.0 ± 1.6 | NaN | NaN | NaN |
| 1 (0–30 µm) | 95.4 ± 1.5 | 94.6 | 0.62 | 0.32 |
| 2 (30–50 µm) | 95.7 ± 2.0 | 96.4 | 0.59 | 0.55 |
| 3 (50–90 µm) | 95.4 ± 1.8 | 94.3 | 0.59 | 0.29 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podhorská, B.; Vetrík, M.; Chylíková-Krumbholcová, E.; Kománková, L.; Rashedi Banafshehvaragh, N.; Šlouf, M.; Dušková-Smrčková, M.; Janoušková, O. Revealing the True Morphological Structure of Macroporous Soft Hydrogels for Tissue Engineering. Appl. Sci. 2020, 10, 6672. https://doi.org/10.3390/app10196672
Podhorská B, Vetrík M, Chylíková-Krumbholcová E, Kománková L, Rashedi Banafshehvaragh N, Šlouf M, Dušková-Smrčková M, Janoušková O. Revealing the True Morphological Structure of Macroporous Soft Hydrogels for Tissue Engineering. Applied Sciences. 2020; 10(19):6672. https://doi.org/10.3390/app10196672
Chicago/Turabian StylePodhorská, Bohumila, Miroslav Vetrík, Eva Chylíková-Krumbholcová, Lucie Kománková, Niloufar Rashedi Banafshehvaragh, Miroslav Šlouf, Miroslava Dušková-Smrčková, and Olga Janoušková. 2020. "Revealing the True Morphological Structure of Macroporous Soft Hydrogels for Tissue Engineering" Applied Sciences 10, no. 19: 6672. https://doi.org/10.3390/app10196672
APA StylePodhorská, B., Vetrík, M., Chylíková-Krumbholcová, E., Kománková, L., Rashedi Banafshehvaragh, N., Šlouf, M., Dušková-Smrčková, M., & Janoušková, O. (2020). Revealing the True Morphological Structure of Macroporous Soft Hydrogels for Tissue Engineering. Applied Sciences, 10(19), 6672. https://doi.org/10.3390/app10196672