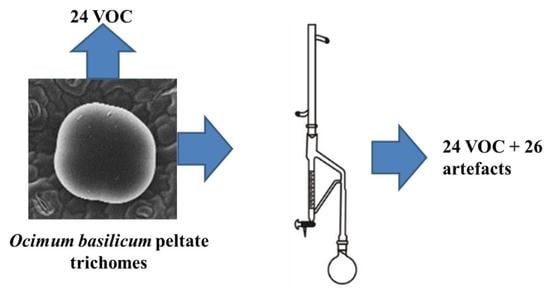
Volatile Organic Compounds of the Glandular Trichomes of Ocimum basilicum and Artifacts during the Distillation of the Leaves
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Sampling from Capitate and Peltate Trichomes
2.3. Isolation of the Essential Oil
2.4. GC-FID e GC-MS Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Chikkaswamy, B.K.; Paramanik, R.C.; Varadaraj, N.; Paramanik, A.; Ramesh, H.L.; Shivashankar, M.; Sivaram, V.R. Determination of genetic variation in Piper species using 4C nuclear DNA and RAPD marker. Int. J. Res. Pharm. Sci. 2013, 4, 58–64. [Google Scholar] [CrossRef] [Green Version]
- Simon, J.E.; Morales, M.R.; Phippen, W.B.; Vieira, R.F.; Hao, Z. Basil: A Source of Aroma Compounds and a Popular Culinary and Ornamental Herb. In Perspectives on New Crops and New Uses; Janick, J., Ed.; ASHS Press: Alexandria, VA, USA, 1999; pp. 499–505. [Google Scholar]
- Carović-Stanko, K.; Liber, Z.; Besendorfer, V.; Javornik, B.; Bohanec, B.; Kolak, I.; Satovic, Z. Genetic relations among basil taxa (Ocimum L.) based on molecular markers, nuclear DNA content, and chromosome number. Plant Syst. Evol. 2010, 285, 13–22. [Google Scholar] [CrossRef]
- Meyers, M. Basil: An Herb Society of America Guide; The Herb Society of America: Kirtland, OH, USA, 2003. [Google Scholar]
- Purushothaman, B.; Prasannasrinivasan, R.; Suganthi, P.; Ranganathan, B.; Gimbun, J.; Shanmugam, K. A comprehensive review on Ocimum basilicum. J. Nat. Remedies 2018, 18, 71–85. [Google Scholar] [CrossRef] [Green Version]
- Dhama, K.; Sharun, K.; Gugjoo, M.B.; Tiwari, R.; Alagawany, M.; Iqbal Yatoo, M.; Thakur, P.; Iqbal, H.M.N.; Chaicumpa, W.; Michalak, I.; et al. A Comprehensive Review on Chemical Profile and Pharmacological Activities of Ocimum basilicum. Food Rev. Int. 2021, 37, 1–29. [Google Scholar] [CrossRef]
- Jayasinghe, C.; Gotoh, N.; Aoki, T.; Wada, S. Phenolics composition and antioxidant activity of sweet basil (Ocimum basilicum L.). J. Agric. Food Chem. 2003, 51, 4442–4449. [Google Scholar] [CrossRef]
- Chiang, L.C.; Ng, L.T.; Cheng, P.W.; Chiang, W.; Lin, C.C. Antiviral activities of extracts and selected pure constituents of Ocimum basilicum. Clin. Exp. Pharmacol. Physiol. 2005, 32, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Manosroi, J.; Dhumtanom, P.; Manosroi, A. Anti-proliferative activity of essential oil extracted from Thai medicinal plants on KB and P388 cell lines. Cancer Lett. 2006, 235, 114–120. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Chemical components and pharmacological benefits of Basil (Ocimum basilicum): A review. Int. J. Food Prop. 2020, 23, 1961–1970. [Google Scholar] [CrossRef]
- Hegnauer, R. (Ed.) Chemotaxonomie der Pflanzen; Birkhiuser: Basel, Switzerland, 1989; Volume 8. [Google Scholar]
- Grayer, R.J.; Kite, G.C.; Goldstone, F.J.; Bryan, S.E.; Paton, A.; Putievsky, E. Infraspecific taxonomy and essential oil chemotypes in sweet basil, Ocimum basilicum. Phytochemistry 1996, 43, 1033–1039. [Google Scholar] [CrossRef]
- Ravid, U.; Putievsky, E.; Katzir, I.; Lewinsohn, E. Enantiomeric composition of linalol in the essential oils of Ocimum species and in commercial basil oils. Flavour Fragr. J. 1997, 12, 293–296. [Google Scholar] [CrossRef]
- Fahn, A. (Ed.) Secretory Tissues in Plants; Academic Press: London, UK, 1979. [Google Scholar]
- Antunes, T.; Sevinate-Pinto, I. Glandular Trichomes of Teucrium scorodonia L. Morphology and Histochemistry. Flora 1991, 185, 65–70. [Google Scholar] [CrossRef]
- Ascensao, L.; Marques, N.; Pais, M.S. Peltate glandular trichomes of Leonotis leonurus leaves: Ultrastructure and histochemical characterization of secretions. Int. J. Plant Sci. 1997, 158, 249–258. [Google Scholar] [CrossRef]
- Ascensão, L.; Marques, N.; Pais, M.S. Glandular trichomes on vegetative and reproductive organs of Leonotis leonurus (Lamiaceæ). Ann. Bot. 1995, 75, 619–626. [Google Scholar] [CrossRef]
- Ascensão, L.; Pais, M.S. The leaf capitate trichomes of Leonotis leonurus: Histochemistry, ultrastructure and secretion. Ann. Bot. 1998, 81, 263–271. [Google Scholar] [CrossRef]
- Bouret, T.M.; Howard, R.J.; O’Keefe, D.P.; Hallahan, D.L. Gland Development on Leaf Surfaces of Nepeta racemosa. Int. J. Plant Sci. 1994, 155, 623–632. [Google Scholar] [CrossRef]
- Voirin, B.; Bayet, C. Developmental changes in the monoterpene composition of Mentha x piperita leaves from individual peltate trichomes. Phytochemistry 1996, 43, 573–580. [Google Scholar] [CrossRef]
- McCaskill, D.; Croteau, R. Monoterpene and sesquiterpene biosynthesis in glandular trichomes of peppermint (Mentha x piperita) rely exclusively on plastid-derived isopentenyl diphosphate. Planta 1995, 197, 49–56. [Google Scholar] [CrossRef]
- Tirillini, B.; Ricci, A.; Pellegrino, R. Secretion constituents of leaf glandular trichome of Salvia officinalis L. J. Essent. Oil Res. 1999, 11, 565–569. [Google Scholar] [CrossRef]
- Falk, K.L.; Gershenzon, J.; Croteau, R. Metabolism of monoterpenes in cell cultures of common sage (Salvia officinalis): Biochemical rationale for the lack of monoterpene accumulation. Plant Physiol. 1990, 93, 1559–1567. [Google Scholar] [CrossRef] [Green Version]
- Venkatachalam, K.V.; Kionaas, R.; Croteau, R. Development and essential oil content of secretory glands of sage (Salvia officinalis). Plant Physiol. 1984, 76, 148–150. [Google Scholar] [CrossRef]
- Yamaura, T.; Tanaka, S.; Tabata, M. Localization of the biosynthesis and accumulation of monoterpenoids in glandular trichomes of thyme. Planta Med. 1992, 58, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Boix, Y.F.; Victório, C.P.; Defaveri, A.C.A.; do Carmo de Oliveira Arruda, R.; Sato, A.; Lage, C.L.S. Glandular trichomes of Rosmarinus officinalis L.: Anatomical and phytochemical analyses of leaf volatiles. Plant Biosyst. 2011, 145, 848–856. [Google Scholar] [CrossRef]
- Sagawa, T.; Ikeda, H.; Hiraoka, T.; Hayakawa, K. Study of rosemary peltate glandular trichomes using combined morphological and chemical approach. Food Sci. Technol. Res. 2013, 19, 491–495. [Google Scholar] [CrossRef] [Green Version]
- Maleci Bini, L.; Gentili, L.; Tirillini, B.; Pellegrino, R. Secretion Constituents of Leaf Glandular Trichomes of Ocìmum basilicum L. In Flavour and Fragrance Chemistry; Lanzotti, V., Taglialatela-Scafati, O., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 143–150. [Google Scholar]
- Tozin, L.R.S.; Marques, M.O.M.; Rodrigues, T.M. Herbivory by leaf-cutter ants changes the glandular trichomes density and the volatile components in an aromatic plant model. AoB Plants 2017, 9, plx057. [Google Scholar] [CrossRef] [Green Version]
- Manan, A.A.; Taha, R.M.; Mubarak, E.E.; Elias, H. In vitro flowering, glandular trichomes ultrastructure, and essential oil accumulation in micropropagated Ocimum basilicum L. Vitr. Cell. Dev. Biol. Plant 2016, 52, 303–314. [Google Scholar] [CrossRef]
- Werker, E.; Putievsky, E.; Ravid, U.; Dudai, N.; Katzir, I. Glandular hairs and essential oil in developing leaves of Ocimum basilicum L. (Lamiaceae). Ann. Bot. 1993, 71, 43–50. [Google Scholar] [CrossRef]
- Schnepf, E. Glands Cells. In Dynamic Ultrastructure; Robards, A.W., Ed.; McGraw-Hill: New York, NY, USA, 1974. [Google Scholar]
- Dong, F.; Fu, X.; Watanabe, N.; Su, X.; Yang, Z. Recent advances in the emission and functions of plant vegetative volatiles. Molecules 2016, 21, 124. [Google Scholar] [CrossRef] [PubMed]
- Boncan, D.A.T.; Tsang, S.S.K.; Li, C.; Lee, I.H.T.; Lam, H.-M.; Chan, T.-F.; Hui, J.H.L. Terpenes and terpenoids in plants: Interactions with environment and insects. Int. J. Mol. Sci. 2020, 21, 7382. [Google Scholar] [CrossRef]
- Abbas, F.; Ke, Y.; Yu, R.; Yue, Y.; Amanullah, S.; Jahangir, M.M.; Fan, Y. Volatile terpenoids: Multiple functions, biosynthesis, modulation and manipulation by genetic engineering. Planta Med. 2017, 246, 803–816. [Google Scholar] [CrossRef]
- Yang, C.; Wang, J.; Li, D. Microextraction techniques for the determination of volatile and semivolatile organic compounds from plants: A review. Anal. Chim. Acta 2013, 799, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Xu, J.; Ke, Y.; Huang, S.; Zeng, F.; Luan, T.; Ouyang, G. Applications of in vivo and in vitro solid-phase microextraction techniques in plant analysis: A review. Anal. Chim. Acta 2013, 794, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Keene, C.K.; Wagner, G.J. Direct demonstration of duvatrienediol biosynthesis in glandular heads of tobacco trichomes. Plant Physiol. 1985, 79, 1026–1032. [Google Scholar] [CrossRef] [Green Version]
- Gershenzon, J.; Duffy, M.A.; Karp, F.; Croteau, R. Mechanized techniques for the selective extraction of enzymes from plant epidermal glands. Anal. Biochem. 1987, 163, 159–164. [Google Scholar] [CrossRef]
- Slone, J.H.; Kelsey, R.G. Isolation and purification of glandular secretory cells from Artemisia tridentata (ssp. vaseyana) by percoll density gradient centrifugation. Amer. J. Bot. 1985, 72, 1445–1451. [Google Scholar] [CrossRef]
- Tirillini, B.; Stoppini, A.M. Injection of a sample by means of microneedles followed by capillary gas chromatography. J. Chromatogr. Sci. 1995, 33, 139–142. [Google Scholar] [CrossRef]
- Mastelic, J.; Jerkovic, I. Application of co-distillation with superheated pentane vapour to the isolation of unstable essential oils. Flavour Fragr. J. 2003, 18, 521–526. [Google Scholar] [CrossRef]
- Baines, D.A.; Jones, R.A.; Webb, T.C.; Campion-Smith, I.H. The chemistry of terpenes-I. The effect of hydrogen ion concentration and oxygen upon the acid catalysed cyclization of citral. Tetrahedron 1970, 26, 4901–4913. [Google Scholar] [CrossRef]
- Banthorpe, D.V.; Charlwood, V.; Francis, M.J.O. The biosynthesis of monoterpenes. Chem. Rev. 1972, 72, 115–155. [Google Scholar] [CrossRef]
- Schratz, E.; Wahlig, T. Gaschromatographische analyse aetherischer ole aus pflanzenextrakten. Planta Med. 1965, 13, 218–225. [Google Scholar] [CrossRef]
- Minteguiaga, M.A.; Frizzo, C.D.; Dellacassa, E.S. Odour-active compounds of Citrus deliciosa Tenore var. Caí essential oils detected by gas chromatography-mass spectrometry and gas chromatography-olfactometry. J. Pharm. Pharmacogn. Res. 2017, 5, 345–353. [Google Scholar]
- Hofmann, L. Einfluss Von Genotyp, Ontogenese Und Äusseren Faktoren Auf Pflanzenbauliche Merkmale Sowie Ätherische Öle und Flavonoide von Klonen der Schafgarbe (Achillea millefolium Aggregat); Technische Universität: München, Germany, 1993. [Google Scholar]
- Tressl, R.; Engel, K.H.; Kossa, M.; Koppler, H. Characterization of tricyclic sesquiterpenes in hop (Humulus lupulus, var Hersbrucker Spat). J. Agric. Food Chem. 1983, 31, 892–897. [Google Scholar] [CrossRef]
- Koedam, A.; Scheffer, J.J.C.; Svendsen, A.B. Monoterpenes in the volatile leaf oil of Abies X Arnoldiana Nitz. J. Agric. Food Chem. 1980, 28, 862–866. [Google Scholar] [CrossRef]
- Toyota, M.; Koyama, H.; Mizljtani, M.; Asakawa, Y. (−)-Ent-spathulenol isolated from liverworts is an artefact. Phytochemistry 1996, 41, 1347–1350. [Google Scholar] [CrossRef]
- Njoroge, S.M.; Ukeda, H.; Sawamura, M. Changes of the volatile profile and artifact formation in daidai (Citrus aurantium) cold-pressed peel oil on storage. J. Agric. Food Chem. 2003, 51, 4029–4035. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Phi, N.T.L.; Sawamura, M. Compositional changes in yuzu (Citrus junos) steam-distilled oil and effects of antioxidants on oil quality during storage. Food Sci. Technol. Res. 2010, 16, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Bryant, R. The Sesquiterpenoids. In Rodd’s Chemistry of Carbon Compounds; Coffey, S., Ed.; Elsevier: Amsterdam, The Netherlands, 1969; pp. 256–366. [Google Scholar]

| Peak | a Compounds | LEO% | VOC% | RI | RI * |
|---|---|---|---|---|---|
| 1 | alpha-pinene | 0.19 ± 0.02 | 0.02 ± 0.01 | 938 | 939 |
| 2 | camphene | 0.02 ± 0.01 | ------ | 954 | 954 |
| 3 | sabinene | 0.23 ± 0.02 | 0.02 ± 0.01 | 976 | 975 |
| 4 | beta-pinene | 0.54 ± 0.05 | 0.04 ± 0.01 | 979 | 979 |
| 5 | octan-3-one | 0.03 ± 0.01 | ------ | 983 | 984 |
| 6 | myrcene | 0.17 ± 0.02 | 0.03 ± 0.01 | 990 | 991 |
| 7 | delta-2-carene | 0.01 ± 0.01 | ------ | 1003 | 1002 |
| 8 | 1.8-cineole | 5.84 ± 0.54 | 5.71 ± 0.53 | 1031 | 1031 |
| 9 | (E)-beta-ocimene | 1.06 ± 0.12 | 0.16 ± 0.02 | 1050 | 1050 |
| 10 | gamma-terpinene | 0.03 ± 0.01 | ------ | 1060 | 1060 |
| 11 | n-octanol | 0.04 ± 0.01 | ------ | 1067 | 1068 |
| 12 | terpinolene | 0.27 ± 0.02 | ------ | 1087 | 1089 |
| 13 | linalool | 32.05 ± 2.57 | 28.99 ± 2.32 | 1097 | 1097 |
| 14 | camphor | 0.07 ± 0.01 | 0.93 ± 0.09 | 1144 | 1146 |
| 15 | borneol | 0.76 ± 0.06 | ------ | 1169 | 1169 |
| 16 | terpinen-4-ol | 0.03 ± 0.01 | ------ | 1179 | 1177 |
| 17 | alpha-terpineol | 0.67 ± 0.07 | 0.55 ± 0.05 | 1187 | 1189 |
| 18 | bornyl acetate | 2.77 ± 0.22 | ------ | 1289 | 1289 |
| 19 | alpha-terpinyl acetate | 0.14 ± 0.01 | ------ | 1349 | 1349 |
| 20 | eugenol | 15.47 ± 1.05 | 41.89 ± 2.83 | 1359 | 1359 |
| 21 | alpha-copaene | 0.18 ± 0.02 | 0.17 ± 0.02 | 1378 | 1377 |
| 22 | beta-cubebene | 0.21 ± 0.02 | ------ | 1388 | 1388 |
| 23 | beta-elemene | 0.69 ± 0.06 | 1.7 ± 0.15 | 1391 | 1391 |
| 24 | beta-longipinene | 0.04 ± 0.01 | ------ | 1401 | 1401 |
| 25 | methyl eugenol | 0.3 ± 0.01 | 0.45 ± 0.05 | 1404 | 1404 |
| 26 | (Z)-caryophyllene | 0.22 ± 0.02 | ------ | 1409 | 1409 |
| 27 | cis-alpha-bergamotene | 9.35 ± 0.79 | 6.34 ± 0.54 | 1414 | 1413 |
| 28 | cadina-3.5-diene | 0.47 ± 0.04 | ------ | 1452 | 1452 |
| 29 | alpha-humulene | 0.72 ± 0.07 | 0.41 ± 0.04 | 1454 | 1455 |
| 30 | cis-muurola-4(14).5-diene | 0.86 ± 0.08 | 0.32 ± 0.03 | 1467 | 1467 |
| 31 | (Z)-beta-bergamotene | 0.35 ± 0.03 | 0.28 ± 0.02 | 1482 | 1483 |
| 32 | alpha-amorphene | 4.24 ± 0.36 | 2.91 ± 0.24 | 1491 | 1490 |
| 33 | cis-beta-guaiene | 0.02 ± 0.01 | ------ | 1494 | 1493 |
| 34 | gamma-amorphene | 0.47 ± 0.04 | ------ | 1496 | 1496 |
| 35 | bicyclogermacrene | 1.51 ± 0.13 | 1.15 ± 0.14 | 1500 | 1500 |
| 36 | gamma-patchoulene | 0.11 ± 0.01 | ------ | 1506 | 1506 |
| 37 | germacrene A | 2.26 ± 0.22 | 0.07 ± 0.01 | 1508 | 1509 |
| 38 | alpha-bulnesene | 1.41 ± 0.09 | 0.14 ± 0.01 | 1510 | 1510 |
| 39 | gamma-cadinene | 3.88 ± 0.35 | 2.64 ± 0.24 | 1514 | 1514 |
| 40 | trans-cycloisolongifol-5-ol | 0.06 ± 0.01 | ------ | 1516 | 1515 |
| 41 | delta-cadinene | 0.11 ± 0.01 | ------ | 1523 | 1523 |
| 42 | beta-sesquiphellandrene | 0.24 ± 0.02 | ------ | 1525 | 1524 |
| 43 | 10-epi-cubebol | 0.06 ± 0.01 | ------ | 1535 | 1535 |
| 44 | cis-muurol-5-en-4-beta-ol | 0.02 ± 0.01 | ------ | 1552 | 1552 |
| 45 | cis-muurol-5-en-4-alpha-ol | 0.03 ± 0.01 | ------ | 1561 | 1561 |
| 46 | spathulenol | 0.02 ± 0.01 | ------ | 1579 | 1578 |
| 47 | 1.10-di-epi-cubenol | 1.29 ± 0.14 | 0.18 ± 0.02 | 1620 | 1619 |
| 48 | epi-alpha-cadinol | 9.97 ± 0.85 | 4.9 ± 0.41 | 1640 | 1640 |
| 49 | alpha-cadinol | 0.14 ± 0.01 | ------ | 1654 | 1654 |
| 50 | 7-epi-alpha-eudesmol | 0.19 ± 0.02 | ------ | 1665 | 1664 |
| Class of compounds | |||||
| Oxygenated monoterpenes | 39.42 ± 3.02 | 36.18 ± 2.77 | |||
| Sesquiterpenes | 27.34 ± 2.12 | 16.13 ± 1.25 | |||
| Allylbenzenes | 15.77 ± 1.34 | 42.34 ± 3.34 | |||
| Oxygenated sesquiterpenes | 11.78 ± 0.96 | 5.08 ± 0.41 | |||
| Monoterpenes | 2.52 ± 0.23 | 0.27 ± 0.02 | |||
| Esters | 2.91 ± 0.27 | ------ | |||
| Ketones | 0.03 ± 0.01 | ------ | |||
| Alcohol | 0.04 ± 0.01 | ------ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tirillini, B.; Maggi, F. Volatile Organic Compounds of the Glandular Trichomes of Ocimum basilicum and Artifacts during the Distillation of the Leaves. Appl. Sci. 2021, 11, 7312. https://doi.org/10.3390/app11167312
Tirillini B, Maggi F. Volatile Organic Compounds of the Glandular Trichomes of Ocimum basilicum and Artifacts during the Distillation of the Leaves. Applied Sciences. 2021; 11(16):7312. https://doi.org/10.3390/app11167312
Chicago/Turabian StyleTirillini, Bruno, and Filippo Maggi. 2021. "Volatile Organic Compounds of the Glandular Trichomes of Ocimum basilicum and Artifacts during the Distillation of the Leaves" Applied Sciences 11, no. 16: 7312. https://doi.org/10.3390/app11167312
APA StyleTirillini, B., & Maggi, F. (2021). Volatile Organic Compounds of the Glandular Trichomes of Ocimum basilicum and Artifacts during the Distillation of the Leaves. Applied Sciences, 11(16), 7312. https://doi.org/10.3390/app11167312