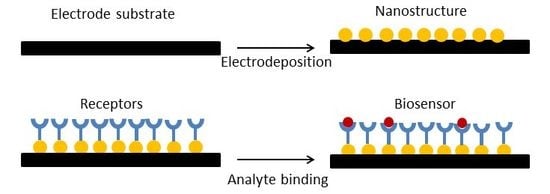
Biosensor Applications of Electrodeposited Nanostructures
Abstract
:1. Introduction
2. Biosensor Application of Electrodeposited Nanoparticles
2.1. Deposition at a Constant Potential for a Fixed Time Period
2.2. Electrodeposition during Potential Sweeps or Cycles
2.3. Constant Current Deposition
2.4. Electrostatic Deposition
3. Biosensor Application of Nanostructures Electrodeposited onto a Template
3.1. Electrodeposition onto Regularly Packed Thin Films of Colloidal or Nanoscale Spheres
3.2. Electrodeposition through Aluminum Oxide and other Templates
3.3. Other Templates for Electrodeposition
4. Biosensor Application of Ramified or Dendritic Electrodeposited Structures
4.1. Dendritic-Like Nanostructures
4.2. Nanostructures Described as ‘Flower-Like’, Irregular, of Unusual Morphology
4.3. Electrodeposition of Single Ramified Nanostructures Through Apertures
5. Biosensor Application of Electrodeposited Composite Nanostructures
5.1. Composite Electrodes Containing Carbon Nanomaterials
5.2. Composite Electrodes Containing Organic Polymers
5.3. Composite Electrodes Containing Prussian Blue
5.4. Composite Electrodes with Nafion
5.5. Composite Electrodes with Chitosan
5.6. Composite Electrodes with Silica
6. Comparison of the Advantages and Performance of Different Nanostructures
7. Conclusions
Funding
Conflicts of Interest
References
- Taurino, I.; Sanzò, G.; Antiochia, R.; Tortolini, C.; Mazzei, F.; Favero, G.; De Micheli, G.; Carrara, S. Recent advances in third generation biosensors based on Au and Pt nanostructured electrodes. Trends Anal. Chem. 2016, 79, 151–159. [Google Scholar] [CrossRef]
- Bhattarai, J.; Neupane, D.; Nepal, B.; Mikhaylov, V.; Demchenko, A.V.; Stine, K.J. Preparation, modification, characterization, and biosensing application of nanoporous gold using electrochemical techniques. Nanomaterials 2018, 8, 171. [Google Scholar] [CrossRef] [PubMed]
- Stine, K.J. Enzyme immobilization on nanoporous gold—A review. Biochem. Insights 2017, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kyaw, H.H.; Al-Harthi, S.H.; Sellai, A.; Dutta, J. Self-organization of gold nanoparticles on silanated surfaces. Beilstein J. Nanotechnol. 2015, 6, 2345–2353. [Google Scholar] [CrossRef] [PubMed]
- Haddada, M.B.; Blanchard, J.; Casale, S.; Krafft, J.-M.; Vallée, A.; Méthivier, C.; Boujday, S. Optimizing the immobilization of gold nanoparticles on functionalized silicon surfaces: Amine- vs thiol-terminated silane. Gold Bull. 2013, 46, 335–341. [Google Scholar] [CrossRef]
- Yu, H.; Zhu, Y.; Yang, H.; Nakanishi, K.; Kanamorib, K.; Guo, X. Facile preparation of silver nanoparticles homogeneously immobilized in hierarchically monolithic silica using ethylene glycol as reductant. Dalton Trans. 2014, 43, 12648–12656. [Google Scholar] [CrossRef] [PubMed]
- Taglietti, A.; Arciola, C.R.; D’Agostino, A.; Dacarro, G.; Montanaro, L.; Campoccia, D.; Cucca, L.; Vercellino, M.; Poggi, A.; Pallavicini, P.; et al. Antibiofilm activity of a monolayer of silver nanoparticles anchored to an amino-silanized glass surface. Biomaterials 2014, 35, 1779–1788. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Sedeño, P.; Pingarrón, J.M. Gold nanoparticle-based electrochemical biosensors. Anal. Bioanal. Chem. 2005, 382, 884–886. [Google Scholar] [CrossRef] [PubMed]
- Chikae, M.; Idegami, K.; Kerman, K.; Nagatani, N.; Ishikawa, M.; Takamura, Y.; Tamiya, E. Direct fabrication of catalytic metal nanoparticles onto the surface of a screen-printed carbon electrode. Electrochem. Commun. 2006, 8, 1375–1380. [Google Scholar] [CrossRef]
- Song, Y.; Ma, Y.; Di, J.; Tu, Y. Electrochemical deposition of gold–platinum alloy nanoparticles on an indium tin oxide electrode and their electrocatalytic applications. Electrochim. Acta 2010, 55, 4909–4914. [Google Scholar] [CrossRef]
- Newman, J.D.S.; Blanchard, G.J. Formation of Gold Nanoparticles Using Amine Reducing Agents. Langmuir 2006, 22, 5882–5887. [Google Scholar] [CrossRef] [PubMed]
- McCreery, R.L. Advanced Carbon Electrode Materials for Molecular Electrochemistry. Chem. Rev. 2008, 108, 2646–2687. [Google Scholar] [CrossRef] [PubMed]
- Haghshenas, E.; Madrakian, T.; Afkhami, A.; Nabiabad, H.S. A label-free electrochemical biosensor based on tubulin immobilized on gold nanoparticle/glassy carbon electrode for the determination of vinblastine. Anal. Bioanal. Chem. 2017, 409, 5269–5278. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Cancel, G.; Suazo-Dávila, D.; Medina-Guzmán, J.; Rosado-González, M.; Díaz-Vázquez, L.M.; Griebenow, K. Chemically glycosylation improves the stability of an amperometric horseradish peroxidase biosensor. Anal. Chim. Acta 2015, 854, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Dimcheva, N.; Horozova, E.; Ivanov, Y.; Godjevargova, T. Self-assembly of acetylcholinesterase on gold nanoparticles electrodeposited on graphite. Cent. Eur. J. Chem. 2013, 11, 1740–1748. [Google Scholar] [CrossRef]
- Yang, S.; Ding, S.; Li, L.; Ding, S.; Cao, Q.; Yang, J.; Xu, W.; Chen, A. One-Step Preparation of Direct Electrochemistry HRP Biosensor via Electrodeposition. J. Electrochem. Soc. 2017, 164, B710–B714. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, X.; Sun, X. A label-free electrochemical aptasensor based on electrodeposited gold nanoparticles and methylene blue for tetracycline detection. Int. J. Electrochem. Sci. 2015, 10, 3668–3679. [Google Scholar]
- Yin, H.; Wang, X.; Guo, Y.; Zhou, Y.; Ai, S. Electrochemical detection of protein kinase activity based on carboxypeptidase Y digestion triggered signal amplification. Biosens. Bioelectron. 2015, 66, 77–83. [Google Scholar] [CrossRef] [PubMed]
- El-Said, W.A.; Lee, J.-H.; Oh, B.-K.; Choi, J.-W. 3-D nanoporous gold thin film for the simultaneous electrochemical determination of dopamine and ascorbic acid. Electrochem. Commun. 2010, 12, 1756–1759. [Google Scholar] [CrossRef]
- Bhattarai, J.K.; Sharma, A.; Fujikawa, K.; Demchenko, A.V.; Stine, K.J. Electrochemical synthesis of nanostructured gold film for the study of carbohydrate–lectin interactions using localized surface plasmon resonance spectroscopy. Carbohydr. Res. 2015, 405, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Deng, J.; Di, J.; Tu, J. Electrodeposition of large size gold nanoparticles on indium tin oxide glass and application as refractive index sensor. Electrochem. Commun. 2009, 11, 1034–1037. [Google Scholar] [CrossRef]
- Bollella, P.; Gorton, L.; Ludwig, R.; Antiochia, R. A third generation glucose biosensor based on cellobiose dehydrogenase immobilized on a glassy carbon electrode decorated with electrodeposited gold nanoparticles: Characterization and Application in Human Saliva. Sensors 2017, 17, 1912. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Zhang, A.; Chen, Y.; Chen, Z.; Chen, Y.; Lu, F.; Chen, Z. A novel probe density controllable electrochemiluminescence biosensor for ultra-sensitive detection of Hg2+ based on DNA hybridization optimization with gold nanoparticles array patterned self-assembly platform. Biosens. Bioelectron. 2013, 49, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Chen, L.; Shen, G.; Yu, R. Platinum nanoparticle-modified carbon fiber ultramicroelectrodes for mediator-free biosensing. Sens. Actuators B 2006, 119, 295–301. [Google Scholar] [CrossRef]
- Zhao, F.; Xie, Q.; Xu, M.; Wang, S.; Zhou, J.; Liu, F. RNA aptamer based electrochemical biosensor for sensitive and selective detection of cAMP. Biosens. Bioelectron. 2015, 66, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Dou, W.; Zhao, G.; Chen, Y. Immunosensor based on electrodeposition of gold-nanoparticles and ionic liquid composite for detection of Salmonella pullorum. J. Microbiol. Methods 2014, 106, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Aydın, E.B.; Sezgintürk, M.K. Indium tin oxide (ITO): A promising material in biosensing technology. Trends Anal. Chem. 2017, 97, 309–315. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Di, J.; Tu, Y. Electrodeposition of gold nanoparticles on indium/tin oxide electrode for fabrication of a disposable hydrogen peroxide biosensor. Talanta 2009, 77, 1454–1459. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wu, Y.; Zhang, Y.; Chen, Z.; Cao, X.; Di, J.; Yang, J. Electrocatalytic behavior and amperometric detection of morphine on ITO electrode modified with directly electrodeposited gold nanoparticles. Electroanalysis 2009, 21, 939–943. [Google Scholar] [CrossRef]
- Deng, J.; Song, Y.; Wang, Y.; Di, J. Label-free optical biosensor based on localized surface plasmon resonance of twin-linked gold nanoparticles electrodeposited on ITO glass. Biosens. Bioelectron. 2010, 26, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Shao, L.; Lia, Q.; Wang, J. Gold nanorods and their plasmonic properties. Chem. Soc. Rev. 2013, 42, 2679–2724. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Song, Y.; Wang, Y.; Di, J. Electrochemical synthesis of gold nanoparticles onto indium tin oxide glass and application in biosensors. Thin Solid Films 2011, 519, 6605–6609. [Google Scholar] [CrossRef]
- Deng, J.; Du, J.; Wang, Y.; Tu, Y.; Di, J. Synthesis of ultrathin silver shell on gold core for reducing substrate effect of LSPR sensor. Electrochem. Commun. 2011, 13, 1517–1520. [Google Scholar] [CrossRef]
- Dong, P.; Lin, Y.; Deng, J.; Di, J. Ultrathin gold-shell coated silver nanoparticles onto a glass platform for improvement of plasmonic sensors. ACS Appl. Mater. Interfaces 2013, 5, 2392–2399. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Di, J.; Yan, X.; Zhao, M.; Lu, Z.; Tu, Y. Direct electrodeposition of gold nanoparticles on indium tin oxide surface and its application. Biosens. Bioelectron. 2009, 24, 1480–1483. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wei, J.; Zhu, H.; Ma, F.; Wang, S. Electrodeposition of gold nanoparticle arrays on ITO glass as electrode with high electrocatalytic activity. Mater. Res. Bull. 2013, 48, 1338–1341. [Google Scholar] [CrossRef]
- Ahmad, M.; Pan, C.; Gan, L.; Nawaz, Z.; Zhu, J. Highly Sensitive Amperometric Cholesterol Biosensor Based on Pt-Incorporated Fullerene-like ZnO Nanospheres. J. Phys. Chem. C 2010, 114, 243–250. [Google Scholar] [CrossRef]
- Ahmadzadeh-Raji, M.; Ghafar-Zadeh, E.; Amoabediny, G. An optically-transparent aptamer-based detection system for colon cancer applications using gold nanoparticles electrodeposited on indium tin oxide. Sensors 2016, 16, 1071. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhai, T.; Zeng, H.; Fang, X.; Bando, X.; Golberg, D. Polystyrene sphere-assisted one-dimensional nanostructure arrays: Synthesis and applications. J. Mater. Chem. 2011, 21, 40–56. [Google Scholar] [CrossRef]
- Bartlett, P.N.; Baumberg, J.J.; Birkin, P.R.; Ghanem, M.A.; Netti, M.C. Highly ordered macroporous gold and platinum films formed by electrochemical deposition through templates assembled from submicron diameter monodisperse polystyrene spheres. Chem. Mater. 2002, 14, 2199–2208. [Google Scholar] [CrossRef]
- Bartlett, P.N.; Birkin, P.R.; Ghanem, M.A. Electrochemical deposition of macroporous platinum, palladium and cobalt films using polystyrene latex sphere templates. Chem. Commun. 2000, 17, 1671–1672. [Google Scholar] [CrossRef]
- Kinkead, B.; van Drunen, J.; Paul, M.T.Y.; Dowling, K.; Jerkiewicz, G.; Gates, B.D. Platinum ordered porous electrodes: Developing a platform for fundamental electrochemical characterization. Electrocatalysis 2013, 4, 179–186. [Google Scholar] [CrossRef]
- Wang, J.; Duan, G.; Liu, G.; Li, Y.; Dai, Z.; Zhang, H.; Cai, W. Gold quasi rod-shaped nanoparticle-built hierarchically micro/nanostructured pore array via clean electrodeposition on a colloidal monolayer and its structurally enhanced SERS performance. J. Mater. Chem. 2011, 21, 8816–8821. [Google Scholar] [CrossRef]
- Wang, C.; Yang, C.; Song, Y.; Gao, W.; Xia, X. Adsorption and direct electron transfer from hemoglobin into a three-dimensionally ordered macroporous gold film. Adv. Funct. Mater. 2005, 15, 1267–1275. [Google Scholar] [CrossRef]
- Laviron, E.A. The use of linear potential sweep voltammetry and of a.c. voltammetry for the study of the surface electrochemical reaction of strongly adsorbed systems and of redox modified electrodes. J. Electroanal. Chem. Interfacial Electrochem. 1979, 100, 263–270. [Google Scholar] [CrossRef]
- Cho, K.; Loget, G.; Corn, R.M. Lithographically patterned nanoscale electrodeposition of plasmonic, bimetallic, semiconductor, magnetic, and polymer nanoring arrays. J. Phys. Chem. C 2014, 118, 28993–29000. [Google Scholar] [CrossRef] [PubMed]
- Toma, M.; Cho, K.; Wood, J.B.; Corn, R.M. Gold nanoring arrays for near infrared plasmonic biosensing. Plasmonics 2014, 9, 765–772. [Google Scholar] [CrossRef]
- Clark, A.W.; Glidle, A.; Cumming, D.R.S.; Cooper, J.M. Plasmonic split-ring resonators as dichroic nanophotonic DNA biosensors. J. Am. Chem. Soc. 2009, 131, 17615–17619. [Google Scholar] [CrossRef] [PubMed]
- Halpern, A.R.; Corn, R.M. Lithographically patterned electrodeposition of gold, silver, and nickel nanoring arrays with widely tunable near-infrared plasmonic resonances. ACS Nano 2013, 7, 1755–1762. [Google Scholar] [CrossRef] [PubMed]
- Thormann, A.; Teuscher, N.; Pfannmçller, M.; Rothe, U.; Heilmann, A. Nanoporous aluminum oxide membranes for filtration and biofunctionalization. Small 2007, 3, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Itaya, K.; Sugawara, S.; Arai, K.; Saito, S. Properties of porous anodic aluminum oxide films as membranes. J. Chem. Eng. Jpn. 1984, 17, 514–520. [Google Scholar] [CrossRef]
- Poinern, G.E.J.; Ali, N.; Fawcett, D. Progress in nano-engineered anodic aluminum oxide membrane development. Materials 2011, 4, 487–526. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Dong, J.; Xu, J. Direct electrodeposition of highly ordered gold nanotube arrays for use in non-enzymatic amperometric sensing of glucose. Microchim. Acta 2016, 183, 1925–1932. [Google Scholar] [CrossRef]
- Hui, J.; Cui, J.; Wang, Y.; Zhang, Y.; Liang, J.; Zhang, X.; Chen, W.; Ogabiela, E.E.; Adeloju, S.B.; Wu, Y. A high throughput glucose biosensor based on FIA and gold nanowire arrays at low potential. J. Electrochem. Soc. 2014, 161, B291–B296. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, K.; Xia, X. Highly ordered platinum-nanotubule arrays for amperometric glucose sensing. Adv. Funct. Mater. 2005, 15, 803–809. [Google Scholar] [CrossRef]
- Takahashia, Y.; Tatsuma, T. Electrodeposition of thermally stable gold and silver nanoparticle ensembles through a thin alumina nanomask. Nanoscale 2010, 2, 1494–1499. [Google Scholar] [CrossRef] [PubMed]
- Kuemmel, M.; Allouche, J.; Nicole, L.; Boissière, C.; Laberty, C.; Amenitsch, H.; Sanchez, C.; Grosso, D. A chemical solution deposition route to nanopatterned inorganic material surfaces. Chem. Mater. 2007, 19, 3717–3725. [Google Scholar] [CrossRef]
- Torati, S.R.; Reddy, V.; Yoon, S.S.; Kim, C. Electrochemical biosensor for Mycobacterium tuberculosis DNA detection based on gold nanotubes array electrode platform. Biosens. Bioelectron. 2016, 78, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Ramulu, T.S.; Venu, R.; Sinha, B.; Lim, B.; Jeon, S.J.; Yoon, S.S.; Kim, C.G. Nanowires array modified electrode for enhanced electrochemical detection of nucleic acid. Biosens. Bioelectron. 2013, 40, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Shariati, M.; Ghorbani, M.; Sasanpour, P.; Karamizefreh, A. An ultrasensitive label free human papilloma virus DNA biosensor using gold nanotubes based on nanoporous polycarbonate in electrical alignment. Anal. Chim. Acta 2019, 1048, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Wu, Y.; Tan, G.; Yu, P.; Zhou, L.; Zhou, Z.; Chen, J.; Wang, Z.; Pang, J.; Ning, C. Palladium nanoparticles entrapped in a self-supporting nanoporous gold wire as sensitive dopamine biosensor. Sci. Rep. 2017, 7, 7941. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Tan, Y. Electrodeposition of enzymes-integrated mesoporous composite films by interfacial templating: A paradigm for electrochemical biosensors. Electrochim. Acta 2014, 116, 495–503. [Google Scholar] [CrossRef]
- Leonardo, S.; Garibo, D.; Fernández-Tejedor, M.; O’Sullivan, C.K.; Campàs, M. Addressed immobilization of biofunctionalized diatoms on electrodes by gold electrodeposition. Biofabrication 2017, 9, 015027. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Nam, O.; Jin, E.; Gu, M.B. A new coccolith modified electrode-based biosensor using a cognate pair of aptamers with sandwich-type binding. Biosens. Bioelectron. 2019, 123, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Pippa, N.; Dokoumetzidis, A.; Demetzos, C.; Macheras, P. On the ubiquitous presence of fractals and fractal concepts in pharmaceutical sciences: A review. Int. J. Pharm. 2013, 456, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.; Paulose, J.; Nelson, D.R.; Manoharan, V.N. Elastic instability of a crystal growing on a curved surface. Science 2014, 343, 634–637. [Google Scholar] [CrossRef] [PubMed]
- Fransaer, J.L.; Penner, R.M. Brownian dynamics simulation of the growth of metal nanocrystal ensembles on electrode surfaces from solution. I. instantaneous nucleation and diffusion-controlled growth. J. Phys. Chem. B 1999, 103, 7643–7653. [Google Scholar] [CrossRef]
- Drews, T.O.; Radisic, A.; Erlebacher, J.; Braatz, R.D.; Searson, P.C.; Alkire, R.C. Stochastic simulation of the early stages of kinetically limited electrodeposition. J. Electrochem. Soc. 2006, 153, C434–C441. [Google Scholar] [CrossRef]
- Hill, H.D.; Millstone, J.E.; Banholzer, M.J.; Mirkin, C.A. The role radius of curvature plays in thiolated oligonucleotide loading on gold nanoparticles. ACS Nano 2009, 3, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.H.; Lin, C.-W.; Liu, H.-H.; Sheu, J.-T.; Hung, W.-H. Potential-controlled electrodeposition of gold dendrites in the presence of cysteine. Chem. Commun. 2011, 47, 2044–2046. [Google Scholar] [CrossRef] [PubMed]
- Nazari-Vanania, R.; Sattarahmady, N.; Yadegari, H.; Helia, H. A novel and ultrasensitive electrochemical DNA biosensor based on an ice crystals-like gold nanostructure for the detection of Enterococcus faecalis gene sequence. Colloids Surf. B Biointerfaces 2018, 166, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Hau, N.Y.; Yang, P.; Liu, C.; Wang, J.; Lee, P.-H.; Feng, S.-P. Aminosilane-assisted electrodeposition of gold nanodendrites and their catalytic properties. Sci. Rep. 2017, 7, 39839. [Google Scholar] [CrossRef] [PubMed]
- Heli, H.; Amirizadeh, O. Non-enzymatic glucose biosensor based on hyperbranched pine-like gold nanostructure. Mater. Sci. Eng. C 2016, 63, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Liu, Y.; Fang, Y.; Li, P.; Yang, Z. A sensor for glycoproteins based on dendritic gold nanoparticles electrodeposited on a gold electrode and modified with a phenylboronic acid. J. Solid State Electrochem. 2015, 19, 563–568. [Google Scholar] [CrossRef]
- Jung, H.; Lee, S.H.; Yang, J.; Cho, M.; Lee, Y. Ni(OH)2@Cu dendrite structure for highly sensitive glucose determination. RSC Adv. 2014, 4, 47714–47720. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, X.; Chen, J. A new third-generation biosensor for superoxide anion based on dendritic gold nanostructure. J. Electroanal. Chem. 2014, 726, 112–118. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, X.-J.; Guo, L.-R.; Li, J.; Xia, X.-H.; Zheng, L.-M. Direct electrochemistry and electrocatalysis of hemoglobin at three-dimensional gold film electrode modified with self-assembled monolayers of 3-mercaptopropylphosphonic acid. Anal. Chim. Acta 2009, 644, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Shu, H.; Cao, L.; Chang, G.; He, H.; Zhang, Y.; He, Y. Direct electrodeposition of gold nanostructures onto glassy carbon electrodes for non-enzymatic detection of glucose. Electrochim. Acta 2014, 132, 524–532. [Google Scholar] [CrossRef]
- Qiu, R.; Zhang, X.L.; Qiao, R.; Li, Y.; Kim, Y.; Kang, Y.S. CuNi dendritic material: Synthesis, mechanism discussion, and application as glucose sensor. Chem. Mater. 2007, 19, 4174–4180. [Google Scholar] [CrossRef]
- Tanga, J.; Tang, D.; Niessner, R.; Knopp, D.; Chen, G. Hierarchical dendritic gold microstructure-based aptasensor for ultrasensitive electrochemical detection of thrombin using functionalized mesoporous silica nanospheres as signal tags. Anal. Chim. Acta 2012, 720, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Chu, Z.; Liu, Y.; Jin, W.; Chen, X. Facile synthesis of hierarchically aloe-like gold micro/nanostructures for ultrasensitive DNA recognition. Biosens. Bioelectron. 2013, 49, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Miao, Z.; Fang, Y.; Zhang, D.; Ma, J.; Zhang, L.; Chen, Q.; Shao, X. Preparation of dendritic nanostructures of silver and their characterization for electroreduction. Langmuir 2012, 28, 5218–5226. [Google Scholar] [CrossRef] [PubMed]
- Sakai, N.; Fujiwara, Y.; Arai, M.; Yu, K.; Tatsuma, T. Electrodeposition of gold nanoparticles on ITO: Control of morphology and plasmon resonance-based absorption and scattering. J. Electroanal. Chem. 2009, 628, 7–15. [Google Scholar] [CrossRef]
- Sanzò, G.; Taurino, I.; Puppo, F.; Antiochia, R.; Gorton, L.; Favero, G.; Mazzei, F.; Carrara, S.; De Micheli, G. A bimetallic nanocoral Au decorated with Pt nanoflowers (bio)sensor for H2O2 detection at low potential. Methods 2017, 129, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Sanzó, G.; Taurino, I.; Antiochia, R.; Gorton, L.; Favero, G.; Mazzei, F.; De Micheli, F.; Carrara, S. Bubble electrodeposition of gold porous nanocorals for the enzymatic and non-enzymatic detection of glucose. Bioelectrochemistry 2016, 112, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Argoubi, W.; Saadaoui, M.; Ben Aoun, S.; Raouafi, N. Optimized design of a nanostructured SPCE-based multipurpose biosensing platform formed by ferrocene-tethered electrochemically-deposited cauliflower-shaped gold nanoparticles. Beilstein J. Nanotechnol. 2015, 6, 1840–1852. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhao, G.; Geng, R.; Hu, H. Facile electrocatalytic redox of hemoglobin by flower-like gold nanoparticles on boron-doped diamond surface. Bioelectrochemistry 2008, 74, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Taurino, I.; Sanzó, G.; Mazzei, F.; Favero, G.; De Micheli, G.; Carrara, S. Fast synthesis of platinum nanopetals and nanospheres for highly-sensitive non-enzymatic detection of glucose and selective sensing of ions. Sci. Rep. 2015, 5, 15277. [Google Scholar] [CrossRef] [PubMed]
- Taurino, I.; Massa, S.; Sanz´o, G.; Aleman, J.; Flavia, B.; Shin, S.R.; Zhang, Y.S.; Dokmeci, M.R.; De Micheli, G.; Carrara, S.; et al. Platinum nanopetal-based potassium sensors for acute cell death monitoring. RSC Adv. 2016, 6, 40517–40526. [Google Scholar] [CrossRef]
- Bu, Y.; Lee, S.-W. Flower-like gold nanostructures electrodeposited on indium tin oxide (ITO) glass as a SERS-active substrate for sensing dopamine. Microchim. Acta 2015, 182, 1313–1321. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, J.-J.; Chen, H.-Y. Shape-controlled gold nanoarchitectures: Synthesis, superhydrophobicity, and electrocatalytic properties. J. Phys. Chem. C 2008, 112, 13886–13892. [Google Scholar] [CrossRef]
- Delshadi-Jahromi, N.; Nazari-Vanani, R.; Yadegaric, H.; Sattarahmady, N.; Hatame, G.R.; Heli, H. Label-free ultrasensitive electrochemical genosensing of Trichomonas vaginalis using anisotropic-shaped gold nanoparticles as a platform, a repeated sequence of the parasite DNA as a probe, and toluidine blue as a redox marker. Sens. Actuators B 2018, 273, 234–241. [Google Scholar] [CrossRef]
- Nazari-Vanania, R.; Sattarahmady, N.; Yadegari, H.; Delshadi, N.; Hatam, G.R.; Helia, H. Electrochemical quantitation of Leishmania infantum based on detection of its kDNA genome and transduction of non-spherical gold nanoparticles. Anal. Chim. Acta 2018, 1041, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Moradi, M.; Sattarahmady, N.; Rahi, A.; Hatam, G.R.; RezayatSorkhabadi, S.M.; Heli, H. A label-free, PCR-free and signal-on electrochemical DNA biosensor for Leishmania major based on gold nanoleaves. Talanta 2016, 161, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Sattarahmady, N.; Rahi, A.; Heli, H. A signal-on built in-marker electrochemical aptasensor for human prostate-specific antigen based on a hairbrush-like gold nanostructure. Sci. Rep. 2017, 7, 11238. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, L.-P.; Wang, S.; Yang, W.; Wen, Y.; Zhang, X. An ultrasensitive electrochemical immunosensor for apolipoprotein E4 based on fractal nanostructures and enzyme amplification. Biosens. Bioelectron. 2015, 71, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Chen, L.; Xu, T.; Liu, H.; Liu, X.; Meng, J.; Yang, G.; Jiang, L.; Wang, S. Programmable fractal nanostructured interfaces for specific recognition and electrochemical release of cancer cells. Adv. Mater. 2013, 25, 3566–3570. [Google Scholar] [CrossRef] [PubMed]
- Elbourne, A.; Coyle, V.E.; Truong, V.K.; Sabri, Y.M.; Kandjani, A.E.; Bhargava, S.K.; Ivanova, E.P.; Crawford, R.J. Multi-directional electrodeposited gold nanospikes for antibacterial surface application. Nanoscale Adv. 2019, 1, 203–212. [Google Scholar] [CrossRef]
- Mahshid, S.; Mepham, A.H.; Mahshid, S.S.; Burgess, I.B.; Safaei, T.S.; Sargent, E.H.; Kelley, S.O. Mechanistic Control of the Growth of Three-Dimensional Gold Sensors. J. Phys. Chem. C 2016, 120, 21123–21132. [Google Scholar] [CrossRef]
- Soleymani, L.; Fang, Z.; Sargent, E.H.; Kelley, S.O. Programming the detection limits of biosensors through controlled nanostructuring. Nat. Nanotechnol. 2009, 4, 844–848. [Google Scholar] [CrossRef] [PubMed]
- Soleymani, L.; Fang, Z.; Kelley, S.O.; Sargent, E.H. Integrated nanostructures for direct detection of DNA at attomolar concentrations. Appl. Phys. Lett. 2009, 95, 143701. [Google Scholar] [CrossRef]
- Soleymani, L.; Fang, Z.; Sun, X.; Yang, H.; Taft, B.J.; Sargent, E.H.; Kelley, S.O. Nanostructuring of patterned microelectrodes To enhance the sensitivity of electrochemical nucleic acids detection. Angew. Chem. Int. Ed. 2009, 48, 8457–8460. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Soleymani, L.; Pampalakis, G.; Yoshimoto, M.; Squire, J.A.; Sargent, E.H.; Kelley, S.O. Direct profiling of cancer biomarkers in tumor tissue using a multiplexed nanostructured microelectrode integrated circuit. ACS Nano 2009, 3, 3207–3213. [Google Scholar] [CrossRef] [PubMed]
- Bin, X.; Sargent, E.H.; Kelley, S.O. Nanostructuring of sensors determines the efficiency of biomolecular capture. Anal. Chem. 2010, 82, 5928–5931. [Google Scholar] [CrossRef] [PubMed]
- Vasilyeva, E.; Lam, B.; Fang, Z.; Minden, M.D.; Sargent, E.H.; Kelley, S.O. Direct Genetic analysis of ten cancer cells: Tuning sensor structure and molecular probe design for efficient mRNA capture. Angew. Chem. Int. Ed. 2011, 50, 4137–4141. [Google Scholar] [CrossRef] [PubMed]
- Das, J.; Kelley, S.O. Protein detection using arrayed microsensor chips: Tuning sensor footprint to achieve ultrasensitive readout of CA-125 in serum and whole blood. Anal. Chem. 2011, 83, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Lam, B.; Fang, Z.; Sargent, E.H.; Kelley, S.O. Polymerase chain reaction-free, sample-to-answer bacterial detection in 30 min with integrated cell lysis. Anal. Chem. 2012, 84, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Soleymani, L.; Fang, Z.; Lam, B.; Bin, X.; Vasilyeva, E.; Ross, A.J.; Sargent, E.H.; Kelley, S.O. Hierarchical nanotextured microelectrodes overcome the molecular transport barrier To achieve rapid, direct bacterial detection. ACS Nano 2011, 5, 3360–3366. [Google Scholar] [CrossRef] [PubMed]
- Narwala, V.; Kumar, P.; Joon, P.; Pundira, C.S. Fabrication of an amperometric sarcosine biosensor based on sarcosine oxidase/chitosan/CuNPs/c-MWCNT/Au electrode for detection of prostate cancer. Enzyme Microb. Technol. 2018, 113, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, L.; Shen, L.; Ge, S.; Yu, J.; Yan, M. Electrochemiluminescence DNA biosensor based on the use of gold nanoparticle modified graphite-like carbon nitride. Microchim. Acta 2017, 184, 2587–2596. [Google Scholar] [CrossRef]
- Wu, F.; Huang, T.; Hu, Y.; Yang, X.; Xie, Q. One-pot electrodeposition of a composite film of glucose oxidase, imidazolium alkoxysilane and chitosan on a reduced graphene oxide–Pt nanoparticle/Au electrode for biosensing. J. Electroanal. Chem. 2016, 781, 296–303. [Google Scholar] [CrossRef]
- Yagati, A.K.; Lee, G.-Y.; Ha, S.; Chang, K.-A.; Pyun, J.-C.; Cho, S. Impedimetric tumor necrosis factor-α sensor based on a reduced graphene oxide nanoparticle-modified electrode array. J. Nanosci. Nanotechnol. 2016, 16, 11921–11927. [Google Scholar] [CrossRef]
- Yu, G.; Wu, W.; Pan, X.; Zhao, Q.; Wei, X.; Lu, Q. High sensitive and selective sensing of hydrogen peroxide released from Pheochromocytoma cells based on Pt-Au bimetallic nanoparticles electrodeposited on reduced graphene sheets. Sensors 2015, 15, 2709–2722. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-Y.; Tsai, T.-H.; Chen, S.-M.; Lou, B.-S.; Liu, X. Development of a multiple biosensor and its application of biofuel cell. Int. J. Electrochem. Sci. 2015, 10, 579–588. [Google Scholar]
- Wu, X.; Zhong, X.; Chai, Y.; Yuan, R. Electrochemiluminescence acetylcholine biosensor based on biofunctional AMs-AChE-ChO biocomposite and electrodeposited graphene-Au-chitosan nanocomposite. Electrochim. Acta 2014, 147, 735–742. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, A.; Lina, H.; Fub, L.; Cai, W. A sensitive electrochemical sensor for direct Phoxim detection based on electrodeposited reduced graphene oxide-gold nanocomposite. RSC Adv. 2015, 5, 15425–15430. [Google Scholar] [CrossRef]
- Shi, L.; Yu, Y.; Chen, Z.; Zhang, L.; He, S.; Shia, Q.; Yang, H. A label-free hemin/G-quadruplex DNAzyme biosensor developed on electrochemically modified electrodes for detection of a HBV DNA segment. RSC Adv. 2015, 5, 11541–11548. [Google Scholar] [CrossRef]
- Numnuam, A.; Thavarungkul, P.; Kanatharana, P. An amperometric uric acid biosensor based on chitosan-carbon nanotubes electrospun nanofiber on silver nanoparticles. Anal. Bioanal. Chem. 2014, 406, 3763–3772. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Fan, L.; Ren, J.; Hong, B. Gold nanoclusters electrodeposited on multi-walled carbon nanotubes: Enhanced electrocatalytic activity of hemoglobin. J. Solid State Electrochem. 2014, 18, 1099–1109. [Google Scholar] [CrossRef]
- Li, G.; Li, T.; Deng, Y.; Cheng, Y.; Shi, F.; Sun, W.; Sun, Z. Electrodeposited nanogold decorated graphene modified carbon ionic liquid electrode for the electrochemical myoglobin biosensor. J. Solid State Electrochem. 2013, 17, 2333–2340. [Google Scholar] [CrossRef]
- Benvidi, A.; Dehghani-Firouzabadi, A.; Mazloum-Ardakani, M.; Fatemeh Mirjalili, B.-B.; Zare, R. Electrochemical deposition of gold nanoparticles on reduced graphene oxide modified glassy carbon electrode for simultaneous determination of levodopa, uric acid and folic acid. J. Electroan. Chem. 2015, 736, 22–29. [Google Scholar] [CrossRef]
- Yiwei, X.; Wen, Z.; Jiyong, S.; Xiaobo, Z.; Yanxiao, L.; Tahir, H.E.; Xiaowei, H.; Zhihua, L.; Xiaodong, Z.; Xuetao, H. Electrodeposition of gold nanoparticles and reduced graphene oxide on an electrode for fast and sensitive determination of methylmercury in Fish. Food Chem. 2017, 237, 423–430. [Google Scholar]
- Chu, Z.; Liu, Y.; Xu, Y.; Shi, L.; Peng, J.; Jin, W. In-situ fabrication of well-distributed gold nanocubes on thiol graphene as a third-generation biosensor for ultrasensitive glucose detection. Electrochim. Acta 2015, 176, 162–171. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, H.; Xu, S.; Cai, J.; Luo, J.; Wei, W.; Liu, X.; Zhu, Y. Preparation of molecularly imprinted polymer/Au nanohybrids as an effective biosensing material. Colloids Surf. A 2018, 555, 95–102. [Google Scholar] [CrossRef]
- Karazehir, T.; Guler Gokce, Z.; Ates, M.; Sarac, A.S. Gold nanoparticle/nickel oxide/poly(pyrrole-N-propionic acid) hybrid multilayer film: Electrochemical study and its application in biosensing. eXPRESS Polym. Lett. 2017, 11, 449–466. [Google Scholar] [CrossRef]
- Hui, Y.; Ma, X.; Qu, F.; Chen, F.; Yu, J.; Gao, Y. Electropolymerization of carboxymethyl-β-cyclodextrin based on co-electrodeposition gold nanoparticles electrode: Electrocatalysis and nonenzymatic glucose sensing. J. Solid State Electrochem. 2016, 20, 1377–1389. [Google Scholar] [CrossRef]
- Miao, Z.; Wang, P.; Zhong, M.; Yang, M.; Xua, Q.; Hao, S.; Hu, X. Development of a glucose biosensor based on electrodeposited gold nanoparticles–polyvinylpyrrolidone–polyaniline nanocomposites. J. Electroanal. Chem. 2015, 756, 153–160. [Google Scholar] [CrossRef]
- Che, X.; Yuan, R.; Chai, Y.; Ma, L.; Li, W.; Li, J. Hydrogen peroxide sensor based on horseradish peroxidase immobilized on an electrode modified with DNA-l-cysteine-gold-platinum nanoparticles in polypyrrole film. Microchim. Acta 2009, 167, 159–165. [Google Scholar] [CrossRef]
- Zhao, H.; He, H.; Shi, L.; Cai, X.; Lia, H.; Lan, M.; Zhang, Q. Electrochemical detection of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone using a cytochrome P450 2E1 decorated biosensor. J. Electroanal. Chem. 2018, 816, 62–67. [Google Scholar] [CrossRef]
- Mohtar, L.G.; Aranda, P.; Messina, G.A.; Nazareno, M.A.; Pereira, S.V.; Raba, J.; Bertolino, F.A. Amperometric biosensor based on laccase immobilized onto a nanostructured scree-printed electrode for determination of polyphenols in propolis. Microchem. J. 2019, 144, 13–18. [Google Scholar] [CrossRef]
- Karyakin, A.A. Prussian blue and its analogues: Electrochemistry and analytical applications. Electroanalysis 2001, 13, 813–819. [Google Scholar] [CrossRef]
- Chao, L.; Wang, W.; Dai, M.; Ma, Y.; Sun, L.; Qin, X.; Xie, Q. Step-by-step electrodeposition of a high-performance Prussian blue-gold nanocomposite for H2O2 sensing and glucose biosensing. J. Electroanal. Chem. 2016, 778, 66–73. [Google Scholar] [CrossRef]
- Tian, R.; Chen, X.; Liu, D.; Yao, C. A Sensitive Biosensor for Determination of Cu2+ by One-step Electrodeposition. Electroanalysis 2016, 28, 1617–1624. [Google Scholar] [CrossRef]
- Wang, B.; Jin, X.; Zhao, H.; Wang, N.; Li, X.; Ni, R.; Liu, Y. An amperometric β-glucan biosensor based on the immobilization of bi-enzyme on Prussian blue–chitosan and gold nanoparticles–chitosan nanocomposite films. Biosens. Bioelectron. 2014, 55, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Mauritz, K.A.; Moore, R.B. State of understanding of Nafion. Chem. Rev. 2004, 104, 4535–4585. [Google Scholar] [CrossRef] [PubMed]
- Thangavel, S.; Ramaraj, R. Polymer membrane stabilized gold nanostructures modified electrode and its application in nitric oxide detection. J. Phys. Chem. C 2008, 112, 19825–19830. [Google Scholar] [CrossRef]
- Krajewska, B. Application of chitin- and chitosan-based materials for enzyme immobilizations: A review. Enzyme Microb. Technol. 2004, 35, 126–139. [Google Scholar] [CrossRef]
- Dagar, K.; Pundir, C. Covalent immobilization of lactate oxidase onto zirconia coated silica nanoparticles/chitosan hybrid film for amperometric determination of lactate. Biochem. Anal. Biochem. 2016, 5, 301–309. [Google Scholar]
- Zeng, X.; Li, X.; Xing, L.; Liu, X.; Luo, S.; Wei, W.; Kong, B.; Li, Y. Electrodeposition of chitosan–ionic liquid–glucose oxidase biocomposite onto nano-gold electrode for amperometric glucose sensing. Biosens. Bioelectron. 2009, 24, 2898–2903. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Wang, F.; Chen, Z. Electrochemical glucose sensor based on one-step construction of gold nanoparticle–chitosan composite film. Sens. Actuators B 2009, 138, 539–544. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Wang, J.; Di, J. Disposable superoxide anion biosensor based on superoxide dismutase entrapped in silica sol–gel matrix at gold nanoparticles modified ITO electrode. Bioprocess Biosyst. Eng. 2009, 32, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Mao, W.; Ni, D.; Di, J.; Wu, Y.; Tu, Y. Direct electrodeposition of gold nanoparticles onto indium/tin oxide film coated glass and its application for electrochemical biosensor. Electrochem. Commun. 2008, 10, 673–676. [Google Scholar] [CrossRef]
- Du, J.; Yu, X.; Di, J. Comparison of the direct electrochemistry of glucose oxidase immobilized on the surface of Au, CdS, and ZnS nanostructures. Biosens. Bioelectron. 2012, 37, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Breslauer, K.J.; Frank, R.; Blöcker, H.; Marky, L.A. Predicting DNA duplex stability from the base sequence. Proc. Natl. Acad. Sci. USA 1986, 83, 3746–3750. [Google Scholar] [CrossRef] [PubMed]










© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stine, K.J. Biosensor Applications of Electrodeposited Nanostructures. Appl. Sci. 2019, 9, 797. https://doi.org/10.3390/app9040797
Stine KJ. Biosensor Applications of Electrodeposited Nanostructures. Applied Sciences. 2019; 9(4):797. https://doi.org/10.3390/app9040797
Chicago/Turabian StyleStine, Keith J. 2019. "Biosensor Applications of Electrodeposited Nanostructures" Applied Sciences 9, no. 4: 797. https://doi.org/10.3390/app9040797
APA StyleStine, K. J. (2019). Biosensor Applications of Electrodeposited Nanostructures. Applied Sciences, 9(4), 797. https://doi.org/10.3390/app9040797