Auditory and Visual Response Inhibition in Children with Bilateral Hearing Aids and Children with ADHD
Abstract
:1. Introduction
1.1. Modality-(In)Dependent Response Inhibition
1.2. Modality-(In)Dependent Response Inhibition in Auditory Deprivation
1.3. Modality-(In)Dependent Response Inhibition in ADHD
1.4. Current Objectives
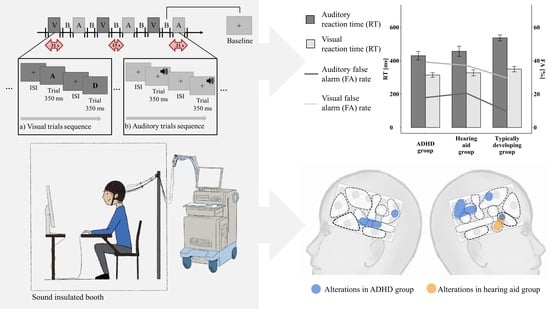
2. Materials and Methods
2.1. Participants
2.2. Set-Up and Go/Nogo Paradigm
2.3. FNIRS
2.3.1. Probe Set Configuration
2.3.2. FNIRS Quality Control and Preprocessing
2.4. Matching Procedure
2.5. Analyses
3. Results
3.1. Behavioral Data
3.1.1. FA Rate and Classical RT Analysis
3.1.2. Maximum Likelihood Estimation of RTs
3.2. FNIRS Analyses
3.2.1. Functional Brain Activation
3.2.2. Effects on Changes in HbO and HbR
3.2.3. Brain-Behavior Correlations
4. Discussion
4.1. Limitations and Future Applications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bari, A.; Robbins, T.W. Inhibition and impulsivity: Behavioral and neural basis of response control. Prog. Neurobiol. 2013, 108, 44–79. [Google Scholar] [CrossRef]
- Diamond, A.; Normal development of prefrontal cortex from birth to young adulthood: Cognitive functions, anatomy, and biochemistry. Princ. Front. Lobe Funct. 2002, 466–503. Available online: http://devcogneuro.com/Publications/ChapterinStuss&Knight.pdf (accessed on 18 July 2018).
- Diamond, A. Executive functions. Annu. Rev. Psychol. 2013, 64, 135–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahrick, L.E.; Todd, J.T.; Soska, K.C. The Multisensory Attention Assessment Protocol (MAAP): Characterizing Individual Differences in Multisensory Attention Skills in Infants and Children and Relations with Language and Cognition. Dev. Psychol. 2018, 54, 2207–2225. [Google Scholar] [CrossRef] [PubMed]
- Piaget, J.; Cook, M. The Origins of Intelligence in Children; International Universities Press New York: New York, NY, USA, 1952; Volume 8. [Google Scholar]
- Quittner, A.L.; Smith, L.B.; Osberger, M.J.; Mitchell, T.V.; Katz, D.B. The impact of audition on the development of visual attention. Psychol. Sci. 1994, 5, 347–353. [Google Scholar] [CrossRef]
- Chmielewski, W.X.; Muckschel, M.; Dippel, G.; Beste, C. Concurrent information affects response inhibition processes via the modulation of theta oscillations in cognitive control networks. Brain Struct. Funct. 2016, 221, 3949–3961. [Google Scholar] [CrossRef]
- Bodmer, B.; Beste, C. On the Dependence of Response Inhibition Processes on Sensory Modality. Hum. Brain Mapp. 2017, 38, 1941–1951. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.K.; Larson, E.; Maddox, R.K.; Shinn-Cunningham, B.G. Using neuroimaging to understand the cortical mechanisms of auditory selective attention. Hear. Res. 2014, 307, 111–120. [Google Scholar] [CrossRef] [Green Version]
- Bahrick, L.E.; Liekliter, R.; Flom, R. Intersensory redundancy guides the development of selective attention, perception, and cognition in infancy. Curr. Dir. Psychol. Sci. 2004, 13, 99–102. [Google Scholar] [CrossRef]
- Lickliter, R.; Bahrick, L.E.; Vaillant-Mekras, J. The intersensory redundancy hypothesis: Extending the principle of unimodal facilitation to prenatal development. Dev. Psychobiol. 2017, 59, 910–915. [Google Scholar] [CrossRef] [Green Version]
- Bell, L.; Wagels, L.; Neuschaefer-Rube, C.; Fels, J.; Gur, R.E.; Konrad, K. The Cross-Modal Effects of Sensory Deprivation on Spatial and Temporal Processes in Vision and Audition: A Systematic Review on Behavioral and Neuroimaging Research since 2000. Neural Plast. 2019, 2019, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonna, K.; Finc, K.; Zimmermann, M.; Bola, Ł.; Mostowski, P.; Szul, M.; Rutkowski, P.; Duch, W.; Marchewka, A.; Jednoróg, K. Early deafness leads to re-shaping of global functional connectivity beyond the auditory cortex. arXiv 2019, arXiv:1903.11915. [Google Scholar]
- Lomber, S.G.; Butler, B.E.; Glick, H.; Sharma, A. Crossmodal Neuroplasticity in Deafness: Evidence from Animal Models and Clinical Populations. In Multisensory Perception; Elsevier: Amsterdam, The Netherlands, 2020; pp. 343–370. [Google Scholar]
- Brimijoin, W.O.; McShefferty, D.; Akeroyd, M.A. Auditory and visual orienting responses in listeners with and without hearing-impairment. J. Acoust. Soc. Am. 2010, 127, 3678–3688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broadbent, D. Perception and Communication; Pergamon Press: Elmsford, NY, USA, 1958. [Google Scholar]
- Driver, J.; Spence, C.J. Spatial Synergies between Auditory and Visual Attention. In Attention and Performance XV: Conscious and Nonconcious Information Processing; MIT Press: Cambridge, MA, USA, 1994. [Google Scholar]
- Conway, C.M.; Pisoni, D.B.; Kronenberger, W.G. The Importance of Sound for Cognitive Sequencing Abilities: The Auditory Scaffolding Hypothesis. Curr. Dir. Psychol. Sci. 2009, 18, 275–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kral, A.; Kronenberger, W.G.; Pisoni, D.B.; O’Donoghue, G.M. Neurocognitive factors in sensory restoration of early deafness: A connectome model. Lancet Neurol. 2016, 15, 610–621. [Google Scholar] [CrossRef]
- Tomblin, J.B.; Oleson, J.J.; Ambrose, S.E.; Walker, E.; Moeller, M.P. The influence of hearing aids on the speech and language development of children with hearing loss. JAMA Otolaryngol. Head Neck Surg. 2014, 140, 403–409. [Google Scholar] [CrossRef]
- Svirsky, M.A. Language development in children with profound and prelingual hearing loss, without cochlear implants. Ann. Otol. Rhinol. Laryngol. Suppl. 2000, 185, 99–100. [Google Scholar] [CrossRef]
- Barker, D.H.; Quittner, A.L.; Fink, N.E.; Eisenberg, L.S.; Tobey, E.A.; Niparko, J.K.; TEAM, C.I. Predicting behavior problems in deaf and hearing children: The influences of language, attention, and parent-child communication. Dev. Psychopathol. 2009, 21, 373–392. [Google Scholar] [CrossRef] [PubMed]
- Le Clercq, C.M.; Labuschagne, L.J.; Franken, M.-C.J.; de Jong, R.J.B.; Luijk, M.P.; Jansen, P.W.; van der Schroeff, M.P. Association of slight to mild hearing loss with behavioral problems and school performance in children. JAMA Otolaryngol. Head Neck Surg. 2020, 146, 113–120. [Google Scholar] [CrossRef]
- Beer, J.; Kronenberger, W.G.; Castellanos, I.; Colson, B.G.; Henning, S.C.; Pisoni, D.B. Executive functioning skills in preschool-age children with cochlear implants. J. Speech Lang. Hear. Res. 2014, 57, 1521–1534. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, M.; Tiddens, E.; Quittner, A.L.; Team, C.D.I. Comparisons of visual attention in school-age children with cochlear implants versus hearing peers and normative data. Hear. Res. 2018, 359, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Theunissen, S.C.; Rieffe, C.; Netten, A.P.; Briaire, J.J.; Soede, W.; Schoones, J.W.; Frijns, J.H. Psychopathology and its risk and protective factors in hearing-impaired children and adolescents: A systematic review. JAMA Pediatr. 2014, 168, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, J.; Kreppner, J.; Pimperton, H.; Worsfold, S.; Kennedy, C. Emotional and behavioural difficulties in children and adolescents with hearing impairment: A systematic review and meta-analysis. Eur. Child Adolesc. Psychiatry 2015, 24, 477–496. [Google Scholar] [CrossRef] [Green Version]
- Tharpe, A.M.; Ashmead, D.H.; Rothpletz, A.M. Visual attention in children with normal hearing, children with hearing aids, and children with cochlear implants. J. Speech Lang. Hear. Res. 2002, 45, 403–413. [Google Scholar] [CrossRef]
- Mitchell, T.V.; Quittner, A.L. Multimethod study of attention and behavior problems in hearing-impaired children. J. Clin. Child Psychol. 1996, 25, 83–96. [Google Scholar] [CrossRef]
- Barkley, R.A. Response inhibition in attention-deficit hyperactivity disorder. Ment. Retard. Dev. Disabil. Res. Rev. 1999, 5, 177–184. [Google Scholar] [CrossRef]
- Nigg, J.T. Is ADHD a disinhibitory disorder? Psychol. Bull. 2001, 127, 571–598. [Google Scholar] [CrossRef]
- Quay, H.C. Inhibition and attention deficit hyperactivity disorder. J. Abnorm. Child Psychol. 1997, 25, 7–13. [Google Scholar] [CrossRef]
- Rubia, K.; Smith, A.B.; Brammer, M.J.; Toone, B.; Taylor, E. Abnormal brain activation during inhibition and error detection in medication-naive adolescents with ADHD. Am. J. Psychiatry 2005, 162, 1067–1075. [Google Scholar] [CrossRef] [Green Version]
- Durston, S.; Tottenham, N.T.; Thomas, K.M.; Davidson, M.C.; Eigsti, I.-M.; Yang, Y.; Ulug, A.M.; Casey, B. Differential patterns of striatal activation in young children with and without ADHD. Biol. Psychiatry 2003, 53, 871–878. [Google Scholar] [CrossRef] [Green Version]
- Miao, S.; Han, J.; Gu, Y.; Wang, X.; Song, W.; Li, D.; Liu, Z.; Yang, J.; Li, X. Reduced Prefrontal Cortex Activation in Children with Attention-Deficit/Hyperactivity Disorder during Go/No-Go Task: A Functional Near-Infrared Spectroscopy Study. Front. Neurosci. 2017, 11, 367. [Google Scholar] [CrossRef]
- Ehlis, A.C.; Baehne, C.G.; Jacob, C.P.; Herrmann, M.J.; Fallgatter, A.J. Reduced lateral prefrontal activation in adult patients with attention-deficit/hyperactivity disorder (ADHD) during a working memory task: A functional near-infrared spectroscopy (fNIRS) study. J. Psychiatr. Res. 2008, 42, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- Liddle, E.B.; Hollis, C.; Batty, M.J.; Groom, M.J.; Totman, J.J.; Liotti, M.; Scerif, G.; Liddle, P.F. Task-related default mode network modulation and inhibitory control in ADHD: Effects of motivation and methylphenidate. J. Child Psychol. Psychiatry 2011, 52, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Hugdahl, K.; Raichle, M.E.; Mitra, A.; Specht, K. On the existence of a generalized non-specific task-dependent network. Front. Hum. Neurosci. 2015, 9, 430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lijffijt, M.; Kenemans, J.L.; Verbaten, M.N.; van Engeland, H. A meta-analytic review of stopping performance in attention-deficit/hyperactivity disorder: Deficient inhibitory motor control? J. Abnorm. Psychol. 2005, 114, 216–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simmonds, D.J.; Pekar, J.J.; Mostofsky, S.H. Meta-analysis of Go/No-go tasks, demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia 2008, 46, 224–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slaats-Willemse, D.; Swaab-Barneveld, H.; de Sonneville, L.; van der Meulen, E.; Buitelaar, J. Deficient response inhibition as a cognitive endophenotype of ADHD. J. Am. Acad. Child Adolesc. Psychiatry 2003, 42, 1242–1248. [Google Scholar] [CrossRef] [PubMed]
- Wodka, E.L.; Mahone, E.M.; Blankner, J.G.; Larson, J.C.; Fotedar, S.; Denckla, M.B.; Mostofsky, S.H. Evidence that response inhibition is a primary deficit in ADHD. J. Clin. Exp. Neuropsychol. 2007, 29, 345–356. [Google Scholar] [CrossRef]
- Soderlund, G.; Sikstrom, S.; Smart, A. Listen to the noise: Noise is beneficial for cognitive performance in ADHD. J. Child Psychol. Psychiatry 2007, 48, 840–847. [Google Scholar] [CrossRef]
- Broadbent, D.E. The Effects of Noise on Behaviour; Pergamon Press, Inc.: New York, NY, USA, 1958. [Google Scholar]
- Smith, A. A Review of the Effects of Noise on Human-Performance. Scand. J. Psychol. 1989, 30, 185–206. [Google Scholar] [CrossRef]
- Moreno-Garcia, I.; Delgado-Pardo, G.; Roldan-Blasco, C. Attention and Response Control in ADHD. Evaluation through Integrated Visual and Auditory Continuous Performance Test. Span. J. Psychol. 2015, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, H.-Y.; Hsieh, H.-C.; Lee, P.; Hong, F.-Y.; Chang, W.-D.; Liu, K.-C. Auditory and Visual Attention Performance in Children With ADHD: The Attentional Deficiency of ADHD Is Modality Specific. J. Atten. Disord. 2014, 1, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Minagawa-Kawai, Y.; Naoi, N.; Kojima, S. Fundamentals of the NIRS System. In New Approach to Functional Neuroimaging: Near Infrared Spectroscopy; Keio University Press: Tokyo, Japan, 2009. [Google Scholar]
- Döpfner, M.; Görtz-Dorten, A.; Lehmkuhl, G.; Breuer, D.; Goletz, H. DISYPS-II. Diagnostik-System für psychische Störungen nach ICD-10 und DSM-IV für Kinder und Jugendliche-II; Manual. [DISYPS-II. Diagnostic assessment system for mental disorders in children and adolescents according to ICD-10 and DSM-IV, 2nd ed. manual]; Verlag Hans Huber: Bern, Germany, 2008. [Google Scholar]
- Achenbach, T.M.; Dumenci, L.; Rescorla, L.A. Ratings of Relations between DSM-IV Diagnostic Categories and Items of the CBCL/6-18, TRF, and YSR; University of Vermont: Burlington, VT, USA, 2001. [Google Scholar]
- International Organization for Standardization. Acoustics—Audiometric Test Methods—Part 1: Pure-Tone Air and Bone Conduction Audiometry; ISO 8253-1; International Organization for Standardization: Geneva, Switzerland, 2010. [Google Scholar]
- International Organization for Standardization. Acoustics—Audiometric Test Methods—Part 2: Sound Field Audiometry with Pure-Tone and Narrow-Band Test Signals; ISO 8253-2; International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- International Organization for Standardization. Acoustics—Audiometric Test Methods—Part 3: Speech Audiometry; ISO 8253-3; International Organization for Standardization: Geneva, Switzerland, 2012. [Google Scholar]
- EBU. EBU Recommendation: Loudness Normalisation and Permitted Maximum Level of Audio Signals; EBU-R128; EBU: Saconnex, Switzerland, 2014. [Google Scholar]
- Jasper, H. Report of the committee on methods of clinical examination in electroencephalography. Electroencephalogr. Clin. Neurophysiol. 1958, 10, 370–375. [Google Scholar]
- Tsuzuki, D.; Jurcak, V.; Singh, A.K.; Okamoto, M.; Watanabe, E.; Dan, I. Virtual spatial registration of stand-alone fNIRS data to MNI space. Neuroimage 2007, 34, 1506–1518. [Google Scholar] [CrossRef] [PubMed]
- Jichi Medical University. Results for Virtual Registration. Available online: http://www.jichi.ac.jp/brainlab/virtual_registration/Result3x5_E.html (accessed on 24 September 2018).
- Lloyd-Fox, S.; Blasi, A.; Volein, A.; Everdell, N.; Elwell, C.E.; Johnson, M.H. Social perception in infancy: A near infrared spectroscopy study. Child Dev. 2009, 80, 986–999. [Google Scholar] [CrossRef] [PubMed]
- Huppert, T.J.; Diamond, S.G.; Franceschini, M.A.; Boas, D.A. HomER: A review of time-series analysis methods for near-infrared spectroscopy of the brain. Appl. Opt. 2009, 48, D280–D298. [Google Scholar] [CrossRef] [Green Version]
- Tak, S.; Uga, M.; Flandin, G.; Dan, I.; Penny, W.D. Sensor space group analysis for fNIRS data. J. Neurosci. Methods 2016, 264, 103–112. [Google Scholar] [CrossRef]
- Willcutt, E.G. The prevalence of DSM-IV attention-deficit/hyperactivity disorder: A meta-analytic review. Neurotherapeutics 2012, 9, 490–499. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Bates, D.; Machler, M.; Bolker, B.M.; Walker, S.C. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Jaeger, B. R2glmm: Computes R Squared for Mixed (Multilevel) Models, R package version 0.1; R Foundation for Statistical Computing: Vienna, Austria, 2017; Volume 2. [Google Scholar]
- Piepho, H.-P. An algorithm for a letter-based representation of all-pairwise comparisons. J. Comput. Graph. Stat. 2004, 13, 456–466. [Google Scholar] [CrossRef]
- Curtin, J. lmSupport: Support for Linear Models, R package version 2.9.2; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Van Belle, J.; van Raalten, T.; Bos, D.J.; Zandbelt, B.B.; Oranje, B.; Durston, S. Capturing the dynamics of response variability in the brain in ADHD. Neuroimage Clin. 2015, 7, 132–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burbeck, S.L.; Luce, R.D. Evidence from auditory simple reaction times for both change and level detectors. Percept. Psychophys. 1982, 32, 117–133. [Google Scholar] [CrossRef]
- Massidda, D. Retimes: Reaction Time Analysis, R package version 0.1-2; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Cui, X. Handy Programs to Visualize NIRS Data (2): PlotTopoMap. Available online: http://www.alivelearn.net/?p=1300 (accessed on 3 September 2018).
- Dawes, P.; Bishop, D.V. Maturation of visual and auditory temporal processing in school-aged children. J. Speech Lang. Hear. Res. 2008, 51, 1002–1015. [Google Scholar] [CrossRef]
- Günther, T.; Konrad, K.; Häusler, J.; Saghraoui, H.; Willmes, K.; Sturm, W. Developmental differences in visual and auditory attention: A cross-sectional study. Z. Neuropsychol. 2014. [Google Scholar] [CrossRef]
- Friedrich, J.; Beste, C. The impact of stimulus modality on the processing of conflicting sensory information during response inhibition. Neuroscience 2019, 410, 191–201. [Google Scholar] [CrossRef]
- Hagmann, P.; Cammoun, L.; Gigandet, X.; Meuli, R.; Honey, C.J.; Wedeen, V.; Sporns, O. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008, 6, 1479–1493. [Google Scholar] [CrossRef]
- Achard, S.; Salvador, R.; Whitcher, B.; Suckling, J.; Bullmore, E.T. A resilient, low-frequency, small-world human brain functional network with highly connected association cortical hubs. J. Neurosci. 2006, 26, 63–72. [Google Scholar] [CrossRef]
- Deng, Y.Q.; Choi, I.; Shinn-Cunningham, B. Topographic specificity of alpha power during auditory spatial attention. Neuroimage 2020, 207. [Google Scholar] [CrossRef]
- Inoue, Y.; Sakihara, K.; Gunji, A.; Ozawa, H.; Kimiya, S.; Shinoda, H.; Kaga, M.; Inagaki, M. Reduced prefrontal hemodynamic response in children with ADHD during the Go/NoGo task: A NIRS study. Neuroreport 2012, 23, 55–60. [Google Scholar] [CrossRef]
- Janssen, T.W.; Heslenfeld, D.J.; van Mourik, R.; Logan, G.D.; Oosterlaan, J. Neural correlates of response inhibition in children with attention-deficit/hyperactivity disorder: A controlled version of the stop-signal task. Psychiatry Res. 2015, 233, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Xiao, Z.; Ke, X.Y.; Hong, S.S.; Yang, H.Y.; Su, Y.L.; Chu, K.K.; Xiao, X.; Shen, J.Y.; Liu, Y.J. Response Inhibition Impairment in High Functioning Autism and Attention Deficit Hyperactivity Disorder: Evidence from Near-Infrared Spectroscopy Data. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Monden, Y.; Dan, I.; Nagashima, M.; Dan, H.; Uga, M.; Ikeda, T.; Tsuzuki, D.; Kyutoku, Y.; Gunji, Y.; Hirano, D.; et al. Individual classification of ADHD children by right prefrontal hemodynamic responses during a go/no-go task as assessed by fNIRS. Neuroimage Clin. 2015, 9, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aron, A.R.; Robbins, T.W.; Poldrack, R.A. Inhibition and the right inferior frontal cortex: One decade on. Trends Cogn. Sci. 2014, 18, 177–185. [Google Scholar] [CrossRef]
- Mostofsky, S.H.; Simmonds, D.J. Response inhibition and response selection: Two sides of the same coin. J. Cogn. Neurosci. 2008, 20, 751–761. [Google Scholar] [CrossRef]
- Murray, M.M.; Camen, C.; Andino, S.L.G.; Bovet, P.; Clarke, S. Rapid brain discrimination of sounds of objects. J. Neurosci. 2006, 26, 1293–1302. [Google Scholar] [CrossRef]
- Ng, A.W.; Chan, A.H. Finger Response Times to Visual, Auditory and Tactile Modality Stimuli. In Proceedings of International Multiconference of Engineers and Computer Scientists, Hong Kong, China, 14–16 March 2018; pp. 1449–1454. [Google Scholar]
- Shelton, J.; Kumar, G.P. Comparison between auditory and visual simple reaction times. Neurosci. Med. 2010, 1, 30–32. [Google Scholar] [CrossRef] [Green Version]
- SanMiguel, I.; Widmann, A.; Bendixen, A.; Trujillo-Barreto, N.; Schroger, E. Hearing Silences: Human Auditory Processing Relies on Preactivation of Sound-Specific Brain Activity Patterns. J. Neurosci. 2013, 33, 8633–8639. [Google Scholar] [CrossRef] [Green Version]
- Lambertz, N.; Gizewski, E.R.; de Greiff, A.; Forsting, M. Cross-modal plasticity in deaf subjects dependent on the extent of hearing loss. Cogn. Brain Res. 2005, 25, 884–890. [Google Scholar] [CrossRef]




| HA | ADHD | TD | Group Comparison | Post hoc | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| M | (SD) | M | (SD) | M | (SD) | χ2/F | p | ||||
| Behavioral analyses analysis Behavioral behavioral task | n | n = 15 | n = 20 | n = 27 | |||||||
| Age | 9.23 | (1.93) | 10.25 | (2.01) | 9.87 | (1.77) | F(2,59) = 1.25 | 0.29 | |||
| CBCL Inattention | 59.50 | (6.43) | 64.00 | (7.81) | 52.85 | (4.70) | F(2,57) = 18.27 | <0.001 | TD < HA < ADHD | ||
| FBB-HKS Total | 61.83 | (26.84) | 77.24 | (23.49) | 42.05 | (29.91) | F(2,47) = 7.99 | 0.001 | TD < ADHD (and TD < HA (padj = 0.07)) | ||
| FBB-HKS Inattention | 72.29 | (22.82) | 85.20 | (19.93) | 48.29 | (30.06) | F(2,52) = 11.51 | <0.001 | TD < HA and TD < ADHD | ||
| FBB-HKS Hyperactivity | 69.75 | (18.23) | 76.16 | (21.51) | 49.62 | (20.10) | F(2,49) = 9.22 | <0.001 | TD < HA and TD < ADHD | ||
| FBB-HKS Impulsivity | 57.80 | (22.07) | 75.40 | (23.18) | 45.67 | (26.43) | F(2,53) = 7.79 | 0.001 | TD < ADHD (and HA < ADHD (padj = 0.06)) | ||
| Gender (m:f) | 5:10 | 14:6 | 17:10 | χ2(2, n = 62) = 5.20 | 0.07 | ||||||
| Neural analyses | n | n = 12 | n = 15 | n = 15 | |||||||
| Age | 9.11 | (1.73) | 10.12 | (2.07) | 10.06 | (1.84) | F(2,39) = 1.31 | 0.33 | |||
| Inattention CBCL (t) | 60.25 | (6.62) | 66.53 | (7.20) | 52.07 | (3.54) | F(2,38) = 21.05 | <0.001 | TD < HA < ADHD | ||
| FBB-HKS Total | 63.30 | (25.67) | 82.92 | (19.47) | 40.73 | (30.69) | F(2,31) = 8.27 | 0.001 | TD < ADHD (and TD < HA (p = 0.07) < ADHD (p = 0.07)) | ||
| FBB-HKS Inattention | 72.64 | (22.75) | 89.87 | (12.87) | 49.27 | (32.08) | F(2,34) = 10.00 | 0.001 | TD < ADHD and TD < HA (and HA < ADHD (p = 0.07)) | ||
| FBB-HKS Hyperactivity | 70.40 | (17.33) | 81.00 | (20.77) | 47.00 | (18.11) | F(2,33) = 10.21 | <0.001 | TD < HA and TD < ADHD | ||
| FBB-HKS Impulsivity | 54.83 | (23.36) | 82.67 | (21.19) | 49.27 | (26.61) | F(2,35) = 7.79 | 0.002 | TD < ADHD and HA < ADHD | ||
| Gender (m:f) | 5:7 | 9:6 | 10:5 | χ2(2, n = 62) = 5.20 | 0.07 | ||||||
| Variable | Modality | HA (n = 15) | ADHD (n = 20) | TD (n = 27) | Group Comparison | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | F | padjusted | Effect Size (R2/η2) | Post hoc (padjusted, d) | ||
| FA rate | Visual | 37.22 | 15.06 | 39.17 | 17.54 | 29.63 | 16.56 | F(2, 58) = 3.64 | 0.03 | 0.11 | ADHD > TD (0.07, −0.63) |
| (%) | Auditory | 20.56 | 24.17 | 17.92 | 18.79 | 9.57 | 13.22 | HA > TD (0.06, −0.85) | |||
| RTgo | Visual | 432.48 | 89.86 | 436.64 | 77.99 | 436.46 | 69.15 | F < 1 | - | - | - |
| Auditory | 611.62 | 125.49 | 576.05 | 96.51 | 636.91 | 69.55 | F(2, 58) = 3.81 | 0.03 (0.06) | 0.12 | ADHD < TD (0.02, 0.81) | |
| SDRTgo | Visual | 121.88 | 63.23 | 141.70 | 79.31 | 110.58 | 41.89 | F(2, 58) = 3.20 | 0.048 | 0.10 | ADHD > TD (0.06, −0.86) |
| Auditory | 187.75 | 64.24 | 184.25 | 70.92 | 143.77 | 42.88 | |||||
| Mu | Visual | 319.37 | 70.58 | 304.18 | 59.24 | 348.42 | 85.05 | F(2, 58) = 6.43 | 0.003 | 0.18 | ADHD < TD (0.003, 1.07) |
| Auditory | 457.86 | 122.61 | 424.08 | 119.11 | 538.66 | 91.90 | HA < TD (0.05, 0.75) | ||||
| Sigma | Visual | 43.52 | 28.36 | 38.65 | 26.38 | 52.15 | 26.28 | F < 1 | - | - | - |
| Auditory | 92.99 | 56.28 | 79.61 | 47.59 | 82.46 | 39.75 | |||||
| Tau | Visual | 106.77 | 59.29 | 126.46 | 76.67 | 82.50 | 43.28 | F(2, 58) = 4.59 | 0.01 | 0.14 | ADHD > TD (0.02, −0.99) HA > TD (0.05, −0.85) |
| Auditory | 152.96 | 92.27 | 152.38 | 80.21 | 100.53 | 52.85 | |||||
| Channel | Brain Region | Statistics | Direction of Effect(s) |
|---|---|---|---|
| Condition effect | |||
| P1 Ch6 | left DLPFC | F(1, 31) = 5.28, p = 0.03, R2 = 0.15 | visual > auditory |
| P1 Ch7 | left DLPFC | F(1, 37) = 7.48, p = 0.01, R2 = 0.17 | visual > auditory |
| P2 Ch13 | right frontopolar cortex | F(1, 36) = 5.19, p = 0.03, R2 = 0.13 | visual > auditory |
| P1 Ch12 | left pre/SMA | F(1, 39) = 4.65, p = 0.04, R2 = 0.11 | auditory > visual |
| P1 Ch16 | left pars opercularis | F(1, 36) = 4.93, p = 0.03, R2 = 0.12 | auditory > visual |
| P1 Ch20 | left IPFG | F(1, 32) = 6.40, p = 0.02, R2 = 0.17 | auditory > visual |
| P2 Ch16 | right STG | F(1, 36) = 7.88, p = 0.008, R2 = 0.18 | auditory > visual |
| Group effect | |||
| P2 Ch1 | right supramarginal gyrus | F(2, 34) = 3.65, p = 0.04, R2 = 0.18 | ADHD > TD (padj = 0.03, d = −1.59) |
| P2 Ch 5 | right supramarginal gyrus | F(2, 33) = 5.01, p = 0.01, R2 = 0.23 | ADHD > TD (padj = 0.01, d = −1.49), ADHD > HA (padj = 0.06, d = −1.27) |
| P2 Ch 6 | right primary somatosensory cortex | F(2, 38) = 3.94, p = 0.03, R2 = 0.17 | ADHD > TD (padj = 0.03, d = −1.22) |
| Condition × Group effect | |||
| P1 Ch9 | left supramarginal gyrus | F(2, 35) = 3.32, p = 0.048, R2 = 0.16 | group effect in the auditory condition (F(2, 35) = 3.60, p = 0.04, padj = 0.07, η2 = 0.17) ADHD > TD (padj = 0.048, d = −0.86) ADHD > HA (padj = 0.048, d = −1.01) |
| P2 Ch21 | right temporopolar cortex | F(2, 33) = 6.31, p = 0.005, R2 = 0.28 | condition effect for the HA group: auditory > visual (p = 0.004, padj = 0.01, R2 = 0.66); condition effect for the ADHD group:auditory < visual (p = 0.04, padj = 0.06, R2 = 0.33) |
| Channel | Brain Region | Statistics | Direction of Effect(s) |
|---|---|---|---|
| Condition effect | |||
| P1 Ch7 | left DLPFC | F(1, 37) = 7.90, p = 0.008, R2 = 0.18 | auditory < visual |
| P2 Ch14 | right STG | F(1, 37) = 4.34, p = 0.04, R2 = 0.11 | auditory < visual |
| P2 Ch16 | right STG | F(1, 36) = 4.88, p = 0.03, R2 = 0.12 | auditory < visual |
| P2 Ch20 | right MTG | F(1, 38) = 10.57, p = 0.002, R2 = 0.22 | auditory < visual |
| Group effect | |||
| P1 Ch8 | left primary somatosensory cortex | F(2, 37) = 4.88, p = 0.01, R2 = 0.21 | ADHD > HA (padj = 0.01, d = −1.40) |
| P1 Ch16 | left pars opercularis | F(2, 36) = 6.79, p = 0.003, R2 = 0.27 | ADHD > HA (padj = 0.004, d = −1.64), ADHD > TD (padj = 0.01, d = −1.21) |
| P1 Ch17 | left STG | F(2, 39) = 4.62, p = 0.02, R2 = 0.19 | ADHD > HA (padj = 0.02, d = −1.04), ADHD > TD (padj = 0.02, d = −0.98) |
| P2 Ch2 | right pre/SMA | F(2, 36) = 3.87, p = 0.03, R2 = 0.18 | ADHD > HA (padj = 0.03, d = −1.28) |
| P2 Ch17 | right IPFG | F(2, 32) = 3.41, p = 0.045, R2 = 0.18 | ADHD > TD (padj = 0.05, d = −0.18), HA < TD (padj = 0.05, d = 1.10) |
| Condition × Group effect | |||
| P2 Ch3 | right DLPFC | F(2, 31) = 4.13, p = 0.03, R2 = 0.21 | group effect in the auditory condition (F(2, 31) = 6.55, p = 0.004, padj = 0.008, η2 = 0.19): ADHD > HA (padj = 0.008, d = −1.47), ADHD > TD (padj = 0.01, d = −1.11) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bell, L.; Scharke, W.; Reindl, V.; Fels, J.; Neuschaefer-Rube, C.; Konrad, K. Auditory and Visual Response Inhibition in Children with Bilateral Hearing Aids and Children with ADHD. Brain Sci. 2020, 10, 307. https://doi.org/10.3390/brainsci10050307
Bell L, Scharke W, Reindl V, Fels J, Neuschaefer-Rube C, Konrad K. Auditory and Visual Response Inhibition in Children with Bilateral Hearing Aids and Children with ADHD. Brain Sciences. 2020; 10(5):307. https://doi.org/10.3390/brainsci10050307
Chicago/Turabian StyleBell, Laura, Wolfgang Scharke, Vanessa Reindl, Janina Fels, Christiane Neuschaefer-Rube, and Kerstin Konrad. 2020. "Auditory and Visual Response Inhibition in Children with Bilateral Hearing Aids and Children with ADHD" Brain Sciences 10, no. 5: 307. https://doi.org/10.3390/brainsci10050307
APA StyleBell, L., Scharke, W., Reindl, V., Fels, J., Neuschaefer-Rube, C., & Konrad, K. (2020). Auditory and Visual Response Inhibition in Children with Bilateral Hearing Aids and Children with ADHD. Brain Sciences, 10(5), 307. https://doi.org/10.3390/brainsci10050307