Concentrations of Essential Trace Metals in the Brain of Animal Species—A Comparative Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Brain Tissues
2.2.1. Preparation of Brain Homogenate
2.2.2. ICP-MS Measurement
2.3. Statistics
3. Results
3.1. Fixation of Tissue Alters Trace Metal Levels in Brain Tissue
3.2. Trace Metal Levels in Brain Tissue of Vertebrates
3.3. Trace Metal Levels in Brain Tissue of Invertebrates
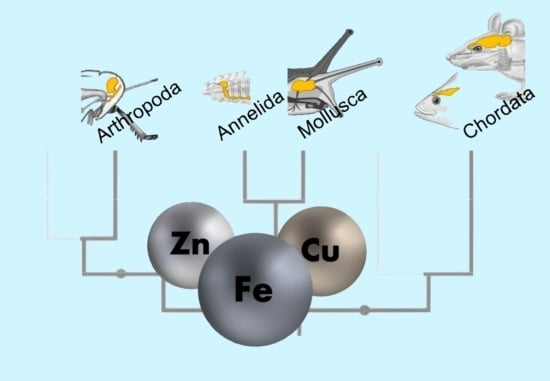
3.4. The Trace Element Levels in Brain Tissue Across All Phyla Indicate Active Species-Specific Regulation
3.5. The Trace Metals Levels in Brain Tissue Are Not Correlated with the Brain Weight
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Trace Elements in Human Nutrition and Health; World Health Organization: Geneva, Switzerland, 1996. [Google Scholar]
- Mehri, A. Trace Elements in Human Nutrition (II)—An Update. Int. J. Prev. Med. 2020, 11, 2. [Google Scholar] [PubMed]
- Kobayashi, M.; Shimizu, S. Cobalt proteins. Eur. J. Biochem. 1999, 261, 1–9. [Google Scholar] [CrossRef]
- Warren, M.J.; Raux, E.; Schubert, H.L.; Escalante-Semerena, J.C. The biosynthesis of adenosylcobalamin (vitamin B12). Nat. Prod. Rep. 2002, 19, 390–412. [Google Scholar] [CrossRef]
- Banerjee, R.; Ragsdale, S.W. The many faces of vitamin B12: Catalysis by cobalamin-dependent Enzymes. Annu. Rev. Biochem. 2003, 72, 209–247. [Google Scholar] [CrossRef]
- De Renzo, E.C.; Heytler, P.G.; Kaleita, E. Further evidence that molybdenum is a co-factor of xanthine oxidase. Arch. Biochem. Biophys. 1954, 49, 242–244. [Google Scholar] [CrossRef]
- Mahler, H.R.; Mackler, B.; Green, D.E.; Bock, R.M. Studies on metalloflavoproteins III. Aldehyde oxidase: A molybdoflavoprotein. J. Biol. Chem. 1954, 210, 465–480. [Google Scholar]
- Nicholas, D.J.; Nason, A.; McLeroy, W.D. Molybdenum and nitrate reductase. Ind. J. Biol. Chem. 1954, 207, 241–251. [Google Scholar]
- Ramos, P.; Santos, A.; Pinto, N.R.; Mendes, R.; Magalhães, T.; Almeida, A. Iron levels in the human brain: A post-mortem study of anatomical region differences and age-related changes. J. Trace Elem. Med. Biol. 2014, 28, 13–17. [Google Scholar] [CrossRef]
- Ramos, P.; Santos, A.; Pinto, N.R.; Mendes, R.; Magalhães, T.; Almeida, A. Anatomical region differences and age-related changes in copper, zinc, and manganese levels in the human brain. Biol. Trace Elem. Res. 2014, 161, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The Physiological, Biochemical, and Molecular Roles of Zinc Transporters in Zinc Homeostasis and Metabolism. Physiol. Rev. 2015, 95, 749–784. [Google Scholar] [CrossRef] [PubMed]
- Kardos, J.; Héja, L.; Simon, Á.; Jablonkai, I.; Kovács, R.; Jemnitz, K. Copper signalling: Causes and consequences. Cell Commun. Signal. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yehuda, S.; Youdim, M.B. Brain iron: A lesson from animal models. Am. J. Clin. Nutr. 1989, 50, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Eisenstein, R.S. Iron regulatory proteins and the molecular control of mammalian iron metabolism. Annu. Rev. Nutr. 2000, 20, 627–662. [Google Scholar] [CrossRef] [PubMed]
- Wigglesworth, J.M. Iron dependent enzymes in the brain. In Brain Iron: Neurochemical and Behavioral Aspects; Taylor and Francis: Abingdon, UK, 1998; pp. 25–66. [Google Scholar]
- Kwik-Uribe, C.L.; Gietzen, D.; German, J.B.; Golub, M.S.; Keen, C.L. Chronic marginal iron intakes during early development in mice result in persistent changes in dopamine metabolism and myelin composition. J. Nutr. 2000, 130, 2821–2830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larkin, E.C.; Ananda Rao, G. Importance of fetal and neonatal iron: Adequacy for normal development of central nervous system. In Brain, Behaviour, and Iron in the Infant Diet; Dobbing, J., Ed.; Springer: London, UK, 1990; pp. 43–57. [Google Scholar]
- Clardy, S.L.; Wang, X.; Zhao, W.; Liu, W.; Chase, G.A.; Beard, J.L.; Felt, B.T.; Connor, J.R. Acute and chronic effects of developmental iron deficiency on mRNA expression patterns in the brain. In Oxidative Stress and Neuroprotection; Parvez, H., Riederer, P., Eds.; Springer: Vienna, Austria, 2006; pp. 173–196. [Google Scholar]
- Dallman, P.R. Biochemical basis for the manifestations of iron deficiency. Annu. Rev. Nutr. 1986, 6, 13–40. [Google Scholar] [CrossRef]
- Georgieff, M.K. The role of iron in neurodevelopment: Fetal iron deficiency and the developing hippocampus. Biochem. Soc. Trans. 2008, 36, 1267–1271. [Google Scholar] [CrossRef] [Green Version]
- Bredholt, M.; Frederiksen, J.L. Zinc in multiple sclerosis: A systematic review and meta-analysis. ASN Neuro 2016, 8, 1759091416651511. [Google Scholar] [CrossRef] [PubMed]
- Tapiero, H.; Tew, K.D. Trace elements in human physiology and pathology: Zinc and metallothioneins. Biomed. Pharmacother. 2003, 57, 399–411. [Google Scholar] [CrossRef]
- Prasad, A.S. Clinical, immunological, anti-inflammatory and antioxidant roles of zinc. Exp. Gerontol. 2008, 43, 370–377. [Google Scholar] [CrossRef]
- Levenson, C.W.; Morris, D. Zinc and neurogenesis: Making new neurons from development to adulthood. Adv. Nutr. 2011, 2, 96–100. [Google Scholar] [CrossRef]
- Fukada, T.; Yamasaki, S.; Nishida, K.; Murakami, M.; Hirano, T. Zinc homeostasis and signaling in health and diseases. JBIC J. Biol. Inorg. Chem. 2011, 16, 1123–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeda, A. Zinc homeostasis and functions of zinc in the brain. Biometals 2001, 14, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S. Impact of the discovery of human zinc deficiency on health. J. Am. Coll. Nutr. 2009, 28, 257–265. [Google Scholar] [CrossRef] [PubMed]
- DiGirolamo, A.M.; Ramirez-Zea, M. Role of zinc in maternal and child mental health. Am. J. Clin. Nutr. 2009, 89, 940S–945S. [Google Scholar] [CrossRef] [PubMed]
- Peña, M.M.; Lee, J.; Thiele, D.J. A delicate balance: Homeostatic control of copper uptake and distribution. J. Nutr. 1999, 129, 1251–1260. [Google Scholar] [CrossRef] [Green Version]
- Rucker, R.B.; Kosonen, T.; Clegg, M.S.; Mitchell, A.E.; Rucker, B.R.; Uriu-Hare, J.Y.; Keen, C.L. Copper, lysyl oxidase, and extracellular matrix protein cross-linking. Am. J. Clin. Nutr. 1998, 67, 996S–1002S. [Google Scholar] [CrossRef] [Green Version]
- Kaler, S.G. Diagnosis and therapy of Menkes syndrome, a genetic form of copper deficiency. Am. J. Clin. Nutr. 1998, 67, 1029S–1034S. [Google Scholar] [CrossRef] [Green Version]
- Kodama, H.; Fujisawa, C. Copper metabolism and inherited copper transport disorders: Molecular mechanisms, screening, and treatment. Metallomics 2009, 1, 42–52. [Google Scholar] [CrossRef]
- Yasuda, H.; Yoshida, K.; Yasuda, Y.; Tsutsui, T. Infantile zinc deficiency: Association with autism spectrum disorders. Sci. Rep. 2011, 1, 129. [Google Scholar] [CrossRef]
- Grabrucker, A.M. Environmental factors in autism. Front. Psychiatry 2013, 3, 118. [Google Scholar] [CrossRef] [Green Version]
- Bjørklund, G. The role of zinc and copper in autism spectrum disorders. Acta Neurobiol. Exp. (Wars) 2013, 73, 225–236. [Google Scholar]
- Pal, A.; Prasad, R. Regional distribution of copper, zinc and iron in brain of Wistar rat model for non-Wilsonian brain copper toxicosis. Indian J. Clin. Biochem. 2016, 31, 93–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yakimoskii, A.F.; Shantyr, I.I.; Vlasenko, M.A.; Yakovleva, M.V. Effects of Acyzol on Zinc Content in Rat Brain and Blood Plasma. Bull. Exp. Biol. Med. 2017, 162, 293–294. [Google Scholar] [CrossRef] [PubMed]
- Takeda, A.; Takefuta, S.; Okada, S.; Oku, N. Relationship between brain zinc and transient learning impairment of adult rats fed zinc-deficient diet. Brain Res. 2000, 859, 352–357. [Google Scholar] [CrossRef]
- Fayed, A.H.A. Brain trace element concentration of rats treated with the plant alkaloid, vincamine. Biol. Trace Elem. Res. 2010, 136, 314–319. [Google Scholar] [CrossRef]
- Knauer, B.; Majka, P.; Watkins, K.J.; Taylor, A.W.R.; Malamanova, D.; Paul, B.; Yu, H.H.; Bush, A.I.; Hare, D.J.; Reser, D.H. Whole-brain metallomic analysis of the common marmoset (Callithrix jacchus). Metallomics 2017, 9, 411–423. [Google Scholar] [CrossRef] [Green Version]
- Portbury, S.D.; Hare, D.J.; Bishop, D.P.; Finkelstein, D.I.; Doble, P.A.; Adlard, P.A. Trehalose elevates brain zinc levels following controlled cortical impact in a mouse model of traumatic brain injury. Metallomics 2018, 10, 846–853. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Jing, X.P.; Zhang, S.P.; Gu, R.X.; Tang, F.X.; Wang, X.L.; Xiong, Y.; Qiu, M.; Sun, X.Y.; Ke, D.; et al. High dose zinc supplementation induces hippocampal zinc deficiency and memory impairment with inhibition of BDNF signaling. PLoS ONE 2013, 8, e55384. [Google Scholar] [CrossRef]
- Paul, B.; Hare, D.J.; Bishop, D.P.; Paton, C.; Cole, N.; Niedwiecki, M.M.; Andreozzi, E.; Vais, A.; Billings, J.L.; Bray, L.; et al. Visualising mouse neuroanatomy and function by metal distribution using laser ablation-inductively coupled plasma-mass spectrometry imaging. Chem. Sci. 2015, 6, 5383–5393. [Google Scholar] [CrossRef] [Green Version]
- Matusch, A.; Depboylu, C.; Palm, C.; Wu, B.; Höglinger, G.U.; Schäfer, M.K.H.; Becker, J.S. Cerebral bioimaging of Cu, Fe, Zn, and Mn in the MPTP mouse model of Parkinson’s disease using laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS). J. Am. Soc. Mass Spectrom. 2010, 21, 161–171. [Google Scholar] [CrossRef] [Green Version]
- Bakirdere, S.; Kizilkan, N.; Yaman, M. Determination of zinc, copper, iron, and manganese in different regions of lamb brain. Biol. Trace Elem. Res. 2011, 142, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Krebs, N.; Langkammer, C.; Goessler, W.; Ropele, S.; Fazekas, F.; Yen, K.; Scheurer, E. Assessment of trace elements in human brain using inductively coupled plasma mass spectrometry. J. Trace Elem. Med. Biol. 2014, 28, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Höck, A.; Demmel, U.; Schicha, H.; Kasperek, K.; Feinendegen, L.E. Trace element concentration in human brain: Activation analysis of cobalt, iron, rubidium, selenium, zinc, chromium, silver, cesium, antimony and scandium. Brain 1975, 98, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.S.; Zoriy, M.V.; Pickhardt, C.; Palomero-Gallagher, N.; Zilles, K. Imaging of copper, zinc, and other elements in thin section of human brain samples (hippocampus) by laser ablation inductively coupled plasma mass spectrometry. Anal. Chem. 2005, 77, 3208–3216. [Google Scholar] [CrossRef] [Green Version]
- Rajan, M.T.; Rao, K.J.; Mamatha, B.M.; Rao, R.V.; Shanmugavelu, P.; Menon, R.B.; Pavithran, M.V. Quantification of trace elements in normal human brain by inductively coupled plasma atomic emission spectrometry. J. Neurol. Sci. 1997, 146, 153–166. [Google Scholar] [CrossRef]
- Andrasi, E.; Orosz, L.; Bezur, L.; Ernyei, L.; Molnár, Z. Normal human brain analysis. Microchem. J. 1995, 51, 99–105. [Google Scholar] [CrossRef]
- Vilella, A.; Belletti, D.; Sauer, A.K.; Hagmeyer, S.; Sarowar, T.; Masoni, M.; Stasiak, N.; Mulvihill, J.J.; Ruozi, B.; Forni, F.; et al. Reduced plaque size and inflammation in the APP23 mouse model for Alzheimer’s disease after chronic application of polymeric nanoparticles for CNS targeted zinc delivery. J. Trace Elem. Med. Biol. 2018, 49, 210–221. [Google Scholar] [CrossRef]
- Corona, C.; Masciopinto, F.; Silvestri, E.; Del Viscovo, A.; Lattanzio, R.; La Sorda, R.; Ciavardelli, D.; Goglia, F.; Piantelli, M.; Canzoniero, L.M.T.; et al. Dietary zinc supplementation of 3xTg-AD mice increases BDNF levels and prevents cognitive deficits as well as mitochondrial dysfunction. Cell Death Dis. 2010, 1, e91. [Google Scholar] [CrossRef]
- Fourie, C.; Vyas, Y.; Lee, K.; Jung, Y.; Garner, C.C.; Montgomery, J.M. Dietary Zinc Supplementation Prevents Autism Related Behaviors and Striatal Synaptic Dysfunction in Shank3 Exon 13–16 Mutant Mice. Front. Cell. Neurosci. 2018, 12, 374. [Google Scholar] [CrossRef] [Green Version]
- Cezar, L.C.; Kirsten, T.B.; da Fonseca, C.C.N.; de Lima, A.P.N.; Bernardi, M.M.; Felicio, L.F. Zinc as a therapy in a rat model of autism prenatally induced by valproic acid. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2018, 84, 173–180. [Google Scholar] [CrossRef]
- Kirsten, T.B.; Queiroz-Hazarbassanov, N.; Bernardi, M.M.; Felicio, L.F. Prenatal zinc prevents communication impairments and BDNF disturbance in a rat model of autism induced by prenatal lipopolysaccharide exposure. Life Sci. 2015, 130, 12–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Xie, X.; Smart, T.G. Modulation of long-term potentiation in rat hippocampal pyramidal neurons by zinc. Pflügers Arch. 1994, 427, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Pfaender, S.; Grabrucker, A.M. Characterization of biometal profiles in neurological disorders. Metallomics 2014, 6, 960–977. [Google Scholar] [CrossRef] [PubMed]
- Sauer, A.K.; Hagmeyer, S.; Grabrucker, A.M. Zinc Deficiency. In Nutritional Deficiency; Erekolu, P., Ed.; InTech Open Science: Riijeka, Croatia, 2016; pp. 23–46. [Google Scholar]
- Petrilli, M.A.; Kranz, T.M.; Kleinhaus, K.; Joe, P.; Getz, M.; Johnson, P.; Malaspina, D. The emerging role for zinc in depression and psychosis. Front. Pharmacol. 2017, 8, 414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, S.; Haratake, M.; Fuchigami, T.; Nakayama, M. Selenium in Seafood Materials. J. Health Sci. 2011, 57, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Lobanov, A.V.; Hatfield, D.L.; Gladyshev, V.N. Eukaryotic selenoproteins and selenoproteomes. Biochim. Biophys. Acta 2009, 1790, 1424–1428. [Google Scholar] [CrossRef] [Green Version]
- Stürzenbaum, S.R.; Kille, P.; Morgan, A.J. The identification, cloning and characterization of earthworm metallothionein. FEBS Lett. 1998, 431, 437–442. [Google Scholar] [CrossRef]
- Carlson, S.D.; Juang, J.L.; Hilgers, S.L.; Garment, M.B. Blood barriers of the insect. Annu. Rev. Entomol. 2000, 45, 151–174. [Google Scholar] [CrossRef]
- Van Holde, K.E.; Miller, K.I.; Decker, H. Hemocyanins and invertebrate evolution. J. Biol. Chem. 2001, 276, 15563–15566. [Google Scholar] [CrossRef] [Green Version]
- Keen, C.L.; Reinstein, N.H.; Goudey-Lefevre, J.; Lefevre, M.; Lönnerdal, B.; Schneeman, B.O.; Hurley, L.S. Effect of dietary copper and zinc levels on tissue copper, zinc, and iron in male rats. Biol. Trace Elem. Res. 1985, 8, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Urgast, D.S.; Hill, S.; Kwun, I.S.; Beattie, J.H.; Goenaga-Infante, H.; Feldmann, J. Zinc isotope ratio imaging of rat brain thin sections from stable isotope tracer studies by LA-MC-ICP-MS. Metallomics 2012, 4, 1057–1063. [Google Scholar] [CrossRef]
- Panayi, A.E.; Spyrou, N.M.; Ubertalli, L.C.; White, M.A.; Part, P. Determination of trace elements in porcine brain by inductively coupled plasma-mass spectrometry, electrothermal atomic absorption spectrometry, and instrumental neutron activation analysis. Biol. Trace Elem. Res. 1999, 71, 529–540. [Google Scholar] [CrossRef]
- Carpene, E.; Cattani, O.; Serrazanetti, G.P.; Fedrizzi, G.; Cortesi, P. Zinc and copper in fish from natural waters and rearing ponds in Northern Italy. J. Fish Biol. 1990, 37, 293–299. [Google Scholar] [CrossRef]
- Drozd, Ł.; Ziomek, M.; Szkucik, K.; Paszkiewicz, W.; Maćkowiak-Dryka, M.; Bełkot, Z.; Gondek, M. Selenium, copper, and zinc concentrations in the raw and processed meat of edible land snails harvested in Poland. J. Vet. Res. 2017, 61, 293–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Augustyniak, M.; Juchimiuk, J.; Przybyłowicz, W.J.; Mesjasz-Przybyłowicz, J.; Babczyńska, A.; Migula, P. Zinc-induced DNA damage and the distribution of metals in the brain of grasshoppers by the comet assay and micro-PIXE. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2006, 144, 242–251. [Google Scholar] [CrossRef]
- Zheng, J.; Goessler, W.; Geiszinger, A.; Kosmus, W.; Chen, B.; Zhuang, G.; Sui, G. Multi-element determination in earthworms with instrumental neutron activation analysis and inductively coupled plasma mass spectrometry: A comparison. J. Radioanal. Nucl. Chem. 1997, 223, 149–155. [Google Scholar] [CrossRef]
- Grabrucker, A.M.; Rowan, M.; Garner, C.C. Brain-delivery of zinc-ions as potential treatment for neurological diseases: Mini review. Drug Deliv. Lett. 2011, 1, 13–23. [Google Scholar]





| Reference | Species | Brain or Brain Region- Zinc mg/kg 1 | Brain or Brain Region- Iron mg/kg 1 | Brain or Brain Region- Copper mg/kg 1 | Method |
|---|---|---|---|---|---|
| [36] | Wistar Rat | Cortex 23.30 ± 0.91 | Cortex 28.87 ± 0.11 | Cortex 2.20 ± 0.06 | AAS |
| Striatum 25.34 ± 0.67 | Striatum. 25.14 ± 0.07 | Striatum 2.18 ± 0.07 | |||
| Cerebellum 32.93 ± 0.12 | Cerebellum 30.13 ± 0.16 | Cerebellum 2.69 ± 0.08 | |||
| [37] | Wistar Rat | Cerebellum 15.00 ± 5.50 | - | - | ICP-MS |
| [38] | Albino Rat | Cortex 11.67 ± 1.04 | - | - | AAS |
| Hippocamp. 12.33 ± 2.75 | - | - | |||
| Cerebellum 10.50 ± 3.78 | - | - | |||
| [39] | Wistar Rat | Whole brain 3.13 ± 0.07 | Whole brain 2.81 ± 0.10 | Whole Brain 1.79 ± 0.21 | AAS |
| [40] | Marmoset | Cortex 15.91 | Cortex 26.73 | - | LA-ICP-MS |
| Hippocampus 13.53 | Hippocampus 20.77 | - | |||
| Thalamus 14.62 | Thalamus 27.52 | - | |||
| [41] | Mouse | Whole brain 10−15 | - | - | LA-ICP-MS |
| [42] | Mouse | Hippocampus 30 | - | - | AAS |
| Cortex 16 | - | - | |||
| Whole brain 18 | - | - | |||
| [43] | Mouse | Hippocampus > 4 | Hippocampus 2–38 | Hippocampus 0–1 | LA-ICP-MS |
| [44] | Mouse | Cortex 8.3 ± 1.3 | Cortex 3.5 ± 0.2 | Cortex 1.7 ± 0.2 | LA-ICP-MS |
| Sub. Nigra 6.6 ± 1.5 | Sub. Nigra 4.2 ± 0.7 | Sub. Nigra 1.7 ± 0.3 | |||
| Striatum 7.3 ± 0.5 | Striatum 3.7 ± 0.5 | Striatum 1.6 ± 0.1 | |||
| [45] | Lamb | Prefrontal cort. 4.03 ± 0.37 | Prefrontal cort. 34.17 ± 1.43 | Prefrontal cort16.83 ± 0.82 | AAS |
| Hippocamp. 2.72 ± 0.30 | Hippocamp. 13.77 ± 0.72 | Hippocamp. 17.07 ± 0.55 | |||
| Thalamus 2.41 ± 0.25 | Thalamus 17.38 ± 0.68 | Thalamus 9.83 ± 0.51 | |||
| [46] | Human | Thalamus 12.3 ± 3.23 | Thalamus 49.0 ± 10.9 | Thalamus 4.83 ± 1.53 | ICP-MS |
| Sub. Nigra 14.6 ± 5.20 | Sub. Nigra 210.3 ± 74.6 | Sub. Nigra 16.45 ± 6.13 | |||
| Frontal cortex 12.2 ± 3.48 | Frontal cortex 27.4 ± 8.7 | Frontal cortex 3.94 ± 0.99 | |||
| Basal ganglia 12.3 ± 2.67 | Basal ganglia 147.1 ± 49.4 | Basal ganglia 6.50 ± 1.61 | |||
| [47] | Human | Hippocampus 9.63 ± 0.47 | Hippocampus 3.38 ± 0.19 | - | INAA |
| [48] | Human | Hippocampus 10 | - | Hippocampus 14 | LA-ICP-MS |
| [49] | Human | Hippocampus 6.5 ± 0.5 | Hippocampus 26.6 ± 7.6 | Hippocampus 4.0 ± 1.0 | ICP-AES |
| Cerebellum 8 ± 1 | Cerebellum 45.4 ± 19.3 | Cerebellum 7.4 ± 2.5 | |||
| [50] | Human | Hippocampus 64 ± 5–15 | Hippocampus 342 ± 5–15 | Hippocampus 65 ± 5–15 | ICP-AES |
| [10] | Human | Frontal cortex 62 ± 12 | - | Frontal cortex 21 ± 7 | ICP-MS |
| Hippocampus 70 ± 10 | - | Hippocampus 19 ± 9 | |||
| Cerebellum 36 ± 8 | - | Cerebellum 22 ± 7 |
| Species | Zn in Literature | Zn in This Study | Zn p-Value | Fe in Literature | Fe in This Study | Fe p-Value | Cu in Literature | Cu in This Study | Cu p-Value |
|---|---|---|---|---|---|---|---|---|---|
| Human [10,46,47,48,49,50] | 19.5 ± 21.30 | - | - | 14.99 ± 16.42 | - | - | 12.33 ± 7.64 | - | - |
| Pig [68] | 9.24 ± 1.70 | 12 ± 1.4 | <0.0001 | 58.78 ± 0.4 | 16 ± 2.8 | <0.0001 | 0.306 ± 1.51 | 3.5 ± 0.61 | 0.0315 |
| Mouse [41,42,44] | 15.25 ± 3.89 | 12 ± 1.2 | 0.2456 | 3.8 ± 0.36 | 13.67 ± 3.79 | <0.0001 | 1.67 ± 0.06 | 3.4 ± 0.2 | <0.0001 |
| Rat [36,37,38,39] | 17.49 ± 8.22 | 14 ± 1.9 | 0.5717 | 21.74 ± 12.80 | 16 ± 1.6 | 0.5615 | 2.22 ± 0.37 | 2.3 ± 0.22 | 0.7707 |
| Fish [69] | 12.76 ± 4.62 | 12 ± 0.76 | 0.6993 | - | 18 ± 2.6 | - | - | 1.6 ± 0.2 | - |
| Snail [70] | 19.81 ± 5.14 | 7 ± 0.65 | <0.0001 | - | 5.4 ± 0.8 | - | 14.8 ± 10.1 | 5.8 ± 0.3 | <0.0001 |
| Locust [71] | 89.0 ± 3.0 | 25 ± 1 | <0.0001 | 65 ± 2.0 | 24 ± 5.3 | <0.0001 | 16.3 ± 0.7 | 1.5 ± 1 | <0.0001 |
| Earthworm [72] | 284.0 ± 111.4 | 74 ± 1.4 | <0.0001 | 1383 ± 577.6 | 170 ± 13 | <0.0001 | 10.08 ± 1.429 | 3.5 ± 0.26 | <0.0001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
DeBenedictis, C.A.; Raab, A.; Ducie, E.; Howley, S.; Feldmann, J.; Grabrucker, A.M. Concentrations of Essential Trace Metals in the Brain of Animal Species—A Comparative Study. Brain Sci. 2020, 10, 460. https://doi.org/10.3390/brainsci10070460
DeBenedictis CA, Raab A, Ducie E, Howley S, Feldmann J, Grabrucker AM. Concentrations of Essential Trace Metals in the Brain of Animal Species—A Comparative Study. Brain Sciences. 2020; 10(7):460. https://doi.org/10.3390/brainsci10070460
Chicago/Turabian StyleDeBenedictis, Chiara Alessia, Andrea Raab, Ellen Ducie, Shauna Howley, Joerg Feldmann, and Andreas Martin Grabrucker. 2020. "Concentrations of Essential Trace Metals in the Brain of Animal Species—A Comparative Study" Brain Sciences 10, no. 7: 460. https://doi.org/10.3390/brainsci10070460
APA StyleDeBenedictis, C. A., Raab, A., Ducie, E., Howley, S., Feldmann, J., & Grabrucker, A. M. (2020). Concentrations of Essential Trace Metals in the Brain of Animal Species—A Comparative Study. Brain Sciences, 10(7), 460. https://doi.org/10.3390/brainsci10070460