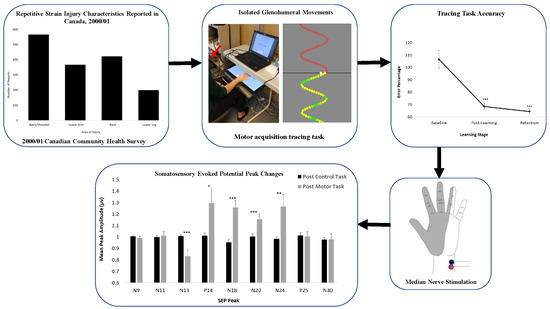
Proximal Upper Limb Sensorimotor Integration in Response to Novel Motor Skill Acquisition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Participants
2.3. Stimulation of the Median Nerve
2.4. SEPS Recording Parameters
2.5. Data Collection
2.6. Motor Acquisition Tracing Task Design
2.7. Motor Acquisition Task Intervention
2.8. Data Analysis
3. Results
3.1. Neurophysiological Data
3.1.1. N9 SEP Peak (Erb’s Point)
3.1.2. N13 SEP Peak (C5 Spinous Process)
3.1.3. P14 SEP Peak (Rossi Site)
3.1.4. N18 SEP Peak (Rossi Site)
3.1.5. N20 SEP Peak (Cc’)
3.1.6. N24 SEP Peak (Rossi Site)
3.2. Behavioural Data
4. Discussion
4.1. Neurophysiological Measures
4.1.1. N13 SEP Peak
4.1.2. P14 SEP Peak
4.1.3. N18 SEP Peak
4.1.4. N20 SEP Peak
4.1.5. N24 SEP Peak
4.1.6. N30 SEP Peak
4.2. Behavioural Measures
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Makin, T.R.; Bensmaia, S.J. Stability of Sensory Topographies in Adult Cortex. Trends Cogn. Sci. 2017, 21, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Byl, N.; Wilson, F.; Merzenich, M.; Melnick, M.; Scott, P.; Oakes, A.; McKenzie, A. Sensory Dysfunction Associated With Repetitive Strain Injuries of Tendinitis and Focal Hand Dystonia: A Comparative Study. J. Orthop. Sports Phys. Ther. 1996, 23, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Altenmüller, E.O.; Ioannou, C.I. Maladaptive Plasticity Induces Degradation of Fine Motor Skills in Musicians. Z. Psychol. 2016, 224, 80–90. [Google Scholar] [CrossRef]
- Tjepkema, M. Repetitive strain injury. Health Rep. 2003, 14, 11. [Google Scholar]
- Da Costa, B.R.; Vieira, E.R. Risk factors for work-related musculoskeletal disorders: A systematic review of recent longitudinal studies. Am. J. Ind. Med. 2009, 53, 285–323. [Google Scholar] [CrossRef] [PubMed]
- Sousa, N.; Mendes, R.; Monteiro, G.; Abrantes, C. Progressive resistance strength training and the related injuries in older adults: The susceptibility of the shoulder. Aging Clin. Exp. Res. 2013, 26, 235–240. [Google Scholar] [CrossRef]
- Linaker, C.H.; Walker-Bone, K. Shoulder disorders and occupation. Best Pract. Res. Clin. Rheumatol. 2015, 29, 405–423. [Google Scholar] [CrossRef] [Green Version]
- Andrew, D.; Haavik, H.; Dancey, E.; Yielder, P.; Murphy, B.A. Somatosensory evoked potentials show plastic changes following a novel motor training task with the thumb. Clin. Neurophysiol. 2015, 126, 575–580. [Google Scholar] [CrossRef]
- Andrew, D.; Yielder, P.; Murphy, B.A. Do pursuit movement tasks lead to differential changes in early somatosensory evoked potentials related to motor learning compared with typing tasks? J. Neurophysiol. 2015, 113, 1156–1164. [Google Scholar] [CrossRef] [Green Version]
- Haavik, H.; Murphy, B. Selective changes in cerebellar-cortical processing following motor training. Exp. Brain Res. 2013, 231, 397–403. [Google Scholar] [CrossRef]
- Woodworth, R.S.; Thorndike, E.L. The influence of improvement in one mental function upon the efficiency of other functions. (I). Psychol. Rev. 1901, 8, 247–261. [Google Scholar] [CrossRef] [Green Version]
- Sanes, J.N.; Donoghue, J.P. Plasticity and Primary Motor Cortex. Annu. Rev. Neurosci. 2000, 23, 393–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spampinato, C.; Palazzo, S.; Giordano, D.; Aldinucci, M.; Leonardi, R. Deep learning for automated skeletal bone age assessment in X-ray images. Med. Image Anal. 2017, 36, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Aune, T.K.; Aune, M.A.; Ingvaldsen, R.P.; Vereijken, B. Transfer of Motor Learning Is More Pronounced in Proximal Compared to Distal Effectors in Upper Extremities. Front. Psychol. 2017, 8, 1530. [Google Scholar] [CrossRef] [Green Version]
- Dancey, E.; Murphy, B.; Srbely, J.; Yielder, P. The effect of experimental pain on motor training performance and sensorimotor integration. Exp. Brain Res. 2014, 232, 2879–2889. [Google Scholar] [CrossRef] [Green Version]
- Altenmüller, E. Neurology of musical performance. Clin. Med. 2008, 8, 410–413. [Google Scholar] [CrossRef]
- Mutha, P.K.; Haaland, K.Y.; Sainburg, R.L. Rethinking Motor Lateralization: Specialized but Complementary Mechanisms for Motor Control of Each Arm. PLoS ONE 2013, 8, e58582. [Google Scholar] [CrossRef]
- Caplan, B.; Mendoza, J.E. Edinburgh Handedness Inventory. In Encyclopedia of Clinical Neuropsychology; Springer Science and Business Media LLC: Berlin, Germany, 2011; p. 928. [Google Scholar]
- Taylor, H.H.; Murphy, B.A. Cervical spine manipulation alters sensorimotor integration: A somatosensory evoked potential study. Clin. Neurophysiol. 2007, 118, 391–402. [Google Scholar] [CrossRef]
- Haavik, H.; Murphy, B.A. The role of spinal manipulation in addressing disordered sensorimotor integration and altered motor control. J. Electromyogr. Kinesiol. 2012, 22, 768–776. [Google Scholar] [CrossRef]
- Fujii, M.; Yamada, T.; Aihara, M.; Kokubun, Y.; Noguchi, Y.; Matsubara, M.; Yeh, M.H. The effects of stimulus rates upon median, ulnar and radial nerve somatosensory evoked potentials. Electroencephalogr. Clin. Neurophysiol. Potentials Sect. 1994, 92, 518–526. [Google Scholar] [CrossRef]
- Rossi, S.; Della Volpe, R.; Ginanneschi, F.; Ulivelli, M.; Bartalini, S.; Spidalieri, R.; Rossi, A. Early somatosensory processing during tonic muscle pain in humans: Relation to loss of proprioception and motor ‘defensive’ strategies. Clin. Neurophysiol. 2003, 114, 1351–1358. [Google Scholar] [CrossRef]
- Magill, R.A.; Hall, K.G. A review of the contextual interference effect in motor skill acquisition. Hum. Mov. Sci. 1990, 9, 241–289. [Google Scholar] [CrossRef]
- Holland, L.; Murphy, B.A.; Passmore, S.; Yielder, P. Time course of corticospinal excitability changes following a novel motor training task. Neurosci. Lett. 2015, 591, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Cruccu, G.; Aminoff, M.; Curio, G.; Guérit, J.; Kakigi, R.; Mauguière, F.; Rossini, P.; Treede, R.-D.; García-Larrea, L. Recommendations for the clinical use of somatosensory-evoked potentials. Clin. Neurophysiol. 2008, 119, 1705–1719. [Google Scholar] [CrossRef]
- Nuwer, M.R.; Comi, G.; Emerson, R.; Fuglsang-Frederiksen, A.; Guérit, J.M.; Hinrichs, H.; Ikeda, A.; Luccas, F.J.; Rappelsberger, P. IFCN standards for digital recording of clinical EEG. The International Federation of Clinical Neurophysiology. Electroencephalogr. Clin. Neurophysiol. Suppl. 1999, 52, 11. [Google Scholar]
- Desmedt, J.E.; Cheron, G. Non-cephalic reference recording of early somatosensory potentials to finger stimulation in adult or aging normal: Differentiation of widespread N18 and contralateral N20 from the prerolandic p22 and N30 components. Electroencephalogr. Clin. Neurophysiol. 1981, 52, 553–570. [Google Scholar] [CrossRef]
- Tinazzi, M.; Fiaschi, A.; Rosso, T.; Faccioli, F.; Grosslercher, J.; Aglioti, S.M. Neuroplastic Changes Related to Pain Occur at Multiple Levels of the Human Somatosensory System: A Somatosensory-Evoked Potentials Study in Patients with Cervical Radicular Pain. J. Neurosci. 2000, 20, 9277–9283. [Google Scholar] [CrossRef] [Green Version]
- Noel, P.; Ozaki, I.; Desmedt, J.E. Origin of N18 and P14 far-fields of median nerve somatosensory evoked potentials studied in patients with a brain-stem lesion. Electroencephalogr. Clin. Neurophysiol. 1996, 98, 167–170. [Google Scholar] [CrossRef]
- Sonoo, M. Anatomic Origin and Clinical Application of the Widespread N18 Potential in Median Nerve Somatosensory Evoked Potentials. J. Clin. Neurophysiol. 2000, 17, 258–268. [Google Scholar] [CrossRef]
- Sonoo, M.; Sakuta, M.; Shimpo, T.; Genba, K.; Mannen, T. Widespread N18 in median nerve SEP is preserved in a pontine lesion. Electroencephalogr. Clin. Neurophysiol. Potentials Sect. 1991, 80, 238–240. [Google Scholar] [CrossRef]
- Desmedt, J.E.; Cheron, G. Central somatosensory conduction in man: Neural generators and interpeak latencies of the far-field components recorded from neck and right or left scalp and earlobes. Electroencephalogr. Clin. Neurophysiol. 1980, 50, 382–403. [Google Scholar] [CrossRef]
- Desmedt, J.E.; Ozaki, I. SEPs to finger joint input lack the N20-P20 response that is evoked by tactile inputs: Contrast between cortical generators in areas 3b and 2 in humans. Electroencephalogr. Clin. Neurophysiol. Potentials Sect. 1991, 80, 513–521. [Google Scholar] [CrossRef]
- Restuccia, D.; Valeriani, M.; Barba, C.; Le Pera, D.; Capecci, M.; Filippini, V.; Molinari, M. Functional changes of the primary somatosensory cortex in patients with unilateral cerebellar lesions. Brain 2001, 124, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Restuccia, D.; Della Marca, G.; Valeriani, M.; Leggio, M.G.; Molinari, M. Cerebellar damage impairs detection of somatosensory input changes. A somatosensory mismatch-negativity study. Brain 2006, 130, 276–287. [Google Scholar] [CrossRef] [Green Version]
- Rossini, P.M.; Cracco, R.Q.; Cracco, J.B.; House, W.J. Short latency somatosensory evoked potentials to peroneal nerve stimulation: Scalp topography and the effect of different frequency filters. Electroencephalogr. Clin. Neurophysiol. 1981, 52, 540–552. [Google Scholar] [CrossRef]
- Akshoomoff, N.A.; Courchesne, E. A new role for the cerebellum in cognitive operations. Behav. Neurosci. 1992, 106, 731. [Google Scholar] [CrossRef]
- Ito, M. Mechanisms of motor learning in the cerebellum11Published on the World Wide Web on 24 November 2000. Brain Res. 2000, 886, 237–245. [Google Scholar] [CrossRef]
- Cebolla, A.; Cheron, G. Sensorimotor and cognitive involvement of the beta–gamma oscillation in the frontal N30 component of somatosensory evoked potentials. Neuropsychologia 2015, 79, 215–222. [Google Scholar] [CrossRef]
- Lelic, D.; Niazi, I.K.; Holt, K.; Jochumsen, M.; Dremstrup, K.; Yielder, P.; Murphy, B.; Drewes, A.M.; Haavik, H. Manipulation of Dysfunctional Spinal Joints Affects Sensorimotor Integration in the Prefrontal Cortex: A Brain Source Localization Study. Neural Plast. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Desrosiers, J.; Hébert, R.; Bravo, G.; Dutil, E. The Purdue Pegboard Test: Normative data for people aged 60 and over. Disabil. Rehabil. 1995, 17, 217–224. [Google Scholar] [CrossRef]
- Peters, M.; Servos, P.; Day, R. Marked sex differences on a fine motor skill task disappear when finger size is used as covariate. J. Appl. Psychol. 1990, 75, 87. [Google Scholar] [CrossRef] [PubMed]
- Newell, A.; Rosenbloom, P.S. Mechanisms of skill acquisition and the law of practice. Cogn. Ski. Their Acquis. 1981, 1, 1–55. [Google Scholar]
- Triccas, L.T.; Meyer, S.; Mantini, D.; Camilleri, K.P.; Falzon, O.; Camilleri, K.; Verheyden, G. A systematic review investigating the relationship of electroencephalography and magnetoencephalography measurements with sensorimotor upper limb impairments after stroke. J. Neurosci. Methods 2019, 311, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Witt, J.K.; Schuck, D.M.; Taylor, J.E.T. Action-specific effects underwater. Perception 2011, 40, 530–537. [Google Scholar] [CrossRef] [Green Version]
- Sonoo, M.; Genba-Shimizu, K.; Mannen, T.; Shimizu, T. Detailed analysis of the latencies of median nerve somatosensory evoked potential components, 2: Analysis of subcomponents of the P13/14 and N20 potentials. Electroencephalogr. Clin. Neurophysiol. Potentials Sect. 1997, 104, 296–311. [Google Scholar] [CrossRef]
- Brashers-Krug, T.; Shadmehr, R.; Bizzi, E. Consolidation in human motor memory. Nature 1996, 382, 252–255. [Google Scholar] [CrossRef]
- Joiner, W.M.; Smith, M.A. Long-term retention explained by a model of short-term learning in the adaptive control of reaching. J. Neurophysiol. 2008, 100, 2948–2955. [Google Scholar] [CrossRef] [Green Version]







| Male | Female | |||||
|---|---|---|---|---|---|---|
| Subject | Baseline | Post-Acquisition | Retention | Baseline | Post-Acquisition | Retention |
| 1 | 68.86 | 58.72 | 51.16 | 137.88 | 81.17 | 73.31 |
| 2 | 110.67 | 58.32 | 66.67 | 104.73 | 63.06 | 59.78 |
| 3 | 79.86 | 65.30 | 57.81 | 104.53 | 76.47 | 59.75 |
| 4 | 84.14 | 79.15 | 60.69 | 129.06 | 71.69 | 76.87 |
| 5 | 141.42 | 64.12 | 71.40 | 138.27 | 81.50 | 67.54 |
| 6 | 92.73 | 74.87 | 64.12 | 99.33 | 69.14 | 58.09 |
| Average | 96.28 | 66.75 | 61.98 | 118.97 | 73.84 | 65.89 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Brien, S.; Andrew, D.; Zabihhosseinian, M.; Yielder, P.; Murphy, B. Proximal Upper Limb Sensorimotor Integration in Response to Novel Motor Skill Acquisition. Brain Sci. 2020, 10, 581. https://doi.org/10.3390/brainsci10090581
O’Brien S, Andrew D, Zabihhosseinian M, Yielder P, Murphy B. Proximal Upper Limb Sensorimotor Integration in Response to Novel Motor Skill Acquisition. Brain Sciences. 2020; 10(9):581. https://doi.org/10.3390/brainsci10090581
Chicago/Turabian StyleO’Brien, Sinead, Danielle Andrew, Mahboobeh Zabihhosseinian, Paul Yielder, and Bernadette Murphy. 2020. "Proximal Upper Limb Sensorimotor Integration in Response to Novel Motor Skill Acquisition" Brain Sciences 10, no. 9: 581. https://doi.org/10.3390/brainsci10090581
APA StyleO’Brien, S., Andrew, D., Zabihhosseinian, M., Yielder, P., & Murphy, B. (2020). Proximal Upper Limb Sensorimotor Integration in Response to Novel Motor Skill Acquisition. Brain Sciences, 10(9), 581. https://doi.org/10.3390/brainsci10090581