Hydrogen-Generating Silica Material Prevents UVA-Ray-Induced Cellular Oxidative Stress, Cell Death, Collagen Loss and Melanogenesis in Human Cells and 3D Skin Equivalents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of ULH-002 Solution
2.2. Oxygen Radical Absorbance Capacity (ORAC) Assay
2.3. Cell Culture
2.4. Flow Cytometry
2.5. Three-Dimensional (3D) Culture of Skin Equivalents
2.5.1. Fibrinogen Gel-Based Method
2.5.2. Collagen Gel-Based Method
2.6. UVA Irradiation
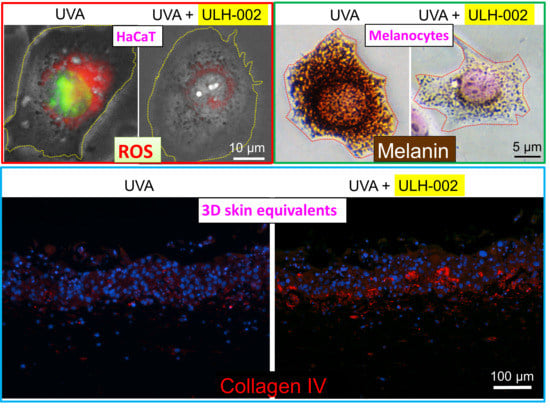
2.7. Cellular ROS Detection
2.8. Apoptosis Detection
2.9. Cell Viability Assay
2.10. Immunohistochemistry
2.11. Determination of Melanin Content
2.12. Statistical Analysis
3. Results
3.1. ULH-002 Has Excellent Antioxidant Capacity under Cell-Free Conditions and Decreases Intracellular ROS in HaCaT Keratinocytes and HGFs
3.2. Characterization of Human Gingival Fibroblasts
3.3. Effects of ULH-002 on UVA-Induced Cell Death in HGFs and HaCaT Cells
3.4. Effects of ULH-002 on UVA-Induced Collagen Synthesis Decline in HGFs
3.5. Effects of ULH-002 on UVA-Induced Collagen Synthesis Decline in 3D Skin Equivalents
3.6. Inhibitory Effect of ULH-002 on Melanin Synthesis in HMV-II Melanocytes and 3D Skin Equivalents
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative stress in aging human skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef] [Green Version]
- Kruk, J.; Duchnik, E. Oxidative stress and skin diseases: Possible role of physical activity. Asian Pac. J. Cancer Prev. 2014, 15, 561–568. [Google Scholar] [CrossRef] [PubMed]
- De Jager, T.L.; Cockrell, A.E.; Du Plessis, S.S. Ultraviolet light induced generation of reactive oxygen species. Adv. Exp. Med. Biol. 2017, 996, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, K. Tobacco smoke: Involvement of reactive oxygen species and stable free radicals in mechanisms of oxidative damage, carcinogenesis and synergistic effects with other respirable particles. Int. J. Environ. Res. Public Health 2009, 6, 445–462. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Quinzii, C.M.; López, L.C.; Gilkerson, R.W.; Dorado, B.; Coku, J.; Naini, A.B.; Lagier-Tourenne, C.; Schuelke, M.; Salviati, L.; Carrozzo, R.; et al. Reactive oxygen species, oxidative stress, and cell death correlate with level of CoQ10 deficiency. FASEB J. 2010, 24, 3733–3743. [Google Scholar] [CrossRef] [Green Version]
- Marionnet, C.; Pierrard, C.; Lejeune, F.; Sok, J.; Thomas, M.; Bernerd, F. Different oxidative stress response in keratinocytes and fibroblasts of reconstructed skin exposed to non extreme daily-ultraviolet radiation. PLoS ONE 2010, 5, e12059. [Google Scholar] [CrossRef] [Green Version]
- Denat, L.; Kadekaro, A.L.; Marrot, L.; Leachman, S.A.; Abdel-Malek, Z.A. Melanocytes as instigators and victims of oxidative stress. J. Investig. Dermatol. 2014, 134, 1512–1518. [Google Scholar] [CrossRef] [Green Version]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef]
- Hong, Y.; Chen, S.; Zhang, J.M. Hydrogen as a selective antioxidant: A review of clinical and experimental studies. J. Int. Med. Res. 2010, 38, 1893–1903. [Google Scholar] [CrossRef] [Green Version]
- Ohta, S. Molecular hydrogen is a novel antioxidant to efficiently reduce oxidative stress with potential for the improvement of mitochondrial diseases. Biochim. Biophys. Acta 2012, 1820, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Sim, M.; Kim, C.S.; Shon, W.J.; Lee, Y.K.; Choi, E.Y.; Shin, D.M. Hydrogen-rich water reduces inflammatory responses and prevents apoptosis of peripheral blood cells in healthy adults: A randomized, double-blind, controlled trial. Sci. Rep. 2020, 10, 12130. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Liao, F.; Fan, Y.; Miwa, N. Enzyme-Digested Colla Corii Asini (E’jiao) accelerates wound healing and prevents ultraviolet A-induced collagen synthesis decline and wrinkle formation in three-dimensional skin equivalents. Hum. Cell 2020, 33, 1056–1067. [Google Scholar] [CrossRef] [PubMed]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, T.; Seki, S.; Matsubara, O.; Ito, S.; Kasuga, T. Specific incorporation of 4-S-cysteinylphenol into human melanoma cells. J. Investig. Dermatol. 1988, 90, 725–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, L.; Matsubayashi, K.; Miwa, N. Inhibitory effect of the water-soluble polymer-wrapped derivative of fullerene on UVA-induced melanogenesis via downregulation of tyrosinase expression in human melanocytes and skin tissues. Arch. Dermatol. Res. 2007, 299, 245–257. [Google Scholar] [CrossRef]
- Mochizuki, M.; Nakahara, T. Establishment of xenogeneic serum-free culture methods for handling human dental pulp stem cells using clinically oriented in-vitro and in-vivo conditions. Stem Cell Res. Ther. 2018, 9, 25. [Google Scholar] [CrossRef] [Green Version]
- Mochizuki, M.; Sagara, H.; Nakahara, T. Type I collagen facilitates safe and reliable expansion of human dental pulp stem cells in xenogeneic serum-free culture. Stem Cell Res. Ther. 2020, 11, 267. [Google Scholar] [CrossRef]
- Xiao, L.; Liao, F.; Ide, R.; Horie, T.; Fan, Y.; Saiki, C.; Miwa, N. Enzyme-Digested Colla Corii Asini (E’jiao) prevents hydrogen peroxide-induced cell death and accelerates amyloid beta clearance in neuronal-like PC12 cells. Neural Regen. Res. 2020, 15, 2270–2272. [Google Scholar] [CrossRef]
- Xiao, L.; Sakagami, H.; Miwa, N. A New Method for Testing Filtration Efficiency of Mask Materials under Sneeze-Like Pressure. In Vivo 2020, 34, 1637–1644. [Google Scholar] [CrossRef]
- Xiao, L.; Miwa, N. Hydrogen-rich water achieves cytoprotection from oxidative stress injury in human gingival fibroblasts in culture or 3D-tissue equivalents, and wound-healing promotion, together with ROS-scavenging and relief from glutathione diminishment. Hum. Cell 2017, 30, 72–87. [Google Scholar] [CrossRef] [PubMed]
- Ebisawa, K.; Kato, R.; Okada, M.; Sugimura, T.; Latif, M.A.; Hori, Y.; Narita, Y.; Ueda, M.; Honda, H.; Kagami, H. Gingival and dermal fibroblasts: Their similarities and differences revealed from gene expression. J. Biosci. Bioeng. 2011, 111, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Scharffetter, K.; Wlaschek, M.; Hogg, A.; Bolsen, K.; Schothorst, A.; Goerz, G.; Krieg, T.; Plewig, G. UVA irradiation induces collagenase in human dermal fibroblasts in vitro and in vivo. Arch. Dermatol. Res. 1991, 283, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Boisnic, S.; Branchet-Gumila, M.C.; Le Charpentier, Y.; Segard, C. Repair of UVA-induced elastic fiber and collagen damage by 0.05% retinaldehyde cream in an ex vivo human skin model. Dermatology 1999, 199, 43–48. [Google Scholar] [CrossRef]
- Otto, A.I.; Riou, L.; Marionnet, C.; Mori, T.; Sarasin, A.; Magnaldo, T. Differential behaviors toward ultraviolet A and B radiation of fibroblasts and keratinocytes from normal and DNA-repair-deficient patients. Cancer Res. 1999, 59, 1212–1218. [Google Scholar]
- Sorrell, J.M.; Caplan, A.I. Fibroblast heterogeneity: More than skin deep. J. Cell Sci. 2004, 117, 667–675. [Google Scholar] [CrossRef] [Green Version]
- Lam, S.; van der Geest, R.N.; Verhagen, N.A.; Daha, M.R.; van Kooten, C. Secretion of collagen type IV by human renal fibroblasts is increased by high glucose via a TGF-β-independent pathway. Nephrol. Dial. Transplant. 2004, 19, 1694–1701. [Google Scholar] [CrossRef] [Green Version]
- Pöschl, E.; Schlötzer-Schrehardt, U.; Brachvogel, B.; Saito, K.; Ninomiya, Y.; Mayer, U. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development 2004, 131, 1619–1628. [Google Scholar] [CrossRef] [Green Version]
- Cadet, J.; Douki, T. Oxidatively generated damage to DNA by UVA radiation in cells and human skin. J. Investig. Dermatol. 2011, 131, 1005–1007. [Google Scholar] [CrossRef]
- Mouret, S.; Baudouin, C.; Charveron, M.; Favier, A.; Cadet, J.; Douki, T. Cyclobutane pyrimidine dimers are predominant DNA lesions in whole human skin exposed to UVA radiation. Proc. Natl. Acad. Sci. USA 2006, 103, 13765–13770. [Google Scholar] [CrossRef] [Green Version]
- Iuchi, K.; Imoto, A.; Kamimura, N.; Nishimaki, K.; Ichimiya, H.; Yokota, T.; Ohta, S. Molecular hydrogen regulates gene expression by modifying the free radical chain reaction-dependent generation of oxidized phospholipid mediators. Sci. Rep. 2016, 6, 18971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kajiyama, S.; Hasegawa, G.; Asano, M.; Hosoda, H.; Fukui, M.; Nakamura, N.; Kitawaki, J.; Imai, S.; Nakano, K.; Ohta, M.; et al. Supplementation of hydrogen-rich water improves lipid and glucose metabolism in patients with type 2 diabetes or impaired glucose tolerance. Nutr. Res. 2008, 28, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Shimouchi, A.; Nose, K.; Yamaguchi, M.; Ishiguro, H.; Kondo, T. Breath hydrogen produced by ingestion of commercial hydrogen water and milk. Biomark. Insights 2009, 4, 27–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardinal, J.S.; Zhan, J.; Wang, Y.; Sugimoto, R.; Tsung, A.; McCurry, K.R.; Billiar, T.R.; Nakao, A. Oral hydrogen water prevents chronic allograft nephropathy in rats. Kidney Int. 2010, 77, 101–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Tanaka, Y.; Miwa, N. Influence of hydrogen-occluding-silica on migration and apoptosis in human esophageal cells in vitro. Med. Gas Res. 2017, 7, 76–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaussain Miller, C.; Septier, D.; Bonnefoix, M.; Lecolle, S.; Lebreton-Decoster, C.; Coulomb, B.; Pellat, B.; Godeau, G. Human dermal and gingival fibroblasts in a three-dimensional culture: A comparative study on matrix remodeling. Clin. Oral Investig. 2002, 6, 39–50. [Google Scholar] [CrossRef]
- Lorimier, S.; Hornebeck, W.; Godeau, G.; Pellat, B.; Gillery, P.; Maquart, F.X.; Laurent-Maquin, D. Morphometric studies of collagen and fibrin lattices contracted by human gingival fibroblasts; comparison with dermal fibroblasts. J. Dent. Res. 1998, 77, 1717–1729. [Google Scholar] [CrossRef] [PubMed]
- Videira, I.F.; Moura, D.F.; Magina, S. Mechanisms regulating melanogenesis. An. Bras. Dermatol. 2013, 88, 76–83. [Google Scholar] [CrossRef] [Green Version]








| Material Composition | Amount | Origin | Gene Recombination | Allergen | BSE |
|---|---|---|---|---|---|
| Seaweed-derived calcium | 0.58% | France | N/A 1 | N/A | N/A |
| Perilla oil | 0.20% | Japan | N/A | N/A | N/A |
| Potassium carbonate | 42.00% | Japan | N/A | N/A | N/A |
| Sodium bicarbonate | 33.00% | Japan | N/A | N/A | N/A |
| Potassium citrate | 21.20% | Japan | N/A | N/A | N/A |
| Fine-grained silicon dioxide | Less than 3% | Korea | N/A | N/A | N/A |
| Magnesium sulfate | 0.02% | Korea | N/A | N/A | N/A |
| Hydrogen-occluded microporous silica | Over 0.01% | Japan | N/A | N/A | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, L.; Mochizuki, M.; Nakahara, T.; Miwa, N. Hydrogen-Generating Silica Material Prevents UVA-Ray-Induced Cellular Oxidative Stress, Cell Death, Collagen Loss and Melanogenesis in Human Cells and 3D Skin Equivalents. Antioxidants 2021, 10, 76. https://doi.org/10.3390/antiox10010076
Xiao L, Mochizuki M, Nakahara T, Miwa N. Hydrogen-Generating Silica Material Prevents UVA-Ray-Induced Cellular Oxidative Stress, Cell Death, Collagen Loss and Melanogenesis in Human Cells and 3D Skin Equivalents. Antioxidants. 2021; 10(1):76. https://doi.org/10.3390/antiox10010076
Chicago/Turabian StyleXiao, Li, Mai Mochizuki, Taka Nakahara, and Nobuhiko Miwa. 2021. "Hydrogen-Generating Silica Material Prevents UVA-Ray-Induced Cellular Oxidative Stress, Cell Death, Collagen Loss and Melanogenesis in Human Cells and 3D Skin Equivalents" Antioxidants 10, no. 1: 76. https://doi.org/10.3390/antiox10010076
APA StyleXiao, L., Mochizuki, M., Nakahara, T., & Miwa, N. (2021). Hydrogen-Generating Silica Material Prevents UVA-Ray-Induced Cellular Oxidative Stress, Cell Death, Collagen Loss and Melanogenesis in Human Cells and 3D Skin Equivalents. Antioxidants, 10(1), 76. https://doi.org/10.3390/antiox10010076