Improved Oxidative Stability and Sensory Quality of Beef Hamburgers Enriched with a Phenolic Extract from Olive Vegetation Water
Abstract
:1. Introduction
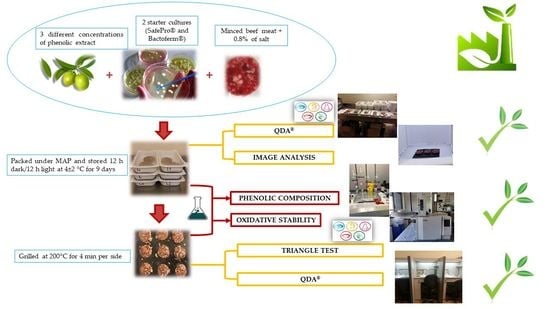
2. Materials and Methods
2.1. OMWW Phenol Extract
2.2. Preparation of Phenol-Enriched Burger Samples
2.3. Proximate Composition
2.4. Phenols Analysis
2.5. Chemical Analysis
2.5.1. Lipid Extraction
2.5.2. Determination of Main Lipid Classes
2.5.3. Determination of Total FA
2.5.4. Determination of Peroxide Value (PV)
2.5.5. Determination of Thiobarbituric Acid Reactive Substances (TBARs)
2.5.6. Determination of Cholesterol and its Oxides (COPs)
2.6. Physical Analysis
Image Analysis
2.7. Sensory Analysis
2.7.1. Descriptive Analysis
2.7.2. Discriminant Test
2.8. Statistical Analysis
3. Results and Discussion
3.1. Proximate Composition
3.2. Evolution of Phenolic Compounds
3.3. Chemical Analysis
3.3.1. Lipid Content and Main Lipid Classes
3.3.2. Total Fatty Acid Profile
3.3.3. Lipid Oxidation
3.3.4. Principal Component Analysis (PCA) of Chemical Data
3.4. Physical Analysis
Image Analysis
3.5. Sensory Analysis
3.5.1. Descriptive Analysis
3.5.2. Discriminant Test
3.5.3. Correlation between Sensory (QDA®) and Instrumental (Electronic Eye) Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Wang, Z.; Zhuang, H.; Nasiru, M.M.; Yuan, Y.; Zhang, J.; Yan, W. Changes in color, myoglobin, and lipid oxi-dation in beef patties treated by dielectric barrier discharge cold plasma during storage. Meat. Sci. 2021, 176, 108456. [Google Scholar] [CrossRef]
- Delgado, J.; Ansorena, D.; Van Hecke, T.; Astiasarán, I.; De Smet, S.; Estévez, M. Meat lipids, NaCl and carnitine: Do they unveil the conundrum of the association between red and processed meat intake and cardiovascular diseases? Invited Review. Meat Sci. 2021, 171, 108278. [Google Scholar] [CrossRef] [PubMed]
- Karwowska, M.; Kononiuk, A.; Wójciak, K.M. Impact of sodium nitrite reduction on lipid oxidation and antioxidant properties of cooked meat products. Antioxidants 2019, 9, 9. [Google Scholar] [CrossRef] [Green Version]
- International Agency for Research on Cancer (IARC). Red Meat and Processed Meat. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2018; Volume 114, pp. 1–511. [Google Scholar]
- Bouvard, V.; Loomis, D.; Guyton, K.Z.; Grosse, Y.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K.; Corpet, D.E. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015, 16, 1599–1600. [Google Scholar] [CrossRef] [Green Version]
- Testa, G.; Rossin, D.; Poli, G.; Biasi, F.; Leonarduzzi, G.M. Implication of oxysterols in chronic inflammatory human diseases. Biochimie 2018, 153, 220–231. [Google Scholar] [CrossRef]
- Duc, D.; Vigne, S.; Pot, C. Oxysterols in autoimmunity. Int. J. Mol. Sci. 2019, 20, 4522. [Google Scholar] [CrossRef] [Green Version]
- Zmysłowski, A.; Szterk, A. Oxysterols as a biomarker in diseases. Clin. Chim. Acta 2019, 491, 103–113. [Google Scholar] [CrossRef]
- Carpentieri, S.; Soltanipour, F.; Ferrari, G.; Pataro, G.; Donsì, F. Emerging green techniques for the extraction of antioxidants from agri-food by-products as promising ingredients for the food industry. Antioxidants 2021, 10, 1417. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Pulido, B.; Bas-Bellver, C.; Betoret, N.; Barrera, C.; Seguí, L. Valorization of vegetable fresh-processing residues as functional powdered ingredients. a review on the potential impact of pretreatments and drying methods on bioactive compounds and their bioaccessibility. Front. Sustain. Food Syst. 2021, 5, 82. [Google Scholar] [CrossRef]
- Carrara, M.; Kelly, M.T.; Roso, F.; Larroque, M.; Margout, D. Potential of olive oil mill wastewater as a source of poly-phenols for the treatment of skin disorders: A review. J. Agr. Food Chem. 2021, 69, 7268–7284. [Google Scholar] [CrossRef]
- Servili, M.; Sordini, B.; Esposto, S.; Urbani, S.; Veneziani, G.; Di Maio, I.; Selvaggini, R.; Taticchi, A. Biological activities of phenolic compounds of extra virgin olive oil. Antioxidants 2013, 3, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Veneziani, G.; Novelli, E.; Taticchi, A.; Servili, M. Application of recovered bioactive compounds in food products. In Olive Mill Waste Recent Advances for Sustainable Management; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 231–253. [Google Scholar]
- Servili, M.; Esposto, S.; Veneziani, G.; Urbani, S.; Taticchi, A.; Di Maio, I.; Selvaggini, R.; Sordini, B.; Montedoro, G.F. Improvement of bioactive phenol content in virgin olive oil with an olive-vegetation water concentrate produced by membrane treatment. Food Chem. 2011, 124, 1308–1315. [Google Scholar] [CrossRef]
- Galanakis, C.M. Phenols recovered from olive mill wastewater as additives in meat products. Trends Food Sci. Technol. 2018, 79, 98–105. [Google Scholar] [CrossRef]
- Caporaso, N.; Formisano, D.; Genovese, A. Use of phenolic compounds from olive mill wastewater as valuable ingredients for functional foods. Crit. Rev. Food Sci. Nutr. 2018, 58, 2829–2841. [Google Scholar] [CrossRef]
- Cui, M.; Chen, B.; Xu, K.; Rigakou, A.; Diamantakos, P.; Melliou, E.; Logothetis, D.E.; Magiatis, P. Activation of specific bitter taste receptors by olive oil phenolics and secoiridoids. Sci. Rep. 2021, 11, 1–11. [Google Scholar]
- De Toffoli, A.; Monteleone, E.; Bucalossi, G.; Veneziani, G.; Fia, G.; Servili, M.; Zanoni, B.; Pagliarini, E.; Gallina Toschi, T.; Dinnella, C. Sensory and chemical profile of a phenolic extract from olive mill waste waters in plant-base food with varied macro-composition. Food Res. Int. 2019, 119, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, S.; Bendini, A.; Valli, E.; Gallina Toschi, T. Do consumers recognize the positive sensorial attributes of extra virgin olive oils related with their composition? A case study on conventional and organic products. J. Food Compos. Anal. 2015, 44, 186–195. [Google Scholar] [CrossRef]
- Ianni, F.; Gagliardi, A.; Taticchi, A.; Servili, M.; Pinna, N.; Schoubben, A.; Sardella, R.; Bruscoli, S. Exploiting food-grade mesoporous silica to preserve the antioxidant properties of fresh olive mill wastewaters phenolic extracts. Antioxidants 2021, 10, 1361. [Google Scholar] [CrossRef] [PubMed]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 15th ed.; Helrich, K., Ed.; AOAC: Arlington, VA, USA, 1990; Volume 2. [Google Scholar]
- Selvaggini, R.; Esposto, S.; Taticchi, A.; Urbani, S.; Veneziani, G.; Di Maio, I.; Sordini, B.; Servili, M. Optimization of the temperature and oxygen concentration conditions in the malaxation during the oil mechanical extraction process of four Italian olive cultivars. J. Agric. Food Chem. 2014, 62, 3813–3822. [Google Scholar] [CrossRef]
- Miraglia, D.; Castrica, M.; Menchetti, L.; Esposto, S.; Branciari, R.; Ranucci, D.; Urbani, S.; Sordini, B.; Veneziani, G.; Servili, M. Effect of an olive vegetation water phenolic extract on the physico-chemical, microbiological and sensory traits of shrimp (Parapenaeus longirostris) during the shelf-life. Foods 2020, 9, 1647. [Google Scholar] [CrossRef]
- Boselli, E.; Velazco, V.; Caboni, M.F.; Lercker, G. Pressurized liquid extraction of lipids for the determination of oxysterols in egg-containing food. J. Chromatogr. A 2001, 917, 239–244. [Google Scholar] [CrossRef]
- Gallina Toschi, T.; Cardenia, V.; Bonaga, G.; Mandrioli, M.; Rodriguez-Estrada, M.T. Coffee silverskin: Characterization, possible uses, and safety aspects. J. Agric. Food Chem. 2014, 62, 10836–10844. [Google Scholar] [CrossRef]
- Luise, D.; Cardenia, V.; Zappaterra, M.; Motta, V.; Bosi, P.; Rodriguez-Estrada, M.T.; Trevisi, P. Evaluation of breed and parity order effects on the lipid composition of porcine colostrum. J. Agric. Food Chem. 2018, 66, 12911–12920. [Google Scholar] [CrossRef] [PubMed]
- Cardenia, V.; Massimini, M.; Poerio, A.; Venturini, M.C.; Rodriguez-Estrada, M.T.; Vecchia, P.; Lercker, G. Effect of dietary supplementation on lipid photooxidation in beef meat, during storage under commercial retail conditions. Meat Sci. 2015, 105, 126–135. [Google Scholar] [CrossRef]
- Ulbricht, T.L.; Southgate, D.A. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, S.G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kang, M.; Yong, H.I.; Bae, Y.S.; Jung, S.; Jo, C. Synergistic Effects of electron-beam irradiation and leek extract on the quality of pork jerky during ambient storage. Asian-Australas. J. Anim. Sci. 2013, 26, 596–602. [Google Scholar] [CrossRef]
- Tarladgis, B.G.; Watts, B.M.; Younathan, M.T.; Dugan, L., Jr. A distillation method for the quantitative determination of malondialdehyde in rancid foods. J. Amer. Oil. Chem. Soc. 1960, 37, 44–48. [Google Scholar] [CrossRef]
- Cardenia, V.; Rodriguez-Estrada, M.T.; Baldacci, E.; Savioli, S.; Lercker, G. Analysis of cholesterol oxidation products by Fast gas chromatography/mass spectrometry. J. Sep. Sci. 2012, 35, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, S.; Soglia, F.; Palagano, R.; Tesini, F.; Bendini, A.; Petracci, M.; Cavani, C.; Gallina Toschi, T. Sensory and rapid instrumental methods as a combined tool for quality control of cooked ham. Heliyon 2016, 2, e00202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meilgaard, M.C.; Civille, G.V.; Carr, B.T. Descriptive analysis techniques. In Sensory Evaluation Techniques; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Heymann, H.; King, E.; Hopfer, H. Classical descriptive analysis. In Novel Techniques in Sensory Characterization and Consumer Profiling; Varela, P., Ares, G., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 9–40. [Google Scholar]
- International Organization for Standardization (ISO). Sensory Analysis—Methodology—Triangle Test. BS EN ISO 4120:2007; British Standards Institution (BSI): London, UK, 2007. [Google Scholar]
- Servili, M.; Rizzello, C.G.; Taticchi, A.; Esposto, S.; Urbani, S.; Mazzacane, F.; Di Maio, I.; Selvaggini, R.; Gobbetti, M.; Di Cagno, R. Functional milk beverage fortified with phenolic compounds extracted from olive vegetation water, and fermented with functional lactic acid bacteria. Int. J. Food Microbiol. 2011, 147, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Esposto, S.; Taticchi, A.; Di Maio, I.; Urbani, S.; Veneziani, G.; Selvaggini, R.; Sordini, B.; Servili, M. Effect of an olive phenolic extract on the quality of vegetable oils during frying. Food Chem. 2015, 176, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Taticchi, A.; Esposto, S.; Urbani, S.; Veneziani, G.; Selvaggini, R.; Sordini, B.; Servili, M. Effect of an olive phenolic extract added to the oily phase of a tomato sauce, on the preservation of phenols and carotenoids during domestic cooking. LWT-Food Sci. Technol. 2017, 84, 572–578. [Google Scholar] [CrossRef]
- Balzan, S.; Taticchi, A.; Cardazzo, B.; Urbani, S.; Servili, M.; Di Lecce, G.; Zabalza, I.B.; Rodriguez-Estrada, M.T.; Novelli, E.; Fasolato, L. Effect of phenols extracted from a by-product of the oil mill on the shelf-life of raw and cooked fresh pork sausages in the absence of chemical additives. LWT-Food Sci. Technol. 2017, 85, 89–95. [Google Scholar] [CrossRef]
- Brenes, M.; García, A.; García, P.; Garrido, A. Acid hydrolysis of secoiridoid aglycons during storage of virgin olive oil. J. Agric. Food Chem. 2001, 49, 5609–5614. [Google Scholar] [CrossRef]
- Obied, H.K.; Prenzler, P.D.; Ryan, D.; Servili, M.; Taticchi, A.; Esposto, S.; Robards, K. Biosynthesis and biotransformations of phenol-conjugated oleosidic secoiridoids from Olea europaea L. Nat. Prod. Rep. 2008, 25, 1167–1179. [Google Scholar] [CrossRef] [PubMed]
- Di Maio, I.; Esposto, S.; Taticchi, A.; Selvaggini, R.; Veneziani, G.; Urbani, S.; Servili, M. HPLC–ESI-MS investigation of tyrosol and hydroxytyrosol oxidation products in virgin olive oil. Food Chem. 2011, 125, 21–28. [Google Scholar] [CrossRef]
- Menchetti, L.; Taticchi, A.; Esposto, S.; Servili, M.; Ranucci, D.; Branciari, R.; Miraglia, D. The influence of phenolic extract from olive vegetation water and storage temperature on the survival of Salmonella Enteritidis inoculated on mayonnaise. LWT-Food Sci. Technol. 2020, 129, 109648. [Google Scholar] [CrossRef]
- Lozano-Castellón, J.; Vallverdú-Queralt, A.; Rinaldi De Alvarenga, J.F.; Illán, M.; Torrado-Prat, X.; Lamuela-Raventós, R.M. Domestic sautéing with EVOO: Change in the phenolic profile. Antioxidants 2020, 9, 77. [Google Scholar] [CrossRef] [Green Version]
- Gruffat, D.; Bauchart, D.; Thomas, A.; Parafita, E.; Durand, D. Fatty acid composition and oxidation in beef muscles as affected by ageing times and cooking methods. Food Chem. 2021, 343, 128476. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: Nutritional implications for chronic diseases. Biomed. Pharm. 2006, 60, 502–507. [Google Scholar] [CrossRef]
- Ortuño, J.; Mateo, L.; Rodríguez-Estrada, M.; Bañón, S. Effects of sous vide vs grilling methods on lamb meat colour and lipid stability during cooking and heated display. Meat Sci. 2021, 171, 108287. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Carpena, J.-G.; Morcuende, D.; Petrón, M.J.; Estévez, M. Inhibition of cholesterol oxidation products (cops) formation in emulsified porcine patties by phenolic-rich avocado (Persea americana Mill.) extracts. J. Agric. Food Chem. 2012, 60, 2224–2230. [Google Scholar] [CrossRef]
- Gray, J.I.; Pearson, A.M. Rancidity and warmed-over flavor. In Advances in Meat Research, Volume 3: Restructured Meat and Poultry Products; Pearson, A.M., Dutson, T.R., Eds.; Van Nostrand Reinhold Co.: New York, NY, USA, 1987; pp. 221–270. [Google Scholar]
- Aouidi, F.; Okba, A.; Hamdi, M. Valorization of functional properties of extract and powder of olive leaves in raw and cooked minced beef meat. J. Sci. Food Agric. 2017, 97, 3195–3203. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, A.R.; Anwar, M.M.; Sallam, E.M. Improving quality and shelf-life of minced beef using irradiated olive leaf extract. J. Food Process. Preserv. 2018, 42, e13789. [Google Scholar] [CrossRef]
- Barriuso, B.; Ansorena, D.; Calvo, M.I.; Cavero, R.Y.; Astiasarán, I. Role of Melissa officinalis in cholesterol oxidation: Antioxidant effect in model systems and application in beef patties. Food Res. Int. 2015, 69, 133–140. [Google Scholar] [CrossRef]



| Protein | Ash | Moisture | Fat | ||
|---|---|---|---|---|---|
| Raw samples | (%) | ||||
| 0 days | |||||
| C | 22.16 ± 1.14 | 1.74 ± 0.14 | 70.23 ± 0.65 | 5.87 ± 0.94 | |
| L1 | 21.86 ± 1.27 | 1.68 ± 0.29 | 70.74 ± 0.80 | 5.73 ± 0.81 | |
| L2 | 23.29 ± 1.15 | 1.85 ± 0.14 | 69.87 ± 0.60 | 4.99 ± 1.06 | |
| 9 days | |||||
| C | 22.65 ± 1.10 | 1.74 ± 0.06 | 70.16 ± 0.43 | 5.44 ± 0.79 | |
| L1 | 22.46 ± 0.91 | 1.83 ± 0.05 | 70.42 ± 0.27 | 5.29 ± 0.92 | |
| L2 | 22.19 ± 1.09 | 1.84 ± 0.12 | 70.31 ± 0.34 | 5.66 ± 0.99 | |
| Factor | F value | ||||
| Form | 0.82 ns | 1.64 ns | 2.64 ns | 0.37 ns | |
| St | 0.00 ns | 0.91 ns | 0.01 ns | 0.04 ns | |
| Form*St | 2.17 ns | 1.06 ns | 1.49 ns | 1.42 ns | |
| 3,4-DHPEA | p-HPEA | VB | 3,4-DHPEA-EDA | Total Phenols | ||
|---|---|---|---|---|---|---|
| Raw samples | (mg/Kg) | |||||
| 0 days | ||||||
| C | - | - | - | - | - | |
| L1 | 29.37 ± 1.15 B,X | 4.46 ± 0.46 B,X | 10.30 ± 0.82 B,X | 40.83 ± 1,93 a,B,X | 84.96 ± 4.37 a,B,X | |
| L2 | 40.98 ± 0.20 b,A,X | 9.23 ± 0.04 A,X | 22.80 ± 0.21 a,A,X | 95.29 ± 1.61 a,A,X | 168.29 ± 2.06 a,A,X | |
| 6 days | ||||||
| C | - | - | - | - | - | |
| L1 | 36.53 ± 1.92 B,X | 3.81 ± 0.10 B,X | 9.12 ± 0.53 B,X | 6.60 ± 0.16 b,B,X | 56.05 ± 2.71 b,B,X | |
| L2 | 43.28 ± 0.56 b,A,X | 9.12 ± 0.02 A,X | 22.76 ± 0.10 a,A,X | 65.91 ± 3.00 b,A,X | 141.07 ± 3.69 b,A,X | |
| 9 days | ||||||
| C | - | - | - | - | - | |
| L1 | 32.08 ± 2.23 B,X | 3.32 ± 0.03 B,X | 9.79 ± 0.75 B,X | 2.82 ± 0.58 b,B,X | 48.01 ± 3.58 b,B,X | |
| L2 | 48.87 ± 1.82 a,A,X | 8.82 ± 0.29 A,X | 20.80 ± 0.53 b,A,X | 28.37 ± 1.43 c,A,X | 106.86 ± 4.07 c,A,X | |
| Grilled samples | 0 days | |||||
| C | - | - | - | - | - | |
| L1 | 11.48 ± 0.87 a,B,Y | 3.35 ± 0.40 a,B,Y | 5.46 ± 0.49 B,Y | ndY | 20.29 ± 1.75 a,B,Y | |
| L2 | 19.31 ± 0.90 a,A,Y | 8.14 ± 0.27 a,A,Y | 21.96 ± 0.65 a,A,Y | ndY | 49.42 ± 1.82 a,A,Y | |
| 6 days | ||||||
| C | - | - | - | - | - | |
| L1 | 4.56 ± 0.40 b,B,Y | 2.46 ± 0.28 ab,B,Y | 4.46 ± 0.17 B,Y | ndY | 11.48 ± 0.85 b,B,Y | |
| L2 | 8.92 ± 1.29 b,A,Y | 7.27 ± 0.06 a,A,Y | 21.95 ± 0.87 a,A,Y | ndY | 38.14 ± 2.23 b,A,Y | |
| 9 days | ||||||
| C | - | - | - | - | - | |
| L1 | ndc,B,Y | 1.38 ± 0.28 b,B,Y | 4.57 ± 0.17 B,Y | ndY | 5.95 ± 0.45 c,B,Y | |
| L2 | 1.31 ± 0.30 c,A,Y | 4.66 ± 0.37 b,A,Y | 15.68 ± 0.68 b,A,Y | ndY | 21.65 ± 1.34 c,A,Y | |
| Factor | F value | |||||
| Form | 2449.72 *** | 4043.62 *** | 6493.57 *** | 3262.06 *** | 4755.42 *** | |
| St | 31.62 *** | 89.59 *** | 40.84 *** | 944.93 *** | 338.66 *** | |
| Gr | 4031.08 *** | 314.37 *** | 244.21 *** | 6484.39 *** | 4794.17 *** | |
| Form*St | 13.18 *** | 24.79 *** | 36.62 *** | 320.75 *** | 108.76 *** | |
| Form*Gr | 1048.99 *** | 91.57 *** | 86.15 *** | 3262.06 *** | 1613.62 *** | |
| St*Gr | 146.39 *** | 29.44 *** | 12.08 *** | 944.93 *** | 55.62 *** | |
| Form*St*Gr | 50.22 *** | 14.75 *** | 8.84 *** | 320.75 *** | 17.07 *** | |
| Lipid Content | FFA | MAG | STE | DAG | E-STE | TAG | ||
|---|---|---|---|---|---|---|---|---|
| (%) | (% of Total Lipids) | |||||||
| Raw samples | 0 days | |||||||
| C | 4.93 ± 0.60 Y | 5.87 ± 0.21 X | 0.26 ± 0.01 X | 1.87 ± 0.44 | 4.26 ± 0.79 | 2.01 ± 0.05 | 85.62 ± 1.18 | |
| L1 | 6.33 ± 0.53 | 4.15 ± 0.70 | 0.13 ± 0.01 | 1.10 ± 0.12 | 3.50 ± 0.50 Y | 1.18 ± 0.64 Y | 89.87 ± 1.01 X | |
| L2 | 5.29 ± 0.45 Y | 3.51 ± 0.45 | 0.10 ± 0.02 | 1.42 ± 0.38 | 4.20 ± 0.59 | 2.12 ± 0.07 | 88.58 ± 1.25 | |
| 6 days | ||||||||
| C | 4.98 ± 0.30 Y | 6.12 ± 0.54 | 0.24 ± 0.03 X | 1.88 ± 0.45 | 4.18 ± 1.01 | 1.68 ± 0.57 | 85.80 ± 1.45 | |
| L1 | 5.13 ± 1.02 Y | 5.50 ± 1.28 | 0.16 ± 0.06 | 1.28 ± 0.36 | 4.49 ± 0.76 | 2.24 ± 0.12 | 86.26 ± 1.91 | |
| L2 | 5.39 ± 0.62 Y | 3.10 ± 0.21 Y | 0.08 ± 0.02 | 1.35 ± 0.15 | 4.51 ± 0.54 | 2.41 ± 0.26 | 88.49 ± 0.46 X | |
| 9 days | ||||||||
| C | 5.04 ± 0.35 Y | 5.55 ± 0.12 | 0.18 ± 0.01 | 1.70 ± 0.06 | 3.83 ± 0.12 | 2.00 ± 0.02 | 86.68 ± 0.17 | |
| L1 | 5.29 ± 0.45 Y | 4.36 ± 0.74 | 0.11 ± 0.00 | 1.14 ± 0.16 | 4.34 ± 0.66 | 2.19 ± 0.02 | 87.79 ± 0.07 | |
| L2 | 6.06 ± 0.18 Y | 5.13 ± 0.56 X | 0.15 ± 0.01 X | 1.13 ± 0.15 | 4.64 ± 0.24 | 2.28 ± 0.01 X | 86.61 ± 0.76 | |
| Grilled samples | 0 days | |||||||
| C | 6.12 ± 0.47 X | 5.38 ± 0.21 Y | 0.19 ± 0.02 Y | 1.97 ± 0.22 | 4.81 ± 0.63 | 2.00 ± 0.07 | 85.57 ± 0.91 | |
| L1 | 6.15 ± 0.22 | 3.56 ± 0.55 | 0.10 ± 0.03 | 1.66 ± 0.27 | 4.84 ± 0.33 X | 2.20 ± 0.19 X | 87.57 ± 0.57 Y | |
| L2 | 6.21 ± 0.10 X | 4.21 ± 0.39 | 0.13 ± 0.02 | 1.56 ± 0.10 | 5.97 ± 0.26 | 2.11 ± 0.23 | 86.86 ± 0.63 | |
| 6 days | ||||||||
| C | 7.09 ± 0.35 X | 6.06 ± 0.37 | 0.18 ± 0.01 Y | 1.65 ± 0.10 | 4.29 ± 0.20 | 1.95 ± 0.31 | 85.79 ± 0.28 | |
| L1 | 6.47 ± 0.90 X | 4.98 ± 0.32 | 0.10 ± 0.04 | 1.66 ± 0.38 | 5.98 ± 0.88 | 2.13 ± 0.12 | 87.08 ± 1.42 | |
| L2 | 6.66 ± 1.15 X | 4.07 ± 0.24 X | 0.11 ± 0.01 | 1.69 ± 0.22 | 5.24 ± 0.51 | 2.18 ± 0.08 | 86.75 ± 0.88 Y | |
| 9 days | ||||||||
| C | 7.49 ± 1.02 X | 5.24 ± 1.03 | 0.17 ± 0.04 | 1.72 ± 0.60 | 4.37 ± 0.99 | 1.79 ± 0.57 | 86.65 ± 2.00 | |
| L1 | 6.47 ± 0.74 X | 3.76 ± 0.32 | 0.10 ± 0.01 | 1.67 ± 0.33 | 4.87 ± 0.46 | 2.06 ± 0.08 | 87.42 ± 0.51 | |
| L2 | 6.89 ± 1.22 X | 3.85 ± 0.24 Y | 0.10 ± 0.01 Y | 1.44 ± 0.33 | 4.45 ± 0.52 | 2.09 ± 0.06 Y | 88.02 ± 1.16 | |
| Factor | F value | |||||||
| Form | 0.30 ns | 46.62 *** | 58.68 *** | 8.30 ** | 1.32 ns | 3.66 * | 11.52 *** | |
| St | 1.05 ns | 2.34 ns | 1.15 ns | 0.82 ns | 0.52 ns | 0.53 ns | 1.81 ns | |
| Gr | 35.04 *** | 3.59 ns | 11.10 ** | 5.90 * | 6.89 * | 0.01 ns | 1.35 ns | |
| Form*St | 0.72 ns | 4.03 ** | 3.21 * | 0.35 ns | 0.74 ns | 1.56 ns | 1.78 ns | |
| Form*Gr | 2.03 ns | 3.07 ns | 4.01 * | 3.68 * | 0.77 ns | 1.74 ns | 0.84 ns | |
| St*Gr | 2.35 ns | 2.05 ns | 0.24 ns | 0.21 ns | 0.33 ns | 2.14 ns | 1.78 ns | |
| Form*St*Gr | 0.34 ns | 3.45 * | 4.58 ** | 0.08 ns | 0.84 ns | 1.91 ns | 2.52 ns | |
| SFA | MUFA | PUFA | n-3 | n-6 | ||
|---|---|---|---|---|---|---|
| Raw samples | (% Total Fatty Acids) | |||||
| 0 days | ||||||
| C | 38.95 ± 1.22 | 57.40 ± 1.13 | 3.65 ± 0.14 | 0.68 ± 0.10 | 2.39 ± 0.21 | |
| L1 | 40.13 ± 0.56 | 55.81 ± 0.50 | 4.06 ± 0.55 a | 0.56 ± 0.18 | 2.80 ± 0.46 | |
| L2 | 38.42 ± 1.42 | 57.56 ± 0.39 | 4.01 ± 1.26 | 0.71 ± 0.16 | 2.50 ± 0.90 | |
| 6 days | ||||||
| C | 36.90 ± 3.79 B | 58.94 ± 3.09 | 4.17 ± 0.95 | 0.65 ± 0.15 | 2.89 ± 0.82 A | |
| L1 | 40.59 ± 1.59 A | 57.58 ± 1.29 | 4.82 ± 0.52 a | 0.73 ± 0.06 | 2.99 ± 0.48 B | |
| L2 | 40.01 ± 3.16 B | 57.71 ± 2.60 | 4.27 ± 0.58 | 0.58 ± 0.03 | 2.43 ± 0.40 AB,Y | |
| 9 days | ||||||
| C | 39.35 ± 1.36 | 56.67 ± 1.64 | 3.98 ± 0.37 | 0.61 ± 0.07 | 2.66 ± 0.44 | |
| L1 | 41.67 ± 0.67 | 56.84 ± 0.85 | 3.50 ± 0.19 b,Y | 0.65 ± 0.17 | 2.77 ± 0.30 Y | |
| L2 | 38.30 ± 0.85 Y | 58.64 ± 1.49 Y | 3.07 ± 1.49 | 0.53 ± 0.01 X | 2.01 ± 1.10 | |
| Grilled samples | 0 days | |||||
| C | 39.70 ± 1.32 | 56.37 ± 0.99 | 3.93 ± 0.95 | 0.64 ± 0.16 | 2.81 ± 0.77 | |
| L1 | 42.49 ± 3.78 | 55.12 ± 1.78 | 2.27 ± 2.32 | 0.51 ± 0.26 | 2.70 ± 1.77 | |
| L2 | 39.26 ± 0.43 b | 57.44 ± 0.91 | 2.82 ± 0.49 | 0.52 ± 0.08 | 2.12 ± 0.49 | |
| 6 days | ||||||
| C | 39.55 ± 2.51 | 56.87 ± 1.97 | 3.58 ± 0.86 | 0.48 ± 0.05 | 2.78 ± 0.75 | |
| L1 | 40.10 ± 1.92 | 55.10 ± 3.34 | 3.12 ± 0.95 | 0.62 ± 0.21 | 2.19 ± 0.62 | |
| L2 | 39.73 ± 0.53 b | 57.33 ± 0.63 | 2.94 ± 0.14 | 0.43 ± 0.11 | 2.32 ± 0.23 X | |
| 9 days | ||||||
| C | 37.80 ± 4.39 | 57.83 ± 3.67 | 4.00 ± 0.16 A | 0.59 ± 0.18 | 2.99 ± 0.28 | |
| L1 | 39.70 ± 2.54 | 56.99 ± 2.09 | 3.31 ± 0.55 AB,X | 0.57 ± 0.23 | 2.40 ± 0.15 X | |
| L2 | 41.18 ± 0.21 a,X | 56.14 ± 0.27 X | 2.69 ±0.38 B | 0.41 ± 0.04 Y | 2.17 ± 0.42 | |
| Factor | F value | |||||
| Form | 2.96 ns | 1.19 ns | 10.08 *** | 1.70 ns | 10.16 *** | |
| St | 0.04 ns | 0.40 ns | 1.88 ns | 0.07 ns | 1.28 ns | |
| Gr | 0.39 ns | 2.37 ns | 0.35 ns | 7.20 * | 4.94 * | |
| Form*St | 0.20 ns | 0.18 ns | 0.50 ns | 2.16 ns | 0.64 ns | |
| Form*Gr | 0.29 ns | 0.10 ns | 0.36 ns | 0.93 ns | 0.21 ns | |
| St*Gr | 0.24 ns | 0.56 ns | 1.80 ns | 0.20 ns | 1.89 ns | |
| Form*St*Gr | 1.61 ns | 0.88 ns | 4.48 ** | 1.09 ns | 3.32 * | |
| n-6/n-3 | UFA/SFA | PUFA/SFA | AI | TI | ||
|---|---|---|---|---|---|---|
| Raw samples | 0 days | |||||
| C | 3.59 ± 0.80 | 1.57 ± 0.08 | 0.09 ± 0.01 | 0.58 ± 0.01 | 1.17 ± 0.06 | |
| L1 | 5.45 ± 2.00 a | 1.49 ± 0.03 | 0.10 ± 0.01 a | 0.60 ± 0.01 | 1.24 ± 0.04 | |
| L2 | 3.69 ± 1.80 | 1.60 ± 0.09 | 0.11 ± 0.04 | 0.56 ± 0.03 | 1.14 ± 0.07 | |
| 6 days | ||||||
| C | 4.59 ± 1.44 A | 1.73 ± 0.29 | 0.12 ± 0.04 A | 0.53 ± 0.08 | 1.08 ± 0.18 | |
| L1 | 4.09 ± 0.81 b,B | 1.47 ± 0.09 | 0.05 ± 0.01 b,B | 0.63 ± 0.04 | 1.22 ± 0.05 | |
| L2 | 4.18 ± 0.68 AB,Y | 1.51 ± 0.19 | 0.06 ± 0.02 AB | 0.62 ± 0.06 | 1.22 ± 0.15 | |
| 9 days | ||||||
| C | 4.42 ± 1.17 | 1.54 ± 0.09 | 0.10 ± 0.01 | 0.58 ± 0.02 | 1.19 ± 0.07 | |
| L1 | 4.26 ± 0.85 b,Y | 1.40 ± 0.04 | 0.04 ± 0.00 b | 0.66 ± 0.01 | 1.27 ± 0.06 | |
| L2 | 3.77 ± 2.04 | 1.61 ± 0.06 X | 0.08 ± 0.04 | 0.57 ± 0.02 Y | 1.15 ± 0.04 Y | |
| Grilled samples | 0 days | |||||
| C | 4.58 ± 1.66 B | 1.52 ± 0.08 | 0.10 ± 0.03 | 0.61 ± 0.04 | 1.21 ± 0.08 | |
| L1 | 5.29 ± 1.92 A | 1.36 ± 0.21 | 0.06 ± 0.06 | 0.69 ± 0.08 | 1.41 ± 0.23 | |
| L2 | 4.14 ± 1.42 B | 1.53 ± 0.03 a | 0.07 ± 0.01 | 0.59 ± 0.03 | 1.20 ± 0.02 b | |
| 6 days | ||||||
| C | 5.85 ± 1.84 | 1.54 ± 0.17 | 0.09 ± 0.03 | 0.60 ± 0.06 | 1.21 ± 0.13 | |
| L1 | 3.99 ± 0.28 | 1.46 ± 0.17 | 0.08 ± 0.03 | 0.58 ± 0.05 | 1.23 ± 0.11 | |
| L2 | 5.71 ± 1.73 X | 1.52 ± 0.03 a | 0.07 ± 0.00 | 0.59 ± 0.02 | 1.22 ± 0.22 b | |
| 9 days | ||||||
| C | 5.42 ± 1.80 | 1.66 ± 0.31 | 0.11 ± 0.02 A | 0.56 ± 0.07 | 1.13 ± 0.20 | |
| L1 | 4.85 ± 2.38 X | 1.53 ± 0.16 | 0.08 ± 0.02 AB | 0.61 ± 0.06 | 1.22 ± 0.15 | |
| L2 | 5.42 ± 1.40 | 1.43 ± 0.01 b,Y | 0.07 ± 0.01 B | 0.63 ± 0.03 X | 1.30 ± 0.01 a,X | |
| Factor | F value | |||||
| Form | 1.37 ns | 3.23 ns | 9.46 *** | 4.01 * | 2.30 ns | |
| St | 0.98 ns | 0.03 ns | 1.31 ns | 0.18 ns | 0.11 ns | |
| Gr | 14.99 *** | 0.57 ns | 0.14 ns | 0.14 ns | 1.42 ns | |
| Form*St | 2.68 * | 0.24 ns | 0.56 ns | 0.69 ns | 0.20 ns | |
| Form*Gr | 0.93 ns | 0.26 ns | 0.45 ns | 0.31 ns | 0.18 ns | |
| St*Gr | 0.48 ns | 0.39 ns | 1.36 ns | 0.48 ns | 0.18 ns | |
| Form*St*Gr | 0.32 ns | 1.47 ns | 3.92 * | 2.73 * | 1.43 ns | |
| PV | TBARs | Cholesterol | Total COPs | COR | ||
|---|---|---|---|---|---|---|
| (meq O2/kg Fat) | (mg MDA/kg Burger) | (mg/kg Burger) | (%) | |||
| Raw samples | 0 days | |||||
| C | 0.91 ± 0.08 c,Y | 0.81 ± 0.16 c,A | 538.27 ± 20.32 Y | 1.59 ± 0.30 b,Y | 0.29 ± 0.05 b,A | |
| L1 | 0.89 ± 0.09 c,Y | 0.53 ± 0.04 b,AB | 743.04 ± 66.67 | 1.28 ± 0.14 Y | 0.17 ± 0.04 B,Y | |
| L2 | 0.84 ± 0.06 b,Y | 0.48 ± 0.06 b,B | 728.32 ± 17.02 Y | 0.90 ± 0.25 Y | 0.12 ± 0.03 B,Y | |
| 6 days | ||||||
| C | 3.57 ± 0.17 b,Y | 4.19 ± 0.20 b,A,X | 560.44 ± 22.92 Y | 5.38 ± 0.07 a,A,Y | 0.97 ± 0.17 a,A | |
| L1 | 3.01 ± 0.20 b,Y | 1.07 ± 0.31 ab,B | 773.74 ± 8.23 Y | 2.03 ± 0.35 B,Y | 0.26 ± 0.04 B,Y | |
| L2 | 2.88 ± 0.15 a,Y | 0.55 ± 0.09 a,C | 827.74 ± 79.71 Y | 1.17 ± 0.36 B,Y | 0.14 ± 0.06 B,Y | |
| 9 days | ||||||
| C | 5.63 ± 0.20 a,A,Y | 4.86 ± 0.57 a,A | 587.53 ± 63.62 Y | 6.32 ± 0.71 a,A,Y | 1.08 ± 0.01 a,A | |
| L1 | 4.05 ± 0.31 a,B,Y | 1.22 ± 0.25 a,B | 778.46 ± 25.49 Y | 2.31 ± 0.48 B | 0.30 ± 0.04 B | |
| L2 | 3.58 ± 0.38 a,B,Y | 0.51 ± 0.03 a,C | 926.07 ± 51.88 Y | 1.19 ± 0.28 B,Y | 0.13 ± 0.04 B,Y | |
| Grilled sample | 0 days | |||||
| C | 8.04 ± 0.68 c,X | 0.93 ± 0.14 c,A | 1040.97 ± 113.46 X | 2.88 ± 0.42 c,X | 0.30 ± 0.07 c | |
| L1 | 6.14 ± 0.55 b,X | 0.63 ± 0.09 B | 1145.94 ± 166.70 | 3.98 ± 0.21 X | 0.35 ± 0.06 X | |
| L2 | 5.65 ± 0.66 c,X | 0.52 ± 0.05 b,C | 1097.02 ± 59.73 X | 2.85 ± 0.24 X | 0.26 ± 0.04 X | |
| 6 days | ||||||
| C | 13.62 ± 0.92 b,A,X | 3.17 ± 0.47 bA,Y | 1146.78 ± 124.46 X | 11.88 ± 0.03 b,A,X | 1.07 ± 0.30 b,A | |
| L1 | 11.44 ± 0.86 a,A,X | 0.97 ± 0.02 B | 1022.39 ± 9.76 X | 4.96 ± 1.01 B,X | 0.49 ± 0.10 B,X | |
| L2 | 9.15 ± 0.72 b,B,X | 0.64 ± 0.04 a,B | 1041.24 ± 10.81 X | 3.04 ± 0.51 B,X | 0.29 ± 0.05 B,X | |
| 9 days | ||||||
| C | 17.74 ± 1.01 a,A,X | 4.07 ± 0.20 a,A | 1126.62 ± 100.13 X | 17.00 ± 1.47 a,A,X | 1.51 ± 0.01 a,A,X | |
| L1 | 14.87 ± 1.09 a,AB,X | 1.02 ± 0.16 B | 1225.14 ± 100.72 X | 3.70 ± 0.30 B | 0.31 ± 0.10 B | |
| L2 | 13.12 ± 0.99 a,B,X | 0.72 ± 0.03 a,B | 1104.97 ± 114.31 X | 3.22 ± 0.76 B,X | 0.30 ± 0.10 B,X | |
| Factor | F value | |||||
| Form | 28.13 *** | 90.43 *** | 2.44 ns | 705.66 *** | 284.00 *** | |
| St | 127.38 *** | 35.94 *** | 0.88 ns | 238.65 *** | 85.21 *** | |
| Gr | 769.06 *** | 4.26 ns | 105.30 *** | 742.57 *** | 49.33 *** | |
| Form*St | 0.86 ns | 17.17 *** | 0.50 ns | 175.40 *** | 60.26 *** | |
| Form*Gr | 5.05 ns | 1.10 ns | 3.50 ns | 110.80 *** | 0.87 ns | |
| St*Gr | 29.68 *** | 1.26 ns | 0.01 ns | 42.23 *** | 0.74 ns | |
| Form*St*Gr | 0.73 ns | 0.94 ns | 1.72 ns | 58.73 *** | 5.78 ** | |
| Sensory Descriptors | ||||||||
|---|---|---|---|---|---|---|---|---|
| Sample Codes | Beef Flavor | Bloody | Salty | Juiciness | Granularity | Tenderness | Fat/Connective Tissue | Color |
| (low; high) * | (low; high) * | (low; high) * | (low; high) * | (low; high) * | (low; high) * | (low; high)* | (red; brown) * | |
| CT0 | 47.2 ab | 26.5 ab | 38.2 b | 55.3 a | 36.7 a | 55.6 a | 42.3 a | 67.2 bc |
| CT6 | 39.1 bc | 28.7 a | 39.9 ab | 52.3 a | 32.9 a | 54.8 a | 43.1 a | 77.9 ab |
| CT9 | 37.7 c | 24.5 a-c | 38.9 b | 37.6 d | 38.6 a | 45.3 ab | 49.6 a | 78.6 a |
| L1T0 | 51.9 a | 19.0 bc | 41.5 ab | 51.3 ab | 29.8 a | 55.7 a | 39.3 a | 42.0 ef |
| L1T6 | 50.2 a | 19.3 bc | 41.4 ab | 51.3 ab | 38.3 a | 51.1 ab | 40.8 a | 47.3 d–f |
| L1T9 | 50.8 a | 24.1 a–c | 42.0 ab | 39.1 cd | 38.2 a | 41.6 b | 40.2 a | 49.9 de |
| L2T0 | 52.0 a | 22.6 a–c | 40.4 ab | 51.3 ab | 34.6 a | 50.6 ab | 43.6 a | 38.0 f |
| L2T6 | 54.9 a | 20.1 bc | 42.6 ab | 49.8 a–c | 33.9 a | 54.2 a | 48.7 a | 39.7 ef |
| L2T9 | 48.6 a | 17.5 c | 45.9 a | 40.0 b–d | 36.7 a | 39.5 b | 48.4 a | 56.4 cd |
| Session n. | Compared Samples | Judges n. | Correct Answers | Significance |
|---|---|---|---|---|
| 1 | CT0 vs. L1T0 | 30 | 15 | 0.05 |
| 2 | CT0 vs. L2T0 | 30 | 17 | 0.01 |
| 3 | L1T0 vs. L2T0 | 30 | 14 | 0.1 |
| 4 | CT0 vs. CT6 | 30 | 16 | 0.05–0.01 |
| 5 | CT0 vs. CT9 | 30 | 13 | 0.2 |
| 6 | CT6 vs. CT9 | 30 | 9 | ns |
| 7 | L1T0 vs. L1T6 | 28 | 10 | ns |
| 8 | L1T0 vs. L1T9 | 28 | 13 | 0.2 |
| 9 | L1T6 vs. L1T9 | 28 | 16 | 0.01 |
| 10 | L2T0 vs. L2T6 | 28 | 12 | 0.3 |
| 11 | L2T0 vs. L2T9 | 28 | 13 | 0.2 |
| 12 | L2T6 vs. L2T9 | 28 | 10 | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbieri, S.; Mercatante, D.; Balzan, S.; Esposto, S.; Cardenia, V.; Servili, M.; Novelli, E.; Taticchi, A.; Rodriguez-Estrada, M.T. Improved Oxidative Stability and Sensory Quality of Beef Hamburgers Enriched with a Phenolic Extract from Olive Vegetation Water. Antioxidants 2021, 10, 1969. https://doi.org/10.3390/antiox10121969
Barbieri S, Mercatante D, Balzan S, Esposto S, Cardenia V, Servili M, Novelli E, Taticchi A, Rodriguez-Estrada MT. Improved Oxidative Stability and Sensory Quality of Beef Hamburgers Enriched with a Phenolic Extract from Olive Vegetation Water. Antioxidants. 2021; 10(12):1969. https://doi.org/10.3390/antiox10121969
Chicago/Turabian StyleBarbieri, Sara, Dario Mercatante, Stefania Balzan, Sonia Esposto, Vladimiro Cardenia, Maurizio Servili, Enrico Novelli, Agnese Taticchi, and Maria Teresa Rodriguez-Estrada. 2021. "Improved Oxidative Stability and Sensory Quality of Beef Hamburgers Enriched with a Phenolic Extract from Olive Vegetation Water" Antioxidants 10, no. 12: 1969. https://doi.org/10.3390/antiox10121969
APA StyleBarbieri, S., Mercatante, D., Balzan, S., Esposto, S., Cardenia, V., Servili, M., Novelli, E., Taticchi, A., & Rodriguez-Estrada, M. T. (2021). Improved Oxidative Stability and Sensory Quality of Beef Hamburgers Enriched with a Phenolic Extract from Olive Vegetation Water. Antioxidants, 10(12), 1969. https://doi.org/10.3390/antiox10121969