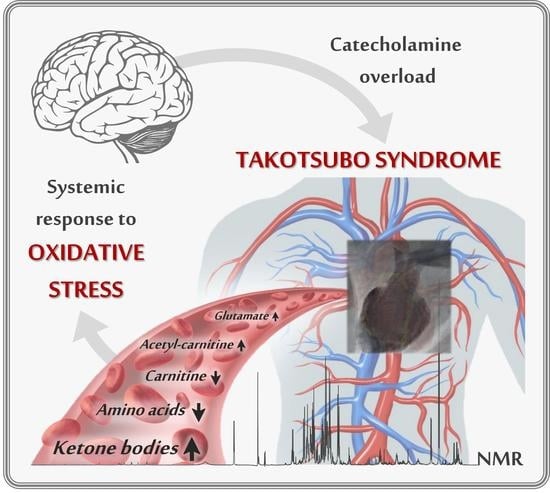
A Pilot Study on the 1H-NMR Serum Metabolic Profile of Takotsubo Patients Reveals Systemic Response to Oxidative Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Venous Serum Collection
2.2. Sample Preparation
2.3. 1H-NMR Spectroscopy
2.4. Data Analysis
2.5. Minimum Sample Size Calculation
3. Results
3.1. Serum Metabolic Profile Differentiate Takotsubo from Control Subjects
3.2. Ketone Bodies, Fatty Acid Metabolism, and Amino Acid Levels Are Unbalanced in Takotsubo Patients with Respect to Normal Values
3.3. The Metabolic Profile Correlates Significantly with the Left Ventricular Ejection Fraction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akashi, Y.J.; Goldstein, D.S.; Barbaro, G.; Ueyama, T. Takotsubo Cardiomyopathy. Circulation 2008, 118, 2754–2762. [Google Scholar] [CrossRef] [Green Version]
- Münzel, T.; Templin, C.; Cammann, V.L.; Hahad, O. Takotsubo Syndrome: Impact of endothelial dysfunction and oxidative stress. Free Radic. Biol. Med. 2021, 169, 216–223. [Google Scholar] [CrossRef]
- Dote, K.; Sato, H.; Tateishi, H.; Uchida, T.; Ishihara, M. Myocardial stunning due to simultaneous multivessel coronary spasms: A review of 5 cases. J. Cardiol. 1991, 21, 203–214. [Google Scholar]
- Trichopoulos, D.; Zavitsanos, X.; Katsouyanni, K.; Tzonou, A.; Dalla-Vorgia, P. Psychological stress and fatal heart attack: The Athens (1981) earthquake natural experiment. Lancet 1983, 321, 441–444. [Google Scholar] [CrossRef]
- Wilbert-Lampen, U.; Leistner, D.; Greven, S.; Pohl, T.; Sper, S.; Völker, C.; Güthlin, D.; Plasse, A.; Knez, A.; Küchenhoff, H.; et al. Cardiovascular Events during World Cup Soccer. N. Engl. J. Med. 2009, 358, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.; Elliott, J.; Troughton, R.; Frampton, C.; Smyth, D.; Crozier, I.; Bridgman, P. Acute Myocardial Infarction and Stress Cardiomyopathy following the Christchurch Earthquakes. PLoS ONE 2013, 8, e68504. [Google Scholar] [CrossRef] [PubMed]
- Alashi, A.; Isaza, N.; Faulx, J.; Popovic, Z.B.; Menon, V.; Ellis, S.G.; Faulx, M.; Kapadia, S.R.; Griffin, B.P.; Desai, M.Y. Characteristics and Outcomes of Patients With Takotsubo Syndrome: Incremental Prognostic Value of Baseline Left Ventricular Systolic Function. J. Am. Heart Assoc. 2020, 9, e016537. [Google Scholar] [CrossRef]
- Wittstein, I.S.; Thiemann, D.R.; Lima, J.A.C.; Baughman, K.L.; Schulman, S.P.; Gerstenblith, G.; Wu, K.C.; Rade, J.J.; Bivalacqua, T.J.; Champion, H.C. Neurohumoral Features of Myocardial Stunning Due to Sudden Emotional Stress. N. Engl. J. Med. 2005, 352, 539–548. [Google Scholar] [CrossRef]
- Pelliccia, F.; Kaski, J.C.; Crea, F.; Camici, P.G. Pathophysiology of Takotsubo Syndrome. Circulation 2017, 135, 2426–2441. [Google Scholar] [CrossRef] [PubMed]
- Odnoshivkina, U.G.; Sytchev, V.I.; Nurullin, L.F.; Giniatullin, A.R.; Zefirov, A.L.; Petrov, A.M. β2-adrenoceptor agonist-evoked reactive oxygen species generation in mouse atria: Implication in delayed inotropic effect. Eur. J. Pharmacol. 2015, 765, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Tanzilli, G.; Truscelli, G.; Arrivi, A.; Carnevale, R.; Placanica, A.; Viceconte, N.; Raparelli, V.; Mele, R.; Cammisotto, V.; Nocella, C.; et al. Glutathione infusion before primary percutaneous coronary intervention: A randomised controlled pilot study. BMJ Open 2019, 9, e025884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golbidi, S.; Li, H.; Laher, I. Oxidative Stress: A Unifying Mechanism for Cell Damage Induced by Noise, (Water-Pipe) Smoking, and Emotional Stress—Therapeutic Strategies Targeting Redox Imbalance. Antioxid. Redox Signal. 2018, 28, 741–759. [Google Scholar] [CrossRef] [PubMed]
- Black, C.N.; Bot, M.; Révész, D.; Scheffer, P.G.; Penninx, B. The association between three major physiological stress systems and oxidative DNA and lipid damage. Psychoneuroendocrinology 2017, 80, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Bertsch, T.; Volke, J.; Schmid, A.; Klingbeil, R.; Metodiev, Y.; Karaca, B.; Kim, S.-H.; Lindner, S.; Schupp, T.; et al. Narrative review of metabolomics in cardiovascular disease. J. Thorac. Dis. 2021, 13, 2532. [Google Scholar] [CrossRef]
- Lema, C.; Andrés, M.; Aguadé-Bruix, S.; Consegal, M.; Rodriguez-Sinovas, A.; Benito, B.; Ferreira-Gonzalez, I.; Barba, I. 1H NMR serum metabolomic profiling of patients at risk of cardiovascular diseases performing stress test. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Nuñez-Gil, I.J.; Andres, M.; Benito, B.; Bernardo, E.; Vedia, O.; Ferreira-Gonzalez, I.; Barba, I. Serum Metabolomic Analysis Suggests Impairment of Myocardial Energy Production in Takotsubo Syndrome. Metabolites 2021, 11, 439. [Google Scholar] [CrossRef]
- Santoro, F.; Núñez Gil, I.J.; Stiermaier, T.; El-Battrawy, I.; Guerra, F.; Novo, G.; Guastafierro, F.; Tarantino, N.; Novo, S.; Mariano, E.; et al. Assessment of the German and Italian Stress Cardiomyopathy Score for Risk Stratification for In-hospital Complications in Patients with Takotsubo Syndrome. JAMA Cardiol. 2019, 4, 892–899. [Google Scholar] [CrossRef]
- Di Marino, S.; Viceconte, N.; Lembo, A.; Summa, V.; Tanzilli, G.; Raparelli, V.; Truscelli, G.; Mangieri, E.; Gaudio, C.; Cicero, D.O. Early metabolic response to acute myocardial ischaemia in patients undergoing elective coronary angioplasty. Open Hear. 2018, 5, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Nagana Gowda, G.A.; Gowda, Y.N.; Raftery, D. Massive Glutamine Cyclization to Pyroglutamic Acid in Human Serum Discovered Using NMR Spectroscopy. Anal. Chem. 2015, 87, 3800–3805. [Google Scholar] [CrossRef] [Green Version]
- Dieterle, F.; Ross, A.; Schlotterbeck, G.; Senn, H. Probabilistic quotient normalization as robust method to account for dilution of complex biological mixtures. Application in1H NMR metabonomics. Anal. Chem. 2006, 78, 4281–4290. [Google Scholar] [CrossRef]
- van den Berg, R.A.; Hoefsloot, H.C.J.J.; Westerhuis, J.A.; Smilde, A.K.; van der Werf, M.J. Centering, scaling, and transformations: Improving the biological information content of metabolomics data. BMC Genom. 2006, 7, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trygg, J.; Wold, S. Orthogonal projections to latent structures (O-PLS). J. Chemom. 2002, 16, 119–128. [Google Scholar] [CrossRef]
- Triba, M.N.; Le Moyec, L.; Amathieu, R.; Goossens, C.; Bouchemal, N.; Nahon, P.; Rutledge, D.N.; Savarin, P. PLS/OPLS models in metabolomics: The impact of permutation of dataset rows on the K-fold cross-validation quality parameters. Mol. Biosyst. 2015, 11, 13–19. [Google Scholar] [CrossRef]
- Das, S.; Mitra, K.; Mandal, M. Sample size calculation: Basic principles. Indian J. Anaesth. 2016, 60, 652–656. [Google Scholar] [CrossRef]
- Serum Metabolome. Available online: https://serummetabolome.ca/ (accessed on 28 November 2021).
- Psychogios, N.; Hau, D.D.; Peng, J.; Guo, A.C.; Mandal, R.; Bouatra, S.; Sinelnikov, I.; Krishnamurthy, R.; Eisner, R.; Gautam, B.; et al. The Human Serum Metabolome. PLoS ONE 2011, 6, e16957. [Google Scholar] [CrossRef] [Green Version]
- Walser, M.; Hill, S.B. Free and protein-bound tryptophan in serum of untreated patients with chronic renal failure. Kidney Int. 1993, 44, 1366–1371. [Google Scholar] [CrossRef] [Green Version]
- Petrella, G.; Montesano, C.; Lentini, S.; Ciufolini, G.; Vanni, D.; Speziale, R.; Salonia, A.; Montorsi, F.; Summa, V.; Vago, R.; et al. Personalized Metabolic Profile by Synergic Use of NMR and HRMS. Molecules 2021, 26, 4167. [Google Scholar] [CrossRef] [PubMed]
- Ong, G.J.; Nguyen, T.H.; Kucia, A.; Liu, S.F.; Surikow, S.Y.; Girolamo, O.; Chong, C.R.; Chirkov, Y.Y.; Schenck-Gustafsson, K.; Frenneaux, M.P.; et al. Takotsubo Syndrome: Finally Emerging From the Shadows? Hear. Lung Circ. 2021, 30, 36–44. [Google Scholar] [CrossRef]
- Dias, A.; Núñez Gil, I.J.; Santoro, F.; Madias, J.E.; Pelliccia, F.; Brunetti, N.D.; Salmoirago-Blotcher, E.; Sharkey, S.W.; Eitel, I.; Akashi, Y.J.; et al. Takotsubo syndrome: State-of-the-art review by an expert panel—Part 1. Cardiovasc. Revascularization Med. 2019, 20, 70–79. [Google Scholar] [CrossRef]
- Siegrist, J.; Sies, H. Disturbed Redox Homeostasis in Oxidative Distress. Circ. Res. 2017, 121, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Morel, O.; Sauer, F.; Imperiale, A.; Cimarelli, S.; Blondet, C.; Jesel, L.; Trinh, A.; De Poli, F.; Ohlmann, P.; Constantinesco, A.; et al. Importance of Inflammation and Neurohumoral Activation in Takotsubo Cardiomyopathy. J. Card. Fail. 2009, 15, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Lommi, J.; Kupari, M.; Koskinen, P.; Näveri, H.; Leinonen, H.; Pulkki, K.; Härkönen, M. Blood ketone bodies in congestive heart failure. J. Am. Coll. Cardiol. 1996, 28, 665–672. [Google Scholar] [CrossRef]
- Opie, L.H.; Knuuti, J. The Adrenergic-Fatty Acid Load in Heart Failure. J. Am. Coll. Cardiol. 2009, 54, 1637–1646. [Google Scholar] [CrossRef] [Green Version]
- Nagao, M.; Toh, R.; Irino, Y.; Mori, T.; Nakajima, H.; Hara, T.; Honjo, T.; Satomi-Kobayashi, S.; Shinke, T.; Tanaka, H.; et al. β-Hydroxybutyrate elevation as a compensatory response against oxidative stress in cardiomyocytes. Biochem. Biophys. Res. Commun. 2016, 475, 322–328. [Google Scholar] [CrossRef]
- Teodoro, J.S.; Rolo, A.P.; Palmeira, C.M. The NAD ratio redox paradox: Why does too much reductive power cause oxidative stress? Toxicol. Mech. Methods 2013, 23, 297–302. [Google Scholar] [CrossRef]
- Poljsak, B.; Milisav, I. NAD+ as the Link Between Oxidative Stress, Inflammation, Caloric Restriction, Exercise, DNA Repair, Longevity, and Health Span. Rejuvenation Res. 2016, 19, 406–413. [Google Scholar] [CrossRef] [Green Version]
- Flores-Guerrero, J.L.; Westenbrink, B.D.; Connelly, M.A.; Otvos, J.D.; Groothof, D.; Shalaurova, I.; Garcia, E.; Navis, G.; de Boer, R.A.; Bakker, S.J.L.; et al. Association of beta-hydroxybutyrate with development of heart failure: Sex differences in a Dutch population cohort. Eur. J. Clin. Investig. 2021, 51, e13468. [Google Scholar] [CrossRef] [PubMed]
- de Koning, M.-S.L.Y.; Westenbrink, B.D.; Assa, S.; Garcia, E.; Connelly, M.A.; van Veldhuisen, D.J.; Dullaart, R.P.F.; Lipsic, E.; van der Harst, P. Association of Circulating Ketone Bodies with Functional Outcomes After ST-Segment Elevation Myocardial Infarction. J. Am. Coll. Cardiol. 2021, 78, 1421–1432. [Google Scholar] [CrossRef] [PubMed]
- Zordoky, B.N.; Sung, M.M.; Ezekowitz, J.; Mandal, R.; Han, B.; Bjorndahl, T.C.; Bouatra, S.; Anderson, T.; Oudit, G.Y.; Wishart, D.S.; et al. Metabolomic Fingerprint of Heart Failure with Preserved Ejection Fraction. PLoS ONE 2015, 10, e0124844. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Morales, P.; Pedraza-Chaverri, J.; Tapia, E. Ketone bodies, stress response, and redox homeostasis. Redox Biol. 2020, 29, 101395. [Google Scholar] [CrossRef] [PubMed]
- John, C.; Newman, E.V. Ketone bodies as signaling metabolites. Trends Endocrinol. Metab. 2014, 25, 42–52. [Google Scholar] [CrossRef] [Green Version]
- Yassen, K.A.; Galley, H.F.; Lee, A.; Webster, N.R. Mitochondrial redox state in the critically ill. Br. J. Anaesth. 1999, 83, 325–327. [Google Scholar] [CrossRef] [Green Version]
- Gall, W.E.; Beebe, K.; Lawton, K.A.; Adam, K.P.; Mitchell, M.W.; Nakhle, P.J.; Ryals, J.A.; Milburn, M.V.; Nannipieri, M.; Camastra, S.; et al. A-Hydroxybutyrate Is an Early Biomarker of Insulin Resistance and Glucose Intolerance in a Nondiabetic Population. PLoS ONE 2010, 5, e10883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz, M.; Labarthe, F.; Fortier, A.; Bouchard, B.; Legault, J.T.; Bolduc, V.; Rigal, O.; Chen, J.; Ducharme, A.; Crawford, P.A.; et al. Circulating acylcarnitine profile in human heart failure: A surrogate of fatty acid metabolic dysregulation in mitochondria and beyond. Am. J. Physiol.—Hear. Circ. Physiol. 2017, 313, 768–781. [Google Scholar] [CrossRef] [Green Version]
- Pauly, D.F.; Pepine, C.J. The role of carnitine in myocardial dysfunction. Am. J. Kidney Dis. 2003, 41, S35–S43. [Google Scholar] [CrossRef]
- Rosenthal, R.E.; Williams, R.; Bogaert, Y.E.; Getson, P.R.; Fiskum, G. Prevention of postischemic canine neurological injury through potentiation of brain energy metabolism by acetyl-l-carnitine. Stroke 1992, 23, 1312–1317. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Rosenthal, R.E.; Starke-Reed, P.; Fiskum, G. Inhibition of postcardiac arrest brain protein oxidation by acetyl-l-carnitine. Free Radic. Biol. Med. 1993, 15, 667–670. [Google Scholar] [CrossRef]
- Niu, Y.C.; Feng, R.N.; Hou, Y.; Li, K.; Kang, Z.; Wang, J.; Sun, C.H.; Li, Y. Histidine and arginine are associated with inflammation and oxidative stress in obese women. Br. J. Nutr. 2012, 108, 57–61. [Google Scholar] [CrossRef] [Green Version]
- Martínez, Y.; Li, X.; Liu, G.; Bin, P.; Yan, W.; Más, D.; Valdivié, M.; Hu, C.-A.A.; Ren, W.; Yin, Y. The role of methionine on metabolism, oxidative stress, and diseases. Amino Acids 2017, 49, 2091–2098. [Google Scholar] [CrossRef]
- Blachier, F.; Mariotti, F.; Huneau, J.F.; Tomé, D. Effects of amino acid-derived luminal metabolites on the colonic epithelium and physiopathological consequences. Amino Acids 2007, 33, 547–562. [Google Scholar] [CrossRef]
- Yamada, J.; Tomiyama, H.; Yambe, M.; Koji, Y.; Motobe, K.; Shiina, K.; Yamamoto, Y.; Yamashina, A. Elevated serum levels of alanine aminotransferase and gamma glutamyltransferase are markers of inflammation and oxidative stress independent of the metabolic syndrome. Atherosclerosis 2006, 189, 198–205. [Google Scholar] [CrossRef]
- Ndrepepa, G.; Kastrati, A. Alanine aminotransferase—A marker of cardiovascular risk at high and low activity levels. J. Lab. Precis. Med. 2019, 4, 29. [Google Scholar] [CrossRef]
- Ormstad, H.; Verkerk, R.; Sandvik, L. Serum Phenylalanine, Tyrosine, and their Ratio in Acute Ischemic Stroke: On the Trail of a Biomarker? J. Mol. Neurosci. 2016, 58, 102–108. [Google Scholar] [CrossRef]
- Fuchs, J.E.; Huber, R.G.; Grafenstein, S.; von Wallnoefer, H.G.; Spitzer, G.M.; Fuchs, D.; Liedl, K.R. Dynamic Regulation of Phenylalanine Hydroxylase by Simulated Redox Manipulation. PLoS ONE 2012, 7, e53005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, A.; Franco, E.; Rubio, M.; Bhalla, V.; Pressman, G.S.; Amanullah, S.; Hebert, K.; Figueredo, V.M. Usefulness of left ventricular strain analysis in patients with takotsubo syndrome during acute phase. Echocardiography 2018, 35, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, K.; Ahearn, T.; Srinivasan, J.; Neil, C.J.; Scally, C.; Rudd, A.; Jagpal, B.; Frenneaux, M.P.; Pislaru, C.; Horowitz, J.D.; et al. Alterations in Cardiac Deformation, Timing of Contraction and Relaxation, and Early Myocardial Fibrosis Accompany the Apparent Recovery of Acute Stress-Induced (Takotsubo) Cardiomyopathy: An End to the Concept of Transience. J. Am. Soc. Echocardiogr. 2017, 30, 745–755. [Google Scholar] [CrossRef] [Green Version]
- Hakuno, D.; Hamba, Y.; Toya, T.; Adachi, T. Plasma Amino Acid Profiling Identifies Specific Amino Acid Associations with Cardiovascular Function in Patients with Systolic Heart Failure. PLoS ONE 2015, 10, e0117325. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.D.; Wei, R.; Liu, E.; Yang, E.; Shi, X.; Martinovic, M.; Farrell, L.; Asnani, A.; Cyrille, M.; Ramanathan, A.; et al. Metabolite profiling of blood from individuals undergoing planned myocardial infarction reveals early markers of myocardial injury. J. Clin. Investig. 2008, 118, 3503–3512. [Google Scholar] [CrossRef]






| CTRL n = 10 | TTS n = 10 | |
|---|---|---|
| Age a | 57 (38–72) | 68 (40–84) |
| Female sex % | 100 | 90 |
| Diabetes % | 20 | 20 |
| Hypertension % | 40 | 60 |
| Smoking % | 30 | 10 |
| LVEF % a,b | 62 (50–80) | 38 (22–50) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanni, D.; Viceconte, N.; Petrella, G.; Biccirè, F.G.; Pelliccia, F.; Tanzilli, G.; Cicero, D.O. A Pilot Study on the 1H-NMR Serum Metabolic Profile of Takotsubo Patients Reveals Systemic Response to Oxidative Stress. Antioxidants 2021, 10, 1982. https://doi.org/10.3390/antiox10121982
Vanni D, Viceconte N, Petrella G, Biccirè FG, Pelliccia F, Tanzilli G, Cicero DO. A Pilot Study on the 1H-NMR Serum Metabolic Profile of Takotsubo Patients Reveals Systemic Response to Oxidative Stress. Antioxidants. 2021; 10(12):1982. https://doi.org/10.3390/antiox10121982
Chicago/Turabian StyleVanni, Domitilla, Nicola Viceconte, Greta Petrella, Flavio Giuseppe Biccirè, Francesco Pelliccia, Gaetano Tanzilli, and Daniel Oscar Cicero. 2021. "A Pilot Study on the 1H-NMR Serum Metabolic Profile of Takotsubo Patients Reveals Systemic Response to Oxidative Stress" Antioxidants 10, no. 12: 1982. https://doi.org/10.3390/antiox10121982
APA StyleVanni, D., Viceconte, N., Petrella, G., Biccirè, F. G., Pelliccia, F., Tanzilli, G., & Cicero, D. O. (2021). A Pilot Study on the 1H-NMR Serum Metabolic Profile of Takotsubo Patients Reveals Systemic Response to Oxidative Stress. Antioxidants, 10(12), 1982. https://doi.org/10.3390/antiox10121982